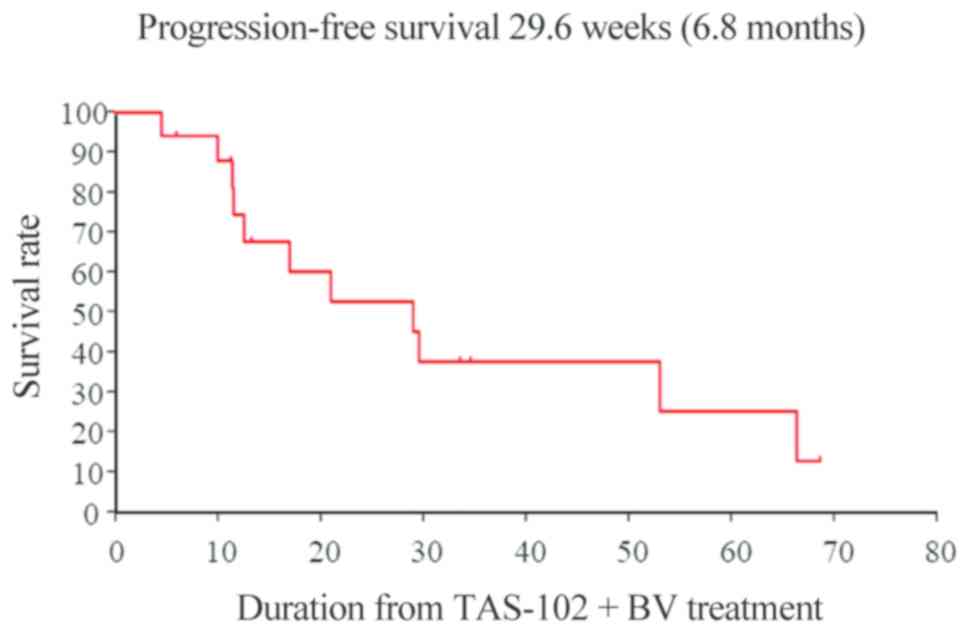
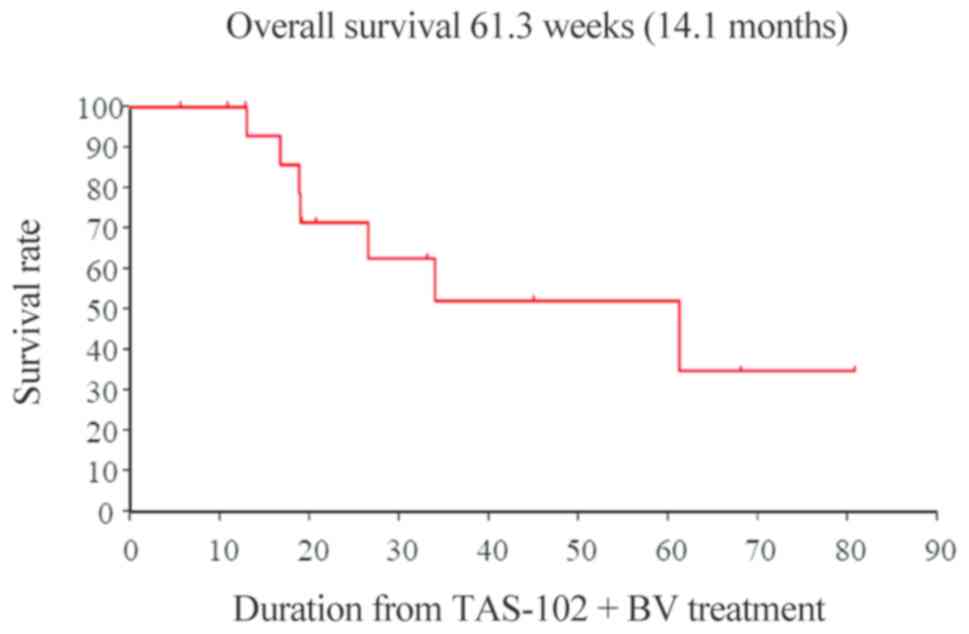
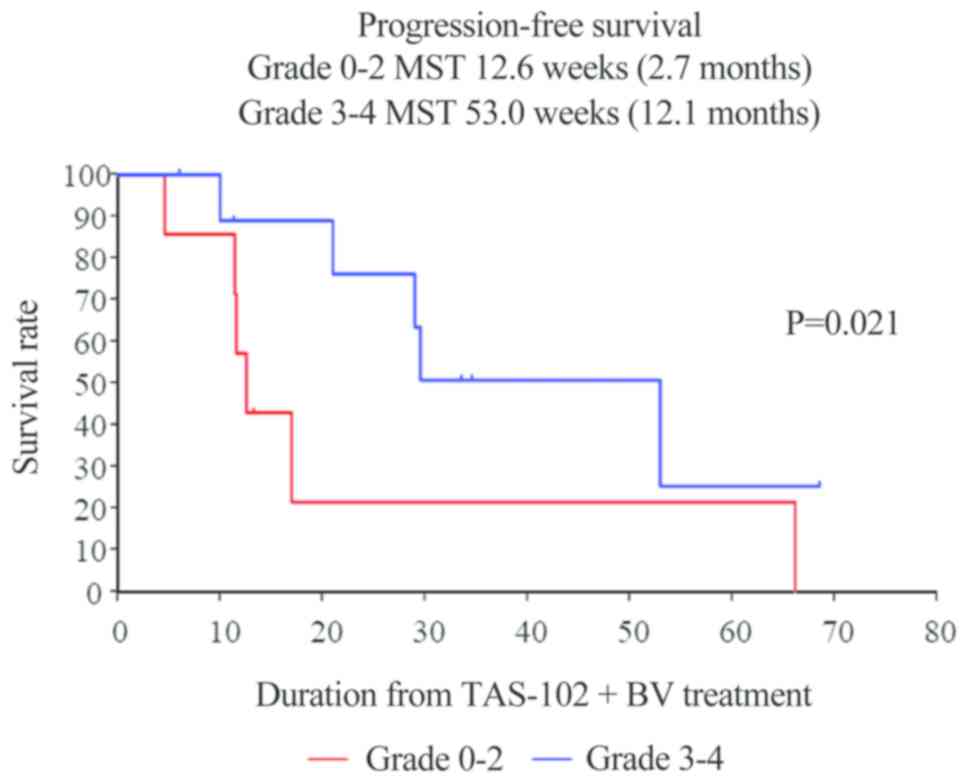
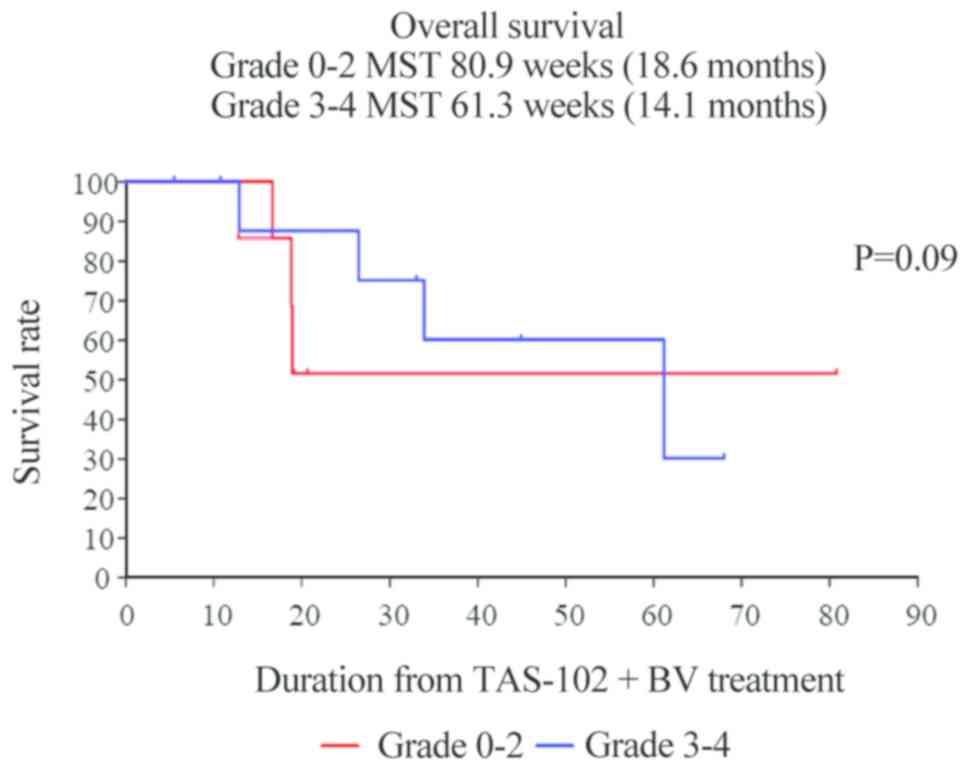
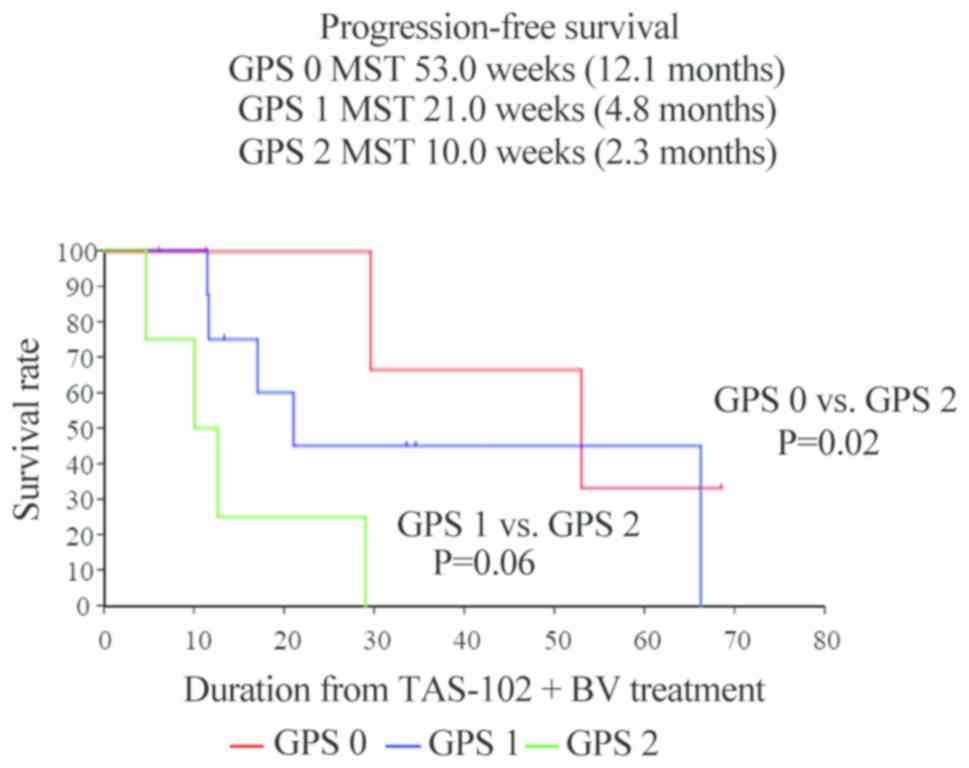
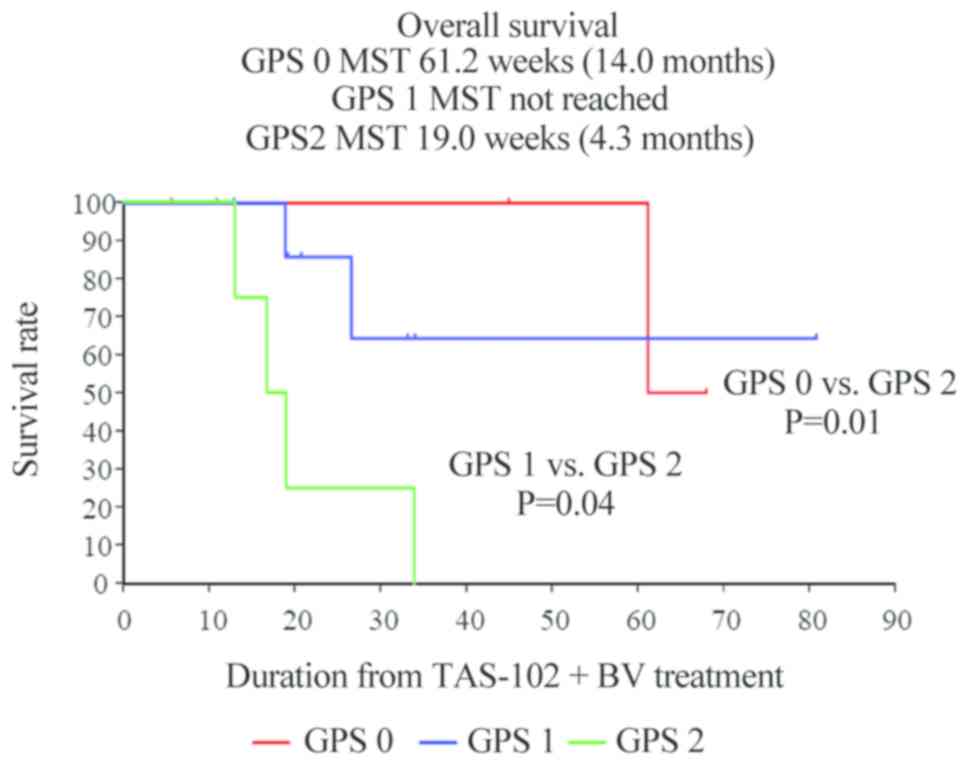
|
1
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng AL, Li J, Vaid AK, Ma BB, The C, Ahn
JB, Bello M, Charoentum C, Chen LT, de Lima Lopes G Jr, et al:
Adaptation of international guidelines for metastatic colorectal
cancer: An Asian consensus. Clin Colorectal Cancer. 13:145–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancersin 185
countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemmens V, van Steenbergen L,
Janssen-Heijnen M, Martijn H, Rutten H and Coebergh JW: Trends in
colorectal cancer in the south of the Netherlands 1975–2007: rectal
cancer survival levels with colon cancer survival. Acta Oncol.
49:784–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Borràs JM, Castells A,
Ciardiello F, Ducreux M, Haq A, Schmoll HJ and Tabernero J:
Improving outcomes in colorectal cancer: where do we go from here?
Eur J Cancer. 49:2476–2485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada Y, Denda T, Gamoh M, Iwanaga I,
Yuki S, Shimodaira H, Nakamura M, Yamaguchi T, Ohori H, Kobayashi
K, et al: S-1 and irinotecan plus bevacizumab vs. mFOLFOX6 or
CapeOX plus bevacizumab as first-line treatment in patients with
metastatic colorectal cancer (TRICOLORE): A randomized, open-label,
phase III, noninferiority trial. Ann Oncol. 29:624–631. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: RECOURSE Study Group. Randomized trial of
TAS-102 for refractory metastatic colorectal cancer. N Engl J.
372:1909–1919. 2015. View Article : Google Scholar
|
|
10
|
Melnyk O, Zimmerman M, Kim KJ and Shuman
M: Neutralizing anti-vascular endothelial growth factor antibody
inhibits further growth of established prostate cancer and
metastases in a pre-clinical model. J Urol. 161:960–963. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshino T, Oki E, Nozawa H,
Eguchi-Nakajima T, Taniguchi H, Morita S, Takenaka N, Ozawa D and
Shirao K: Rationale and design of the TRUSTY study: A randomised,
multicentre, open-label phase II/III study of
trifluridine/tipiracil plus bevacizumab versus irinotecan,
fluoropyrimidine plus bevacizumab as second-line treatment in
patients with metastatic colorectal cancer progressive during or
following first-line oxaliplatin-based chemotherapy. ESMO Open.
3:e0004112018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Douglas E and McMillan DC: Towards a
simple objective framework for the investigation and treatment of
cancer cachexia: The glasgow prognostic score. Cancer Treat Rev.
40:685–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeno S, Hashimoto T, Shibata R, Maki K,
Shiwaku H, Yamana I, Yamashita R and Yamashita Y: The
high-sensitivity modified Glasgow prognostic score is superior to
the modified Glasgow prognostic score as a prognostic predictor in
patients with resectable gastric cancer. Oncology. 87:205–214.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version5.0 Published Nov 27, 2017.
|
|
17
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Gruppo Oncologico Nord Ovest.Phase III trial of
infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan
(FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and
irinotecan (FOLFIRI) as first-line treatment for metastatic
colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol.
25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicenter,
randomised, placebo-controlled phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukihara H, Nakagawa F, Sakamoto K,
Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K and Takechi T:
Efficacy of combination chemotherapy using a novel oral
chemotherapeutic agent, TAS-102, together with bevacizumab,
cetuximab, or panitumumab on human colorectal cancer xenografts.
Oncol Rep. 33:2135–2142. 2015.PubMed/NCBI
|
|
24
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasi PM, Kotani D, Cecchini M, Shitara K,
Ohtsu A, Ramanathan RK, Hochster HS, Grothey A and Yoshino T:
Chemotherapy induced neutropenia at 1-month mark is a predictor of
overall survival in patients receiving TAS-102 for refractory
metastatic colorectal cancer: A cohort study. BMC Cancer.
16:4672016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deans DA, Tan BH, Wigmore SJ, Ross JA, de
Beaux AC, Paterson-Brown S and Fearon KC: The influence of systemic
inflammation, dietary intake and stage of disease on rate of weight
loss in patients with gastro-oesophageal cancer. Br J Cancer.
100:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morley JE, Thomas DR and Wilson MM:
Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr.
83:735–743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation- based prognostic score (mGPS) predicts cancer
survival independent of tumour site: A glasgow inflammation outcome
study. Br J Cancer. 104:726–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prager GW, Braemswig KH, Martel A, Unseld
M, Heinze G, Brodowicz T, Scheithauer W, Kornek G and Zielinski CC:
Baseline carcinoembryonic antigen (CEA) serum levels predict
bevacizumab-based treatment response in metastatic colorectal
cancer. Cancer Sci. 105:996–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|