Introduction
Acute leukemia (AL) is a heterogeneous group of
disorders that result from clonal transformation of hematopoietic
precursors through the acquisition of chromosomal rearrangements
and multiple gene mutations (1).
It was reported that AL accounts for ∼ 20,000 cancer diagnoses and
10,000 deaths annually in the United States (2). However, the pathogenesis of the
majority of AL cases remains unknown. Cytogenetic aberrations and
molecular genetic alterations are currently considered to be the
most significant prognostic factors in determining the response to
chemotherapy and survival outcome (3,4). The
analysis and characterization of the genes and proteins involved in
leukemia development at the molecular level may enhance our
knowledge of potential prognostic factors. These factors may be
roughly divided into tumor suppressor genes, proto-oncogenes and
markers of metastatic propensity and proliferation (5).
The B-cell lymphoma 2 (Bcl-2) protein is located in
the mitochondrial and nuclear membranes and the endoplasmic
reticulum (6,7). It was demonstrated that Bcl-2 is able
to prevent multi-factor-mediated apoptosis and its expression
status may determine cell survival and death. The Bcl-2 protein has
been identified in a number of tumor cells, including lymphoma
(8), breast cancer (9), prostate cancer (10) and neuroblastoma cells (11) and the Bcl-2 expression status in AL
has been a focus of investigation. However, results regarding the
association between Bcl-2 expression and its clinical significance
in AL are heterogeneous. Therefore, the aim of the present
systematic review and meta-analysis was to assess the prognostic
value of Bcl-2 expression in patients with AL.
Materials and methods
Publication selection
Studies eligible for inclusion in this meta-analysis
met the following criteria: i) published up to December, 2012 as
original articles written in English; ii) included only AL
patients; iii) measured Bcl-2 expression (protein, DNA or RNA); and
iv) provided survival data according to Bcl-2 status, including
complete remission (CR), disease-free survival and/or overall
survival. Studies were excluded if they focused exclusively on
acute promyelocytic leukemia. Multiple reports of a single study
were considered as one publication and only the most recent article
was included.
Methodological assessment
A computerized literature search of PubMed, Medline
and EMBASE databases was conducted using the terms ‘acute
leukemia’,‘Bcl-2’ and ‘survival’. The publication period was
limited to before December, 2012 and the language was limited to
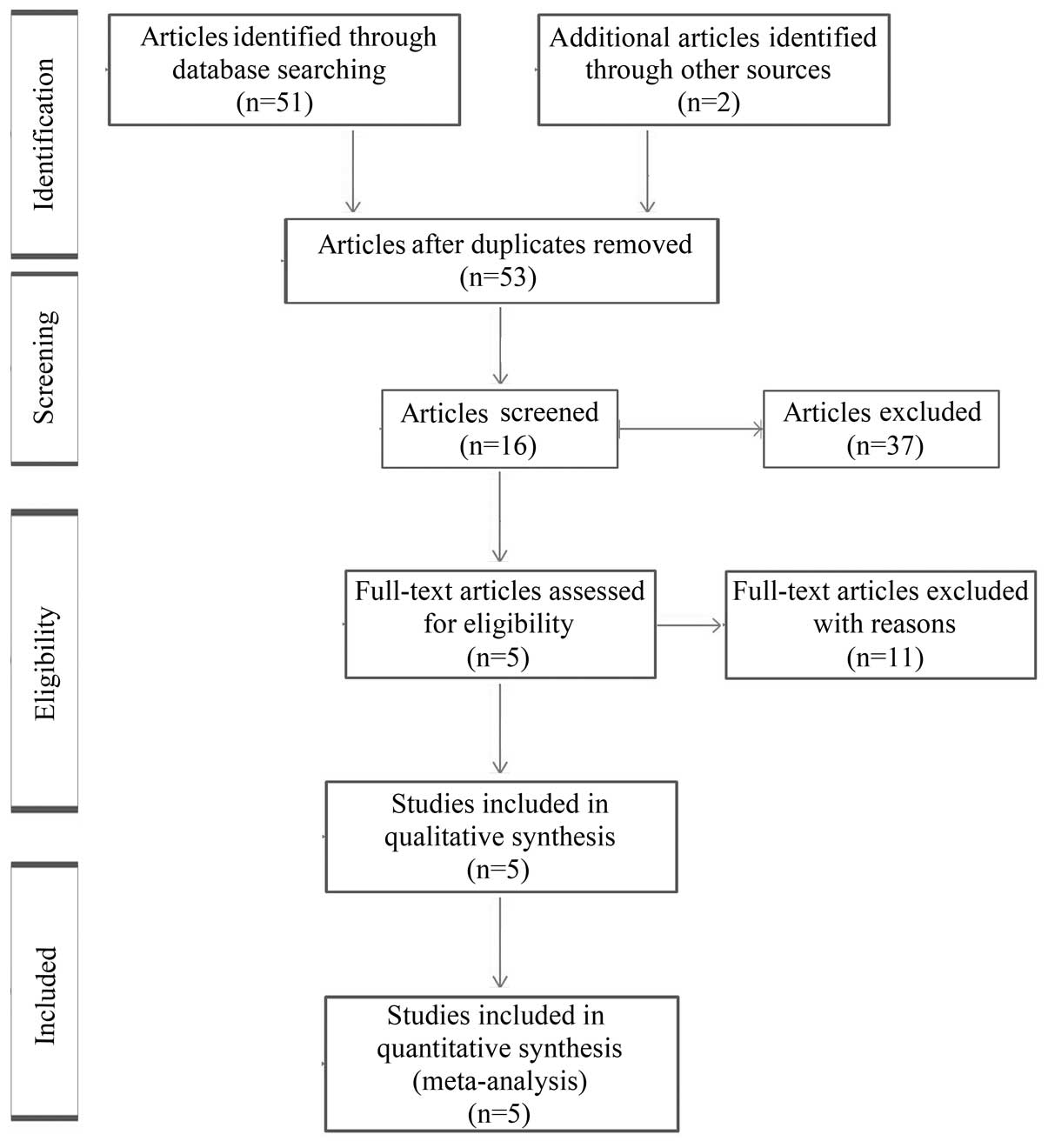
English. The initial search yielded a total of 53 publications.
After reviewing the titles and abstracts of these publications, 37
were excluded, leaving 16 as candidate articles. Of these, 11
full-text articles were excluded due to lack of survival data. A
total of 5 studies met the eligibility criteria and were finally
included in this meta-analysis (Table
I). The process of study selection is shown in Fig. 1.
 | Table I.Studies included in the
meta-analysis. |
Table I.
Studies included in the
meta-analysis.
| Authors | Country | No. of subjects | Bcl-2 expression
(%) | Refs. |
|---|
| Zhao et
al | China | 41 | 5.5–52.4 | (18) |
| Campos et
al | France | 82 | 0–95.0 | (19) |
| Karakas et
al | Germany | 156 | 0–95.0 | (20) |
| Uckun et
al | USA | 338 | 0–61.2 | (21) |
| Maung et
al | UK | 48 | 1.0–99.0 | (22) |
To avoid bias in the data abstraction process, two
co-authors (Mei Zhang and Pengcheng He) independently examined the
data from the articles and subsequently compared the results. All
the data were assessed for internal consistency and disagreements
were resolved through discussion. The characteristics abstracted
from the articles included last name of first author, year of
publication, location of the study, number of subjects, mean or
median values of white blood cell (WBC) count, incidence of Bcl-2
expression, outcome, including hematological CR rate, hazard ratio
(HR) and 95% confidence interval (CI) for CR according to the Bcl-2
status, based on multivariate analysis. When the data required for
the analysis could not be abstracted, attempts were made to contact
the investigators who conducted the studies.
The quality of evidence and the strength of
recommendations were evaluated by GRADEprofiler software, version
3.2 (http://ims.cochrane.org) (12). Any discrepancies in quality
assessments were resolved by consensus amongst authors. The overall
quality of the evidence was graded as moderate.
Quantitative data synthesis
The HR was used to assess the effect of
Bcl-2-negative status compared to that of Bcl-2-positive status on
AL patient survival. The natural logarithm of a crude HR and its
variance within the study was calculated using the abstracted
survival probabilities at each time point with the methods proposed
by Parmar et al (13) as
previously described (14). The HR
was calculated to compare the probability of survival failure
between Bcl-2-negative and Bcl-2-positive patients; an HR of <1
indicated that the Bcl-2-negative status yielded a higher survival
rate compared to the Bcl-2-positive status.
A DerSimonian-Laird random method was used to
calculate summary HRs and their 95% CIs. Begg’s funnel plots
(15) and Egger’s test (16) were used to detect possible
publication bias. The between-study variation (τ2) from
the Q statistic (17) was also
calculated.
Statistical analysis
All the statistical analyses were conducted with
Stata 12 software (StataCorp, College Station, TX, USA). P<0.05
was considered to indicate a statistically significant difference
for a summary HR. To avoid false-negative results due to the small
number of studies entered in the regression analysis, P<0.15 was
defined as significant for a meta-regression test. No adjustment of
multiple comparisons was performed due to the lack of statistical
power of the study and the existence of an a priori
hypothesis.
Results
Study selection and characteristics
A total of 5 studies, including a total of 665
subjects (235 with positive and 433 with negative Bcl-2 expression)
were finally included in our meta-analysis (Table I). The studies originated from
China (18), France (19), Germany (20), the United States (21) and the United Kingdom (22). Immunohistochemical analysis, flow
cytometry or reverse transcription-polymerase chain reaction were
used in all the trials to detect the expression of Bcl-2 protein or
mRNA. The frequency of Bcl-2 expression in AL patients varied
between 0 and 99.00%. There was no graphical or statistical
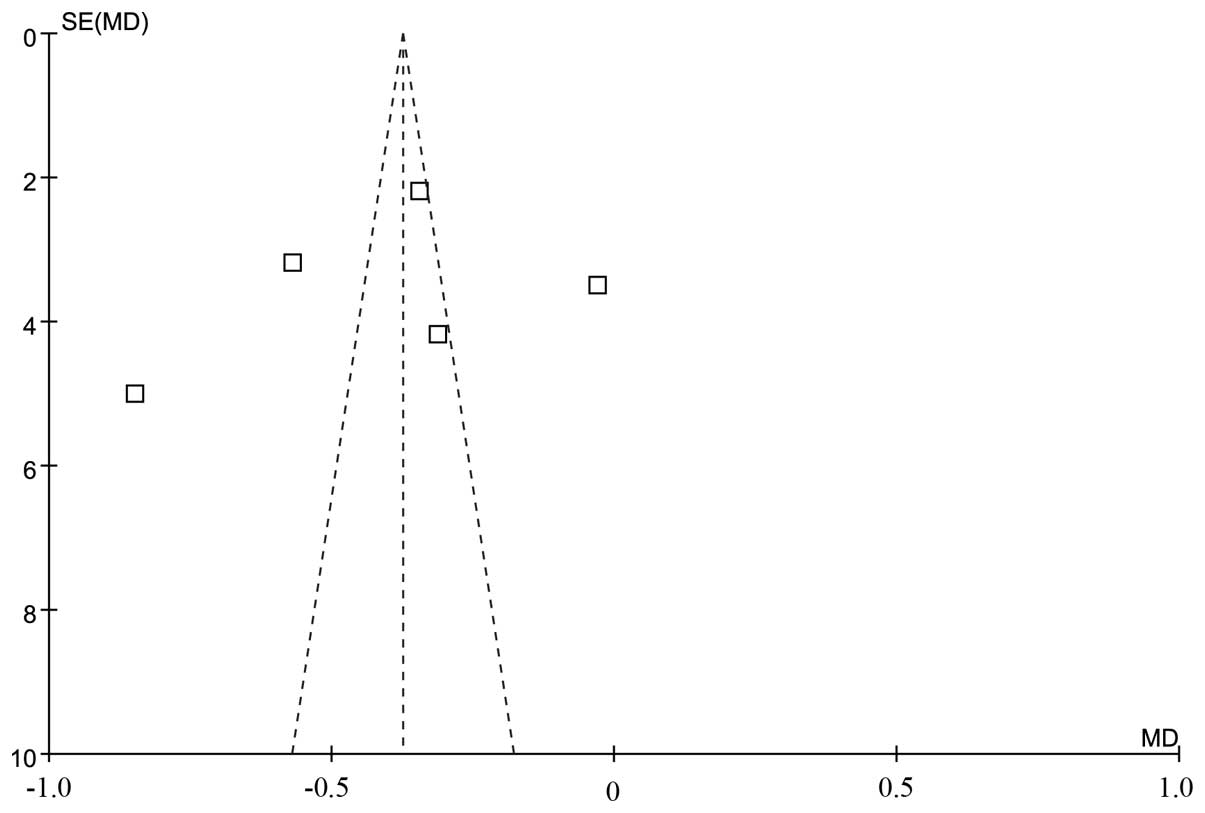
evidence of publication bias for either the WBC count or CR.
Association between Bcl-2 status and WBC
count
The results of the median WBC count according to
Bcl-2 expression status in the different studies are summarized in
Table II. In total, 461 of the 665
subjects (69.32%) revealed that the positive Bcl-2 expression
status was associated with a higher median WBC count compared to
the negative Bcl-2 expression status (three studies were considered
evaluable for meta-analysis).
 | Table II.Median WBC count according to Bcl-2
status. |
Table II.
Median WBC count according to Bcl-2
status.
| Authors | Bcl-2 status | No. of subjects | Median WBC count
(109/l) | Refs. |
|---|
| Zhao et
al | Positive | 14 | 50.24±31.09a | (18) |
| Negative | 27 | 17.45±5.95 |
| Campos et
al | Positive | 28 | 105.28±28.46a | (19) |
| Negative | 54 | 44.85±6.30 |
| Uckun et
al | Positive | 93 | 73.44±20.69a | (21) |
| Negative | 245 | 20.38±8.16 |
Survival analysis
The results of the survival analysis are presented
in Table III. In total, 327 of the
665 subjects (49.17%) indicated that positive Bcl-2 expression was
a poor prognostic factor for survival (all 5 studies were evaluable
for meta-analysis) and 338 subjects (50.83%) indicated that Bcl-2
expression was not a prognostic factor for survival (all 5 studies
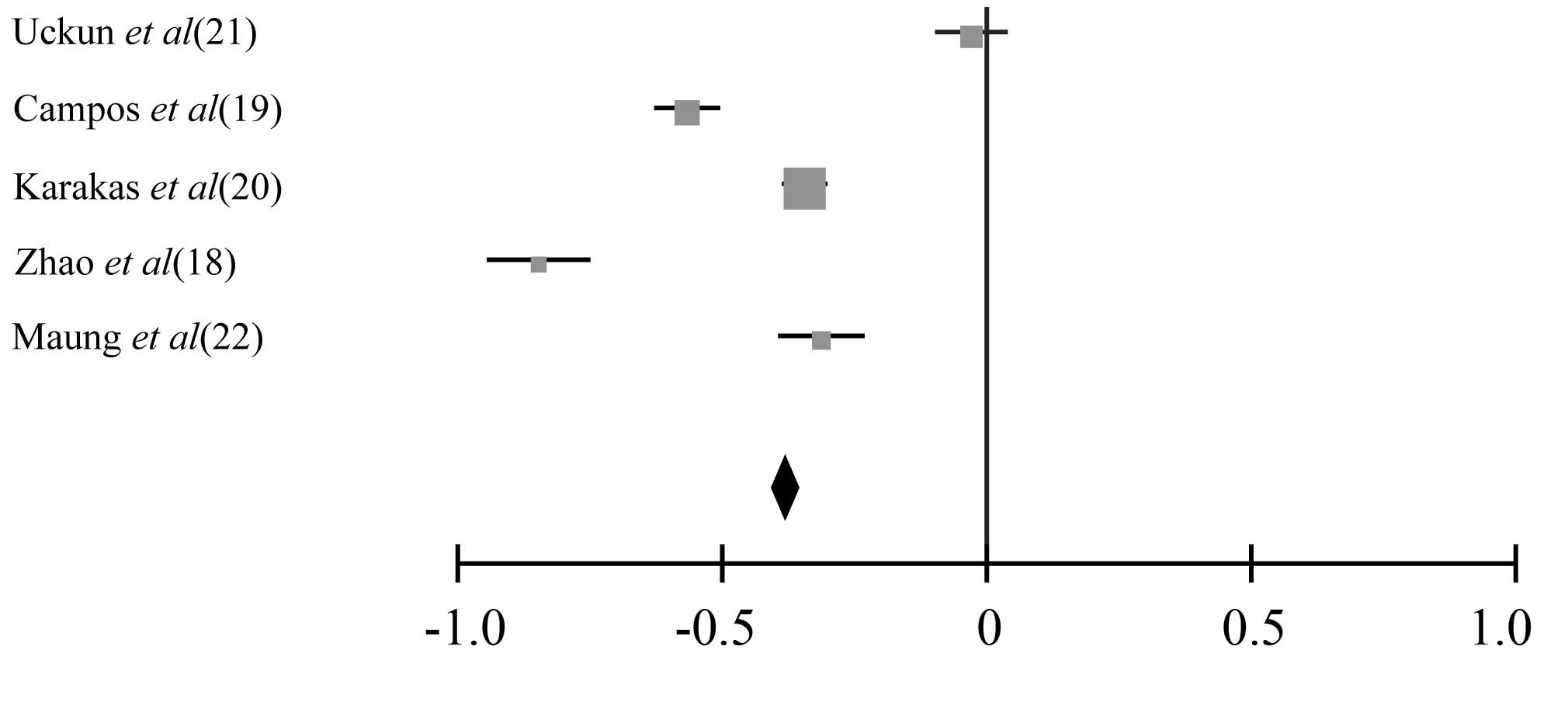
were evaluable for meta-analysis). The summary HR of Bcl-2
negativity/positivity for CR was 0.62 (95% CI: 0.53–0.81,
P<0.001). The test for heterogeneity, which evaluates the
variation in study outcomes among studies in a meta-analysis,
revealed no significant heterogeneity among the studies included in
the CR analysis (Q=4.06, df=3, P=0.36, τ2=24) (Figs. 2 and 3).
 | Table III.Complete remission according to Bcl-2
status. |
Table III.
Complete remission according to Bcl-2
status.
| Authors | Bcl-2 status | No. of subjects | Complete remission
(%) | Refs. |
|---|
| Zhao et
al | Positive | 14 | 0a | (18) |
| Negative | 27 | 85.71 |
| Campos et
al | Positive | 28 | 28.57a | (19) |
| Negative | 54 | 85.19 |
| Karakas et
al | Positive | 61 | 33.01a | (20) |
| Negative | 95 | 67.43 |
| Uckun et
al | Positive | 93 | 67.60 | (21) |
| Negative | 245 | 70.50 |
| Maung et
al | Positive | 39 | 46.51a | (22) |
| Negative | 9 | 77.78 |
Furthermore, we conducted a sensitivity test during
this meta-analysis. The exclusion of any single study did not
affect the overall results in any way.
Discussion
The prognostic significance of Bcl-2 expression in
patients with AL was previously investigated, with some results
suggesting a positive prognostic effect of Bcl-2 (23) and others reporting no differences
in clinical outcome between patients with and those without Bcl-2
expression (21). The aim of the
present systematic review and meta-analysis was to elucidate the
prognostic significance of the Bcl-2 status in patients with AL. A
meta-analysis is a statistical method used for integrating results
from independent studies for a specified outcome. Combining
relevant studies increases the statistical power and enables the
detection of effects that may be overlooked by individual studies
(24). The present meta-analysis
reported that the frequency of Bcl-2 expression was 0–99.00% in the
5 selected studies. Bcl-2-positive patients had a higher median WBC
count compared to Bcl-2-negative patients. Additionally,
Bcl-2-negative patients had >2-fold higher odds of achieving CR
compared to Bcl-2-positive patients. The summary HR of Bcl-2
negativity/positivity for CR was 0.62 (95% CI: 0.53–0.81,
P<0.001).
There were several limitations to our study. First,
the analyses were based on observational rather than prospective
controlled studies or randomized trials. Second, we used abstracted
data, whereas an individual patient data-based meta-analysis may
have provided a more robust estimate of the association. Therefore,
our results should be interpreted with caution by clinical
physicians. In addition, as is often the case with meta-analyses,
the significant effect of heterogeneity requires consideration.
Although gender and age at the time of diagnosis were not
identified as sources of heterogeneity, we cannot exclude the
potential effects of other factors, such as differences in
treatment and distinct cytogenetic categories, which were not
investigated in our analysis. Finally, publication bias, although
not directly detected, may have also affected the accuracy of our
results (17,25).
Despite the abovementioned limitations, our
meta-analysis demonstrated that Bcl-2 expression exerts a poor
effect on the survival outcome of AL patients. Our findings suggest
that it may be beneficial to distinguish Bcl-2-negative from
Bcl-2-positive AL and justify the use of risk-adapted therapeutic
strategies for AL based on the Bcl-2 expression status.
Further prospective studies should include a large
number of patients in order to reach more definitive conclusions.
In addition to the presence or absence of Bcl-2 expression, several
factors associated with Bcl-2 were suggested as being of prognostic
value, including the expression levels of CD34 transcripts and the
expression status of other Bcl family members. These factors
require further investigation in order to achieve a more accurate
estimation of the prognosis of AL.
Acknowledgements
This study was supported by grants
from the Fundamental Research Funds for the Central Universities
and the Shaanxi Province Science and Technology Development Fund,
China (nos. 2010K01-135 and 2012KTCL03-12).
References
|
1.
|
Rubnitz JE, Gibson B and Smith FO: Acute
myeloid leukemia. Hematol Oncol Clin North Am. 24:35–63. 2010.
View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
Harrison CJ, Hills RK, Moorman AV, et al:
Cytogenetics of childhood acute myeloid leukemia: United Kingdom
Medical Research Council Treatment trials AML 10 and 12. J Clin
Oncol. 28:2674–2681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Grimwade D: The clinical significance of
cytogenetic abnormalities in acute myeloid leukaemia. Best Pract
Res Clin Haematol. 14:497–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Martin B, Paesmans M, Berghmans T, et al:
Role of Bcl-2 as a prognostic factor for survival in lung cancer: a
systematic review of the literature with meta-analysis. Br J
Cancer. 89:55–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Leber B, Lin J and Andrews DW: Still
embedded together binding to membranes regulates Bcl-2 protein
interactions. Oncogene. 29:5221–5230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Soundararajan S, Chen W, Spicer EK,
Courtenay-Luck N and Fernandes DJ: The nucleolin targeting aptamer
AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer
cells. Cancer Res. 68:2358–2365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Khor LY, Moughan J, Al-Saleem T, et al:
Bcl-2 and Bax expression predict prostate cancer outcome in men
treated with androgen deprivation and radiotherapy on radiation
therapy oncology group protocol 92-02. Clin Cancer Res.
13:3585–3590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bender A, Opel D, Naumann I, et al: PI3K
inhibitors prime neuroblastoma cells for chemotherapy by shifting
the balance towards pro-apoptotic Bcl-2 proteins and enhanced
mitochondrial apoptosis. Oncogene. 30:494–503. 2011. View Article : Google Scholar
|
|
12.
|
Guyatt GH, Oxman AD, Vist GE, et al GRADE
Working Group: GRADE: an emerging consensus on rating quality of
evidence and strength of recommendations. BMJ. 336:924–926. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Parmar MK, Torri V and Stewart L:
Extracting summary statistics to perform meta-analyses of the
published literature for survival endpoints. Stat Med.
17:2815–2834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Meta-analysis of randomized clinical
trials comparing cisplatin to carboplatin in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 22:3852–3859. 2004.
View Article : Google Scholar
|
|
15.
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar
|
|
16.
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Feng JH, Guo XP, Chen YY, Wang ZJ, Cheng
YP and Tang YM: Prognostic significance of IDH1 mutations in acute
myeloid leukemia: a meta-analysis. Am J Blood Res. 2:254–264.
2012.PubMed/NCBI
|
|
18.
|
Zhao XQ, Li GS, Guo WJ, et al: Expression
of bcl-2 and bax protein and clinical significance in children with
acute leukemia. Chin J Contemp Pediatr. 1:193–195. 1999.(In
Chinese).
|
|
19.
|
Campos L, Rouault JP, Sabido O, et al:
High expression of bcl-2 protein in acute myeloid leukemia cells is
associated with poor response to chemotherapy. Blood. 81:3091–3096.
1993.PubMed/NCBI
|
|
20.
|
Karakas T, Maurer U, Weidmann E, Miething
CC, Hoelzer D and Bergmann L: High expression of bcl-2 mRNA as a
determinant of poor prognosis in acute myeloid leukemia. Ann Oncol.
9:159–165. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Uckun FM, Yang Z, Sather H, et al:
Cellular expression of antiapoptotic BCL-2 oncoprotein in newly
diagnosed childhood acute lymphoblastic leukemia: a Children’s
Cancer Group Study. Blood. 89:3769–3777. 1997.
|
|
22.
|
Maung ZT, MacLean FR, Reid MM, et al: The
relationship between bcl-2 expression and response to chemotherapy
in acute leukaemia. Br J Haematol. 88:105–109. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fakler M, Loeder S, Vogler M, et al: Small
molecule XIAP inhibitors cooperate with TRAIL to induce apoptosis
in childhood acute leukemia cells and overcome Bcl-2-mediated
resistance. Blood. 113:1710–1722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Beitinjaneh A, Jang S, Roukoz H and
Majhail NS: Prognostic significance of FLT3 internal tandem
duplication and tyrosine kinase domain mutations in acute
promyelocytic leukemia: a systematic review. Leuk Res. 34:831–836.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yanada M, Matsuo K, Suzuki T, Kiyoi H and
Naoe T: Prognostic significance of FLT3 internal tandem duplication
and tyrosine kinase domain mutations for acute myeloid leukemia: a
meta-analysis. Leukemia. 19:1345–1349. 2005. View Article : Google Scholar : PubMed/NCBI
|