Spandidos Publications style
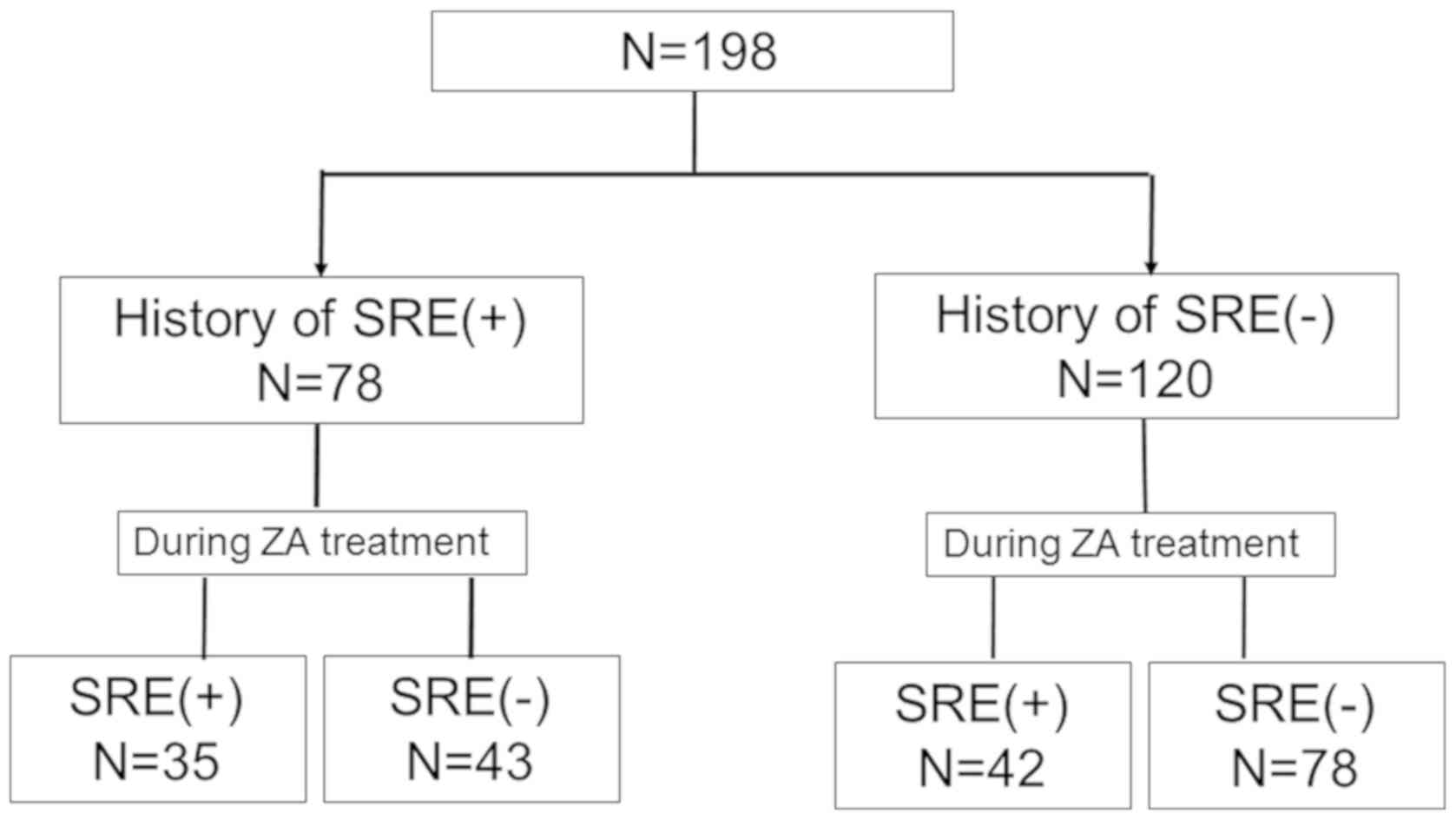
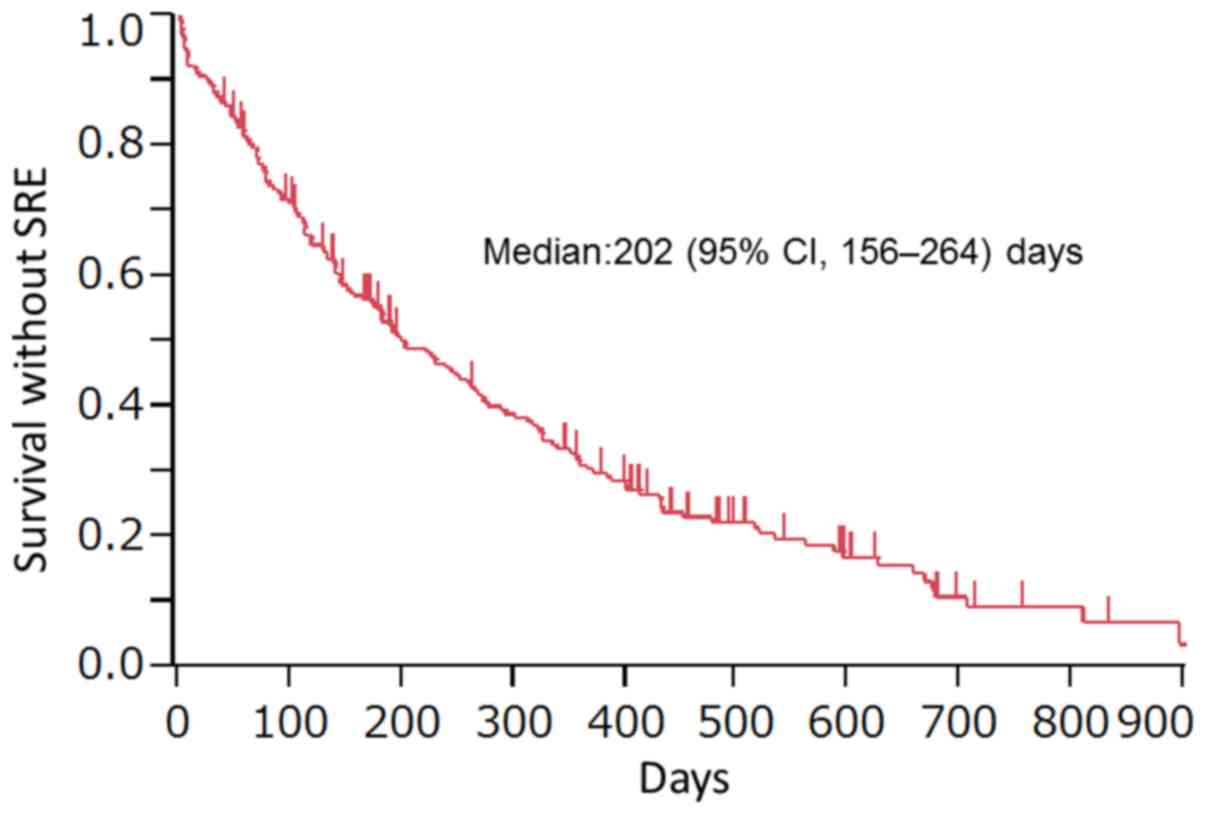
Nakahara Y, Hosomi Y, Shibuya M, Mitsufuji H, Katagiri M, Naoki K, Soejima K, Nogami N, Nagase S, Nishikawa M, Nishikawa M, et al: Multicenter study of zoledronic acid administration in non‑small‑cell lung cancer patients with bone metastasis: Thoracic Oncology Research Group (TORG) 1017. Mol Clin Oncol 11: 349-353, 2019.
APA
Nakahara, Y., Hosomi, Y., Shibuya, M., Mitsufuji, H., Katagiri, M., Naoki, K. ... Okamoto, H. (2019). Multicenter study of zoledronic acid administration in non‑small‑cell lung cancer patients with bone metastasis: Thoracic Oncology Research Group (TORG) 1017. Molecular and Clinical Oncology, 11, 349-353. https://doi.org/10.3892/mco.2019.1903
MLA
Nakahara, Y., Hosomi, Y., Shibuya, M., Mitsufuji, H., Katagiri, M., Naoki, K., Soejima, K., Nogami, N., Nagase, S., Nishikawa, M., Minato, K., Takiguchi, Y., Seki, N., Yamada, K., Seto, T., Okamoto, H."Multicenter study of zoledronic acid administration in non‑small‑cell lung cancer patients with bone metastasis: Thoracic Oncology Research Group (TORG) 1017". Molecular and Clinical Oncology 11.4 (2019): 349-353.
Chicago
Nakahara, Y., Hosomi, Y., Shibuya, M., Mitsufuji, H., Katagiri, M., Naoki, K., Soejima, K., Nogami, N., Nagase, S., Nishikawa, M., Minato, K., Takiguchi, Y., Seki, N., Yamada, K., Seto, T., Okamoto, H."Multicenter study of zoledronic acid administration in non‑small‑cell lung cancer patients with bone metastasis: Thoracic Oncology Research Group (TORG) 1017". Molecular and Clinical Oncology 11, no. 4 (2019): 349-353. https://doi.org/10.3892/mco.2019.1903