Introduction
Breast cancer is one of the leading cause of
cancer-related deaths in women worldwide (1). Surgery is the mainstay of treatment for
breast cancer; however, a large number of patients are unable to
undergo surgery due to advanced cancer or severe underlying
conditions, such as malignant hypertension, diabetes mellitus, poor
heart and/or lung function. Therefore, new treatment strategies are
urgently required for breast cancer patients with a high surgical
risk. Over the past years, several such methods had been
investigated, including high-intensity focused ultrasound, laser
ablation, microwave ablation and radiofrequency ablation (RFA)
(2–5).
RFA is an established procedure that has been widely
used in various types of cancer, such as bone metastases, renal
cell carcinoma, hepatocellular carcinoma (HCC) and breast cancer
(4,6–8). RFA is
a local, minimally invasive approach that is accompanied by fewer
complications compared with other methods. For example, Lee et
al (9) reported that RFA was
associated with a better overall and progression-free survival
compared with transarterial chemoembolization in single HCC. Chen
et al (10), confirmed that
computed tomography (CT)-guided RFA was feasible, effective and
safe for inoperable pulmonary tumors. A meta-analysis of 15 studies
demonstrated that RFA achieved a higher complete ablation rate and
a low complication rate in breast cancer, proving that this method
is effective and safe (11).
However, whether patients diagnosed with advanced breast cancer or
synchronous severe underlying conditions can benefit from RFA
remains to be investigated.
In the present study, the efficacy of magnetic
resonance imaging (MRI)-guided RFA was evaluated in patients with
breast cancer at an advanced stage or with a high surgical
risk.
Patients and methods
Patients
The study was conducted at the Department of Breast
Surgery of Jiangsu Cancer Hospital and all eligible patients
provided written informed consent prior to treatment. Between
January 2015 and December 2015, 10 patients were diagnosed by
coarse-needle puncture and were all confirmed as invasive breast
cancer. All patients were assessed based on MRI, CT and related
data. Patients with bone metastases were evaluated by emission CT
(ECT). The inclusion criteria for RFA were as follows: i) Age
>18 years; ii) diagnosis confirmed by pathology; iii) tumor size
≤3 cm; iv) tumor distance from the chest wall ≥1 cm; v) TNM stage
IV; vi) TNM stage III with severe underlying disease; and vii)
tumor refractory to various treatments and patient refusing to
undergo surgery, chemotherapy and radiotherapy. The exclusion
criteria included tumor size >3 cm, distance from the chest wall
<1 cm, multifocal lesions and good outcome predicted with
systemic chemotherapy or radiotherapy. Prior to the initiation of
this study, all patients provided written informed consent and the
study protocol was approved by the Ethics Committee of Jiangsu
Cancer Hospital and Nanjing Medical University.
RFA procedures
Two professional radiologists performed the RFA
procedures. All patients underwent routine blood tests, coagulation
function and liver function assessment prior to RFA. Tumor stage
was assessed by B-mode ultrasonography, CT and MRI. RFA was
performed using MedSphere S500 (Medsphere, Carslbad, CA, USA) under
MRI guidance (Philips Healthcare, Amsterdam, The Netherlands) and a
17-Ga insulated magnetic-free needle (BD Biosciences, Franklin
Lakes, NJ, USA) with an ablation range of 2.5–3.0 cm.
Local anesthesia and disinfection were performed
prior to RFA and the needle was inserted into the tumor. Then, MRI
scanning at the cross-section, coronal and sagittal planes was
performed to obtain three-dimensional stereoscopic images, which
confirmed that the needlepoint was at least 0.5–1.0 cm inside the
tumor. At the same time, the volume of tumor was calculated by MRI.
Additionally, the power of RFA was gradually adjusted to 50 W and
the temperature of the needle tip was maintained at 100°C over 5–10
min. During the process, 0.9% NaCl2 with 0–5°C was
circulated into the system continuously to ensure uniform energy
distribution. After ablation, the tumor size and blood flow were
accessed by MRI. If there was residual tumor, an additional RFA
session was immediately performed. After RFA, the patients were
observed for 1 h and any complications were recorded.
Follow-up and outcome
measurements
After RFA, all patients were followed up at 1, 3, 6
and 12 months, and annually thereafter: i) The size was assessed by
physical examination (palpation); ii) the size, bleeding and
apparent diffusion coefficient (ADC) value of the tumor were
assessed by MRI; and iii) the size, bleeding and calcification of
the tumor were assessed by breast X-ray. Complete remission (CR)
was defined as complete disappearance of the tumor and no
enhancement on MRI enhanced scan. Partial remission (PR) was
defined as a decrease in the tumor volume of >50% and
non-enhanced area of >50% on MRI. Stable disease (SD) was
defined as a decrease in the tumor volume of <50% or an increase
of <25%, and a non-enhanced area <50%. Progressive disease
(PD) was defined as an increase in the volume of the tumor of
>25%.
Ethics
The procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional or regional) and with the Helsinki
Declaration of 1975, as revised in 2000 (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/).
Statistical analysis
Statistical analysis was performed with SPSS 21.0
software (SPSS Inc., Chicago, IL, USA). The pre-/post-treatment
volumes were calculated as V=πabc/6 (V, volume; a, largest
diameter; and b and c, the two other perpendicular diameters). The
data are presented as mean ± standard error of the mean.
Chi-squared and Fisher's exact tests were used to assess the
significance of each clinicopathological factor. Student's t-test
was used to evaluate the differences in tumor volume, bleeding and
ADC value. P<0.05 was considered to indicate statistically
significant differences.
Results
Patient and clinicopathological
characteristics
A total of 10 breast cancer patients who were unable
to undergo conventional surgery presented to the Jiangsu Cancer
Hospital between January and December 2015. The clinical
information of the 10 patients is summarized in Table I. All the patients were women, with a
median age of 56 years (range, 48–83 years). A total of 6 patients
had stage IV disease (lung metastasis n=3, bone metastasis n=1,
liver metastasis n=1 and mediastinal metastasis n=1) and the
remaining 4 patients had stage III disease accompanied by severe
underlying conditions.
 | Table I.Clinical characteristics of breast
cancers prior to treatment with RFA. |
Table I.
Clinical characteristics of breast
cancers prior to treatment with RFA.
|
|
|
|
|
|
Immunohistochemisty |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Sex | Age (years) | Pathology | ER | PR | HER2 | Reason for RFA | Location | Largest diameter
(cm) | Volume (ml) | TNM stage |
|---|
| 1 | F | 73 | IDC | + | + | + | Lung metastasis | L | 1.5×1.2×3.0 | 2.83 | T2N1M1 |
| 2 | F | 73 | IDC | + | + | − | Lung metastasis | R | 2.69×1.55×1.6 | 3.49 | T2N1M1 |
| 3 | F | 57 | IDC | − | − | + | Lung metastasis | R | 2.5×2.0×1.4 | 3.66 | T2N1M1 |
| 4 | F | 54 | IDC | − | − | + | Bone metastasis | L | 2.6×1.4×1.5 | 2.86 | T2N0M1 |
| 5 | F | 50 | IDC | + | − | − | Mediastinal
metastasis | L | 3.0×2.5×2.1 | 2.75 | T2N0M1 |
| 6 | F | 56 | IDC | + | + | + | Liver metastasis | L | 1.8×1.2×1.5 | 1.70 | T1N1M1 |
| 7 | F | 59 | IDC | + | + | − | Refused surgery | L | 2.4×1.7×3.0 | 6.41 | T2N1M0 |
| 8 | F | 81 | IDC | + | + | − | Refused surgery | L | 2.1×1.1×3.0 | 3.63 | T2N0M0 |
| 9 | F | 80 | IDC | + | + | − | Refused surgery | R | 2.1×1.3×1.8 | 2.57 | T2N1M0 |
| 10 | F | 48 | IDC | + | + | + | Refused surgery | R | 1.8×1.2×1.5 | 1.70 | T1N1M0 |
Outcomes following RFA
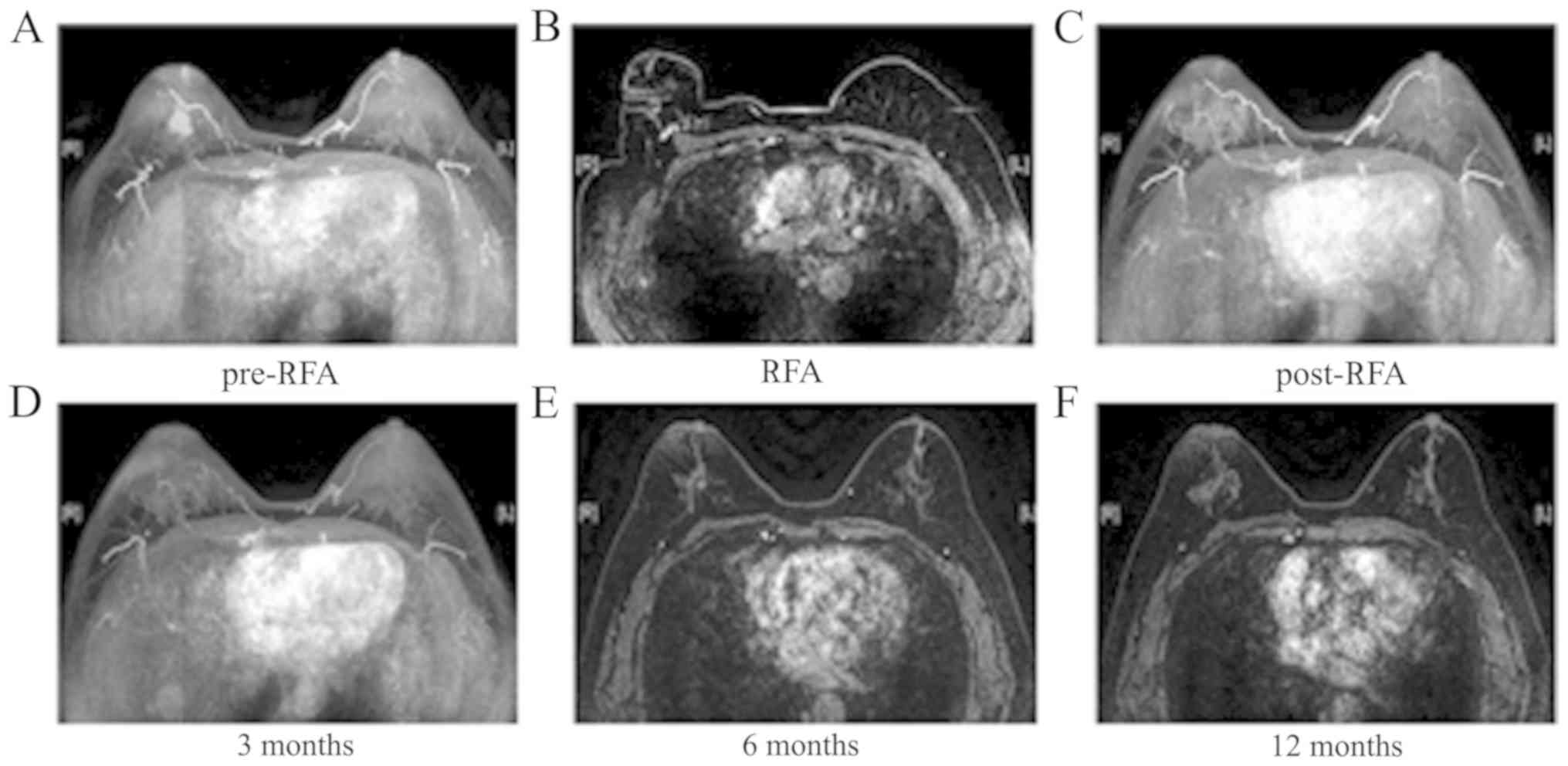
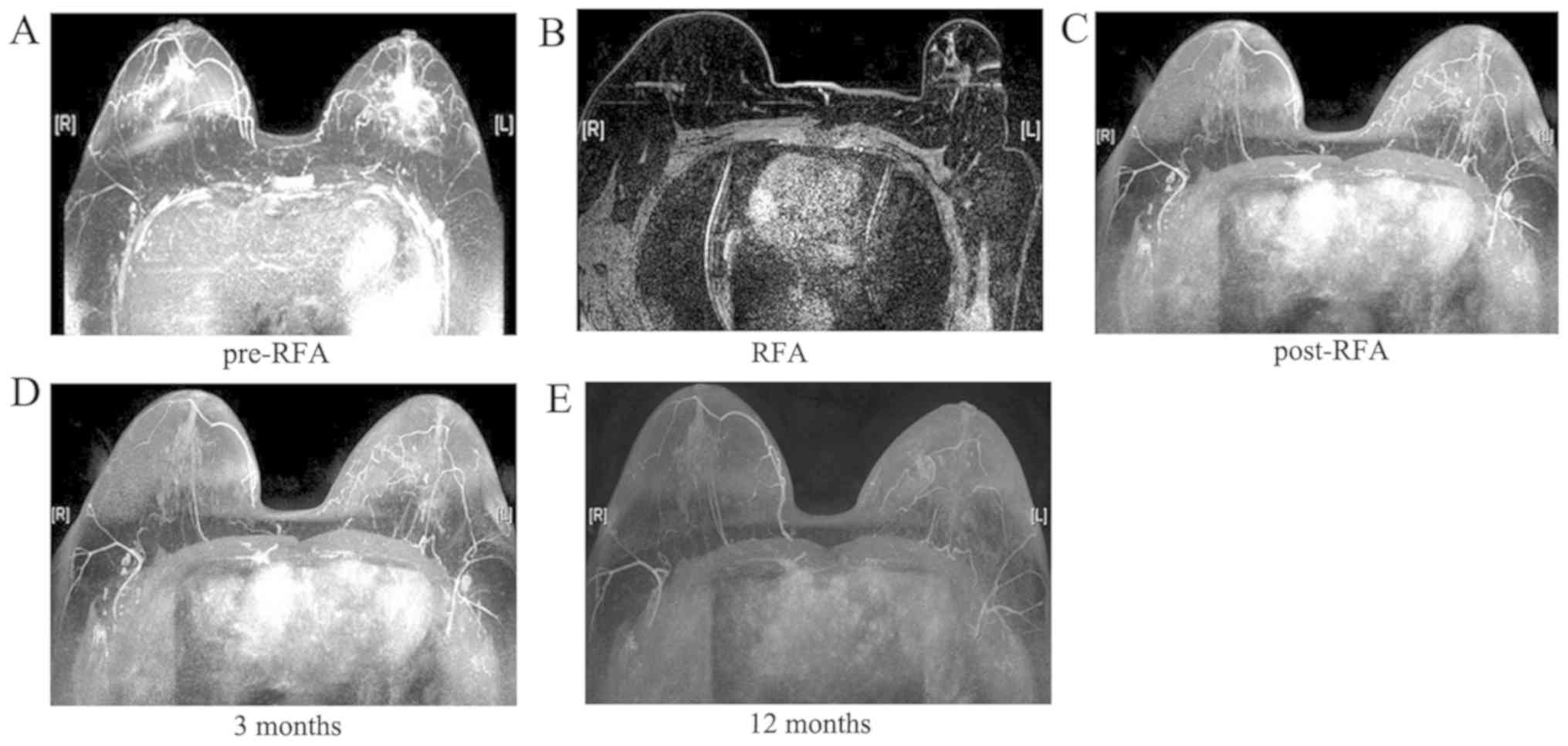
A total of 14 RFA sessions were performed in 10
patients, among whom 7 patients underwent a single RFA session, 2
patients received two sessions, and 1 patient received three
sessions. Successful placement of the RFA probe was achieved in all
patients (100%). After RFA, all the tumors became necrotic, the
blood supply disappeared and no enhancement was observed on MRI
(Figs. 1 and 2).
Compared with pre-RFA, the volume of the tumors at 6
months post-RFA had markedly decreased (Table II). No local tumor recurrence or
metastasis were detected during a mean follow-up of 19.5±3.46
months (range, 15–25 months).
 | Table II.Tumor volume pre- and post-RFA. |
Table II.
Tumor volume pre- and post-RFA.
| Patient no. | Pre-RFA (ml) | 1 month post-RFA
(ml) | 6 months post-RFA
(ml) | Volume reduction
ratio (%) |
|---|
| 1 | 2.83 | 2.80 | 0.22 | 92.2 |
| 2 | 3.49 | 3.01 | 0.28 | 92.0 |
| 3 | 3.66 | 2.81 | 0.11 | 97.0 |
| 4 | 2.86 | 2.00 | 0.00 | 100.0 |
| 5 | 2.75 | 1.85 | 0.00 | 100.0 |
| 6 | 1.70 | 2.20 | 0.00 | 100.0 |
| 7 | 6.41 | 4.12 | 0.45 | 93.0 |
| 8 | 3.63 | 3.50 | 0.24 | 93.4 |
| 9 | 2.57 | 2.23 | 0.13 | 95.0 |
| 10 | 1.70 | 2.44 | 0.00 | 100.0 |
| P-value |
| 0.12 |
<0.01 |
|
Complications
All processes were successfully completed, without
any procedure-related or severe complications. The RFA-related
complications included one case of pain in the breast and one of
perspiration, which were resolved with conservative management.
Discussion
Although combined-modality therapy has decreased the
mortality of breast cancer, a number of patients are unable to
undergo surgery due to the high surgical risk associated with old
age, advanced stage and severe underlying diseases, or refusal to
receive surgery, leading to cancer progression (1). Therefore, different therapeutic
strategies are urgently needed. With the advances in technological
innovations, new minimally invasive therapeutic alternatives for
various solid tumors have been developed, particularly RFA
(10,12,13). A
systematic review of the relevant literature demonstrated that RFA
is a feasible, safe and successful approach to breast cancer
treatment, and is associated with only minor complications
(14,15).
The present study confirmed the efficacy of
MRI-guided RFA in breast cancers with a higher surgical risk. The
RFA procedure was safe and all patients tolerated it well, without
major complications. In terms of efficacy, RFA reduced the size of
the tumor and prevented bleeding. Thus, RFA appears to be a viable
treatment option for patients with breast cancer who are not
surgical candidates. In addition, it has also been demonstrated
that RFA may be used with ultrasound and CT (16). A number of studies have confirmed MRI
as the gold standard for assessing the response of breast cancer to
RFA treatment (17–19); those studies reported that RFA may be
performed in early-stage breast tumors (size range, 0.5–2.0 cm);
however, the present study indicated that MRI-guided RFA may be an
alternative treatment option for breast cancer patients who are not
considered candidates for surgery. In addition, accumulating
evidence indicates that the most common complications of RFA
include pain, skin burns, fever, bleeding and infection, without
serious RFA-related complications (11,20).
Apart from breast cancer, benign breast diseases may also be
treated by RFA. Li et al observed that patients with breast
fibroadenoma exhibited rapid recovery, less extensive injury and
shorter hospitalization after RFA (21).
Moreover, Wang et al demonstrated that RFA
combined with transcatheter arterial chemoembolization prolonged
the progression-free survival, median survival time and survival
rate of breast cancer patients with liver metastasis (22). In a 4T1 breast cancer animal model,
Chiu et al demonstrated that RFA combined with glycated
chitosan controlled tumor progression through inducing potent
antitumor cytokine responses (23).
According to those studies, it may be hypothesized that RFA
combined with various other therapies may be applied as rescue
treatment for advanced cancers preoperatively or postoperatively,
although more evidence-based studies on breast cancer are
required.
There were certain limitations to the present study,
such as the small number of patients and the fact that it was
conducted at a single center. Further multicenter studies with a
larger patient sample population are necessary to confirm our
results.
In summary, RFA appears to be feasible, effective
and safe for breast cancer patients who have surgical
contraindications or refuse surgery. Additionally, the value of
MRI-guided RFA combined with surgery, chemotherapy, radiotherapy
and other treatments warrants further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Key Research and Development Program of China (no.
2016YFC0905900), the ‘333’ Talent Project of Jiangsu Province [no.
4(2016)], the National Key Clinical Specialist Construction
Programs of China [no. 544 (2013)] and the Natural Science
Foundation of Jiangsu Province (no. BK20151579).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
LJ, WD and ZY were responsible for RFA operation. TJ
and ZJ designed the current study and performed statistical
analysis. All authors read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent prior
to enrolment. The study protocol was approved by the Ethics
Committee of Jiangsu Cancer Hospital and Nanjing Medical
University. The procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional or regional) and with the Helsinki
Declaration of 1975, as revised in 2000 (http://www.wma.net/e/policy/17-c_e.html).
Patient consent for publication
All patients approved the publication of the current
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niederkorn A, Sadoghi B and Komericki P:
Pulsed-dye laser therapy for carcinoma in situ of the penis. Br J
Dermatol. 179:195–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lunardi A, Cervelli R, Volterrani D,
Vitali S, Lombardo C, Lorenzoni G, Crocetti L, Bargellini I,
Campani D, Pollina LE, et al: Feasibility of percutaneous
intrahepatic split by microwave ablation (PISA) after portal vein
embolization for hypertrophy of future liver remnant: The
radiological stage-1 ALPPS. Cardiovasc Intervent Radiol.
41:789–798. 2018.PubMed/NCBI
|
|
4
|
Wiksell H, Lofgren L, Schassburger KU,
Grundstrom H, Janicijevic M, Lagerstedt U, Leifland K, Nybom R,
Rotstein S, Saracco A, et al: Feasibility study on the treatment of
small breast carcinoma using percutaneous US-guided preferential
radiofrequency ablation (PRFA). Breast. 19:219–225. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haar GT and Coussios C: High intensity
focused ultrasound: Physical principles and devices. Int J
Hyperthermia. 23:89–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gazis AN, Beuing O, Franke J, Jollenbeck B
and Skalej M: Bipolar radiofrequency ablation of spinal tumors:
Predictability, safety and outcome. Spine J. 14:604–608. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wah TM, Sourbron S, Wilson DJ, Magee D,
Gregory WM, Selby PJ and Buckley DL: Renal cell carcinoma perfusion
before and after radiofrequency ablation measured with dynamic
contrast enhanced MRI: A pilot study. Diagnostics (Basel).
8:E32018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajyaguru DJ, Borgert AJ, Smith AL, Thomes
RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN and Go RS:
Radiofrequency ablation versus stereotactic body radiotherapy for
localized hepatocellular carcinoma in nonsurgically managed
patients: Analysis of the national cancer database. J Clin Oncol.
36:600–608. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Jin YJ and Lee JW: Survival
benefit of radiofrequency ablation for solitary (3–5 cm)
hepatocellular carcinoma: An analysis for nationwide cancer
registry. Medicine (Baltimore). 96:e84862017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T, Jin J and Chen S: Clinical
assessment of computed tomography guided radiofrequency ablation in
the treatment of inoperable patients with pulmonary tumors. J
Thoracic Dis. 9:5131–5142. 2017. View Article : Google Scholar
|
|
11
|
Chen J, Zhang C, Li F, Xu L, Zhu H, Wang
S, Liu X, Zha X, Ding Q, Ling L, et al: A meta-analysis of clinical
trials assessing the effect of radiofrequency ablation for breast
cancer. OncoTargets Ther. 9:1759–1766. 2016.
|
|
12
|
Chen K, Zhan MX, Hu BS, Li Y, He X, Fu SR,
Xin YJ and Lu LG: Combination of the neutrophil to lymphocyte ratio
and the platelet to lymphocyte ratio as a useful predictor for
recurrence following radiofrequency ablation of hepatocellular
carcinoma. Oncol Lett. 15:315–323. 2018.PubMed/NCBI
|
|
13
|
Bale R, Richter M, Dunser M, Levy E,
Buchberger W and Schullian P: Stereotactic radiofrequency ablation
for breast cancer liver metastases. J Vasc Interv Radiol.
29:262–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mauri G, Sconfienza LM, Pescatori LC,
Fedeli MP, Ali M, Di Leo G and Sardanelli F: Technical success,
technique efficacy and complications of minimally-invasive
imaging-guided percutaneous ablation procedures of breast cancer: A
systematic review and meta-analysis. Eur Radiol. 27:3199–3210.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen T, Hattery E and Khatri VP:
Radiofrequency ablation and breast cancer: A review. Gland Surg.
3:128–135. 2014.PubMed/NCBI
|
|
16
|
Shah DR, Green S, Elliot A, McGahan JP and
Khatri VP: Current oncologic applications of radiofrequency
ablation therapies. World J Gastrointest Oncol. 5:71–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oura S, Tamaki T, Hirai I, Yoshimasu T,
Ohta F, Nakamura R and Okamura Y: Radiofrequency ablation therapy
in patients with breast cancers two centimeters or less in size.
Breast Cancer. 14:48–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Earashi M, Noguchi M, Motoyoshi A and
Fujii H: Radiofrequency ablation therapy for small breast cancer
followed by immediate surgical resection or delayed mammotome
excision. Breast Cancer. 14:39–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto N, Fujimoto H, Nakamura R, Arai
M, Yoshii A, Kaji S and Itami M: Pilot study of radiofrequency
ablation therapy without surgical excision for T1 breast cancer:
Evaluation with MRI and vacuum-assisted core needle biopsy and
safety management. Breast Cancer. 18:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hua YQ, Wang P, Zhu XY, Shen YH, Wang K,
Shi WD, Lin JH, Meng ZQ, Chen Z and Chen H: Radiofrequency ablation
for hepatic oligometastatic pancreatic cancer: An analysis of
safety and efficacy. Pancreatology. 17:967–973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li P, Xiao-Yin T, Cui D, Chi JC, Wang Z,
Wang T, Qi XX and Zhai B: Evaluation of the safety and efficacy of
percutaneous radiofrequency ablation for treating multiple breast
fibroadenoma. J Cancer Res Ther. 12 (Suppl):C138–C142. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Liu B, Long H, Zhang F, Wang S and
Li F: Clinical study of radiofrequency ablation combined with TACE
in the treatment of breast cancer with liver metastasis. Oncol
Lett. 14:2699–2702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu HY, Leu JD, Chang CY, Lee YJ and Chen
WR: Combination of radiofrequency ablation and glycated chitosan as
treatment on a syngeneic breast tumor model. Anticancer Res.
37:2965–2974. 2017.PubMed/NCBI
|