Introduction
Brain metastases are the most common intracranial
tumors in adults, accounting for over half of all lesions (1,2).
Whole-brain radiation therapy (WBRT) has been a cornerstone in the
management for brain metastases for decades (3–6). Modern
surgical techniques that minimize surgical morbidity and mortality
have made resection a viable option for a larger number of
patients, including providing superior outcomes for patients with
1–3 metastases and those with symptomatic disease (7). Recently, stereotactic radiosurgery (SRS)
has been considered as a definitive or postoperative approach
instead of WBRT, to minimize the risk of cognitive impairment that
may be associated with WBRT (8).
Case report
A 74-year-old Caucasian woman was diagnosed with a
right upper lung lesion, for which she underwent a right upper
lobectomy on November 7, 2002. The histopathological evaluation
revealed an adenocarcinoma [American Joint Committee on Cancer
stage I (T2N0M0), grade 3, measuring 3.5×3.0×3.0 cm] involving the
bronchial margin. The adjacent lung parenchyma exhibited
emphysematous changes but no tumor involvement; all the resected
lymph nodes were free of disease. After 4 cycles of adjuvant
chemotherapy with paclitaxel and carboplatin, the patient was
closely followed up, including interval physical evaluations and
regular imaging studies, which included computed tomography (CT)
and positron emission tomography (PET) scans. The patient was free
of disease at her last imaging studies on December 20, 2004.
On January 27, 2005, the patient tripped and fell
while walking up a staircase; she denied suffering from headaches,
confusion, dizziness or visual disturbances and was subsequently
treated for a right arm fracture. On March 15, 2005, the patient
presented with altered mental status; an MRI scan of the brain
performed on the same day revealed a large enhancing right frontal
lobe mass, measuring 3.3×3.0×3.1 cm in the anteroposterior,
transverse and craniocaudal dimensions, respectively. The lesion
was associated with extensive surrounding edema in the right
frontal lobe, causing a mass effect on the genu of the corpus
callosum and adjacent left frontal lobe. There was a second
enhancing focus, measuring 6 mm, within the vermis. No other
abnormal parenchymal enhancement was identified. Due to the
significant edema associated with the frontal lesion and the
altered mental status, the patient underwent a right frontal
craniotomy and tumor resection and fared well postoperatively. The
histopathological evaluation revealed a fairly well-differentiated
metastatic adenocarcinoma; the immunohistochemical staining results
were positive for cytokeratin (CK)7 and thyroid transcription
factor-1 and negative for CK 20 and thyroglobulin, which were
compatible with metastasis from a primary lung tumor.
After consenting to the procedure, the patient was
scheduled for postoperative Gamma Knife SRS. On April 1, 2005,
following placement of a stereotactic frame with local anesthetic,
the patient was positioned supine on an MR gantry for a
double-contrast scan. Relevant images were selected and transferred
to the Gamma Knife planning station for treatment planning.
Following a neuroradiology review, no additional lesions were
identified; only the postoperative residual cavity in the right
frontal region and the vermian metastasis were noted on the
pre-procedure MRI. The matrices were set over the target areas. The
initial frontal lesion was planned out using 15 isocenters with a
combination of 4-, 8- and 18-mm collimators with a total volume of
~10.2 cm3. A dose of 16 Gy was selected, prescribed to
the 50% isodose line. A single isocenter with 8-mm collimator was
used for the vermian lesion (with a volume of 0.6 cm3)
and a dose of 20 Gy was selected to the 50% isodose line. The
patient tolerated the procedure well.
The patient was again closely followed up with
serial clinical evaluations and imaging; she fared well post-SRS
and did not experience headaches or any other neurological
complaints. The follow-up brain MRIs, performed every 3 months,
revealed good control of the frontal lobe cavity and the vermian
lesion, with no additional new lesions.
After 8 months (December 9, 2005), on a follow-up
brain MRI, a 4-mm enhancing focus was identified in the left
posterior parietal subcortical white matter, with minimal
associated FLAIR signal abnormality. This lesion was new and
considered to be consistent with a metastatic focus. Otherwise,
there continued to be no evidence of local recurrence or metastatic
disease progression systemically, apart from the new brain lesion.
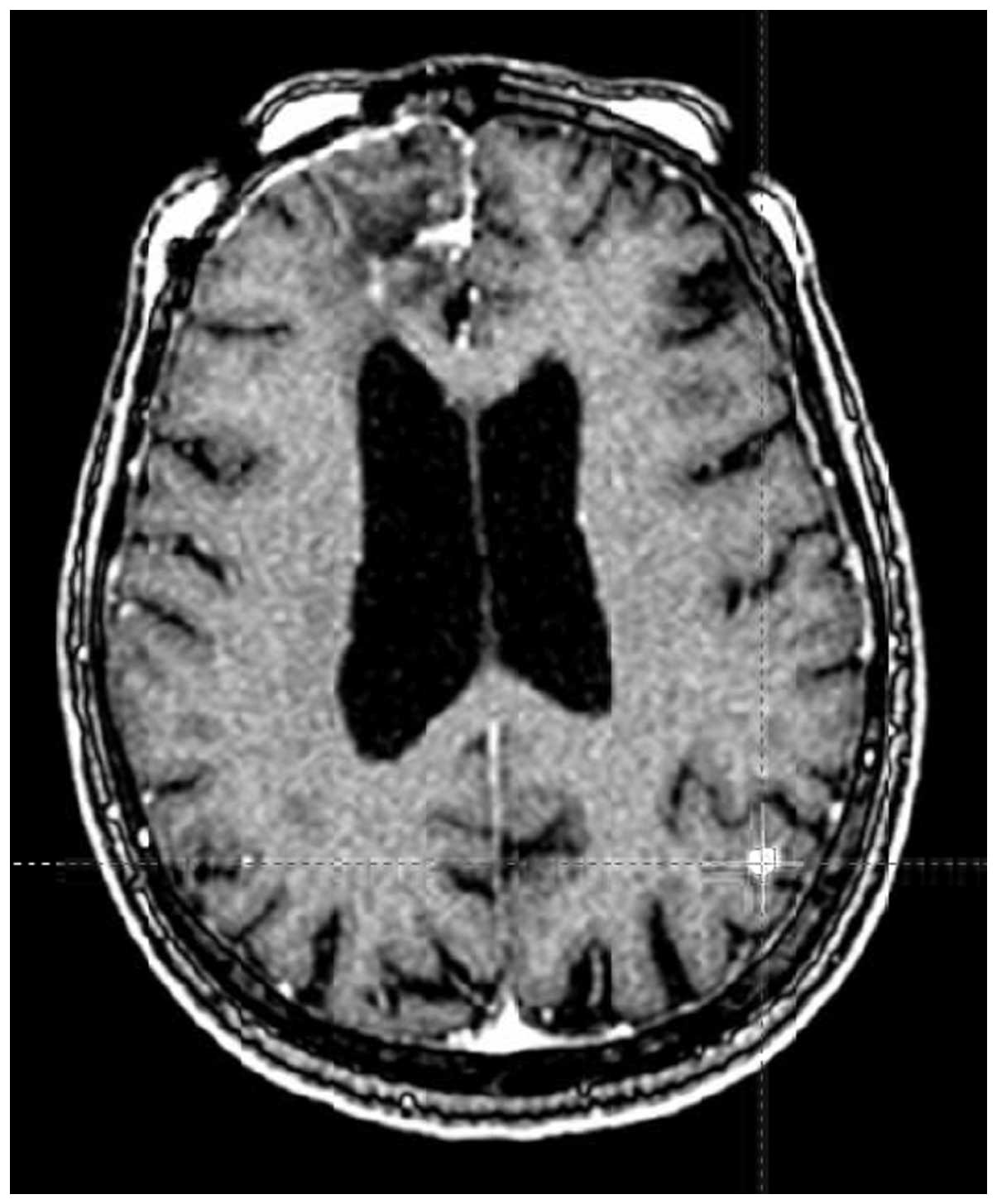
The patient underwent a second Gamma Knife SRS treatment on
December 29, 2005. The pre-SRS MRI reviewed by the neuroradiologist
only showed the metastatic lesion that was treated. The previously
treated cerebellar lesion had largely disappeared; the frontal
lesion remained small and stable, with an excellent collapse of the
cavity. Based on this information, a treatment plan was generated
using an 8-mm collimator over the left parietal occipital
metastatic lesion, with 4 isocenters circumferentially covering the
metastatic lesion. Based on the previous excellent response to 20
Gy for a single metastatic lesion, 20 Gy was again selected
(Fig. 1).
The patient was again closely followed up. All the
physical examinations and imaging studies revealed no disease
progression until April 23, 2007 (16 months after the second SRS
treatment). At that time, although the patient remained clinically
asymptomatic, a brain MRI revealed the development of two new
metastatic lesions: One within the left precentral gyrus, and
another in the posterior occipital area. A follow-up MRI on July 8,
2007 revealed interval development of enhancement in the high left
posterior frontal lobe, along with persistence of the lesions seen
in the previous scan. Based on the patient's good control and
response to the previous Gamma Knife treatments and the fact that
the brain disease remained focal, the patient, the neurosurgeon and
the radiation oncologist concurred in selecting Gamma Knife SRS
over whole-brain radiation therapy (WBRT). On August 7, 2007 (20
months after the second SRS treatment and 28 months since being
first diagnosed with brain metastases), the patient received a
third Gamma Knife SRS treatment.
Following this treatment, the patient's serial MRI
scans revealed completely stable intracranial disease (with
additional scans revealing no lung disease or extracranial
progression) up to September 13, 2010, when a follow-up brain MRI
revealed progression of five intracranial lesions. As the patient
continued to fare well clinically, a repeat brain MRI on April 11,
2011 revealed a marginal increase in the size of the left parietal
metastatic lesion, but a stable appearance of the other metastatic
lesions. The patient was offered WBRT vs. repeat Gamma Knife SRS;
after a thorough discussion of the risks and benefits, she
consented to a repeat course of SRS and received her fourth Gamma
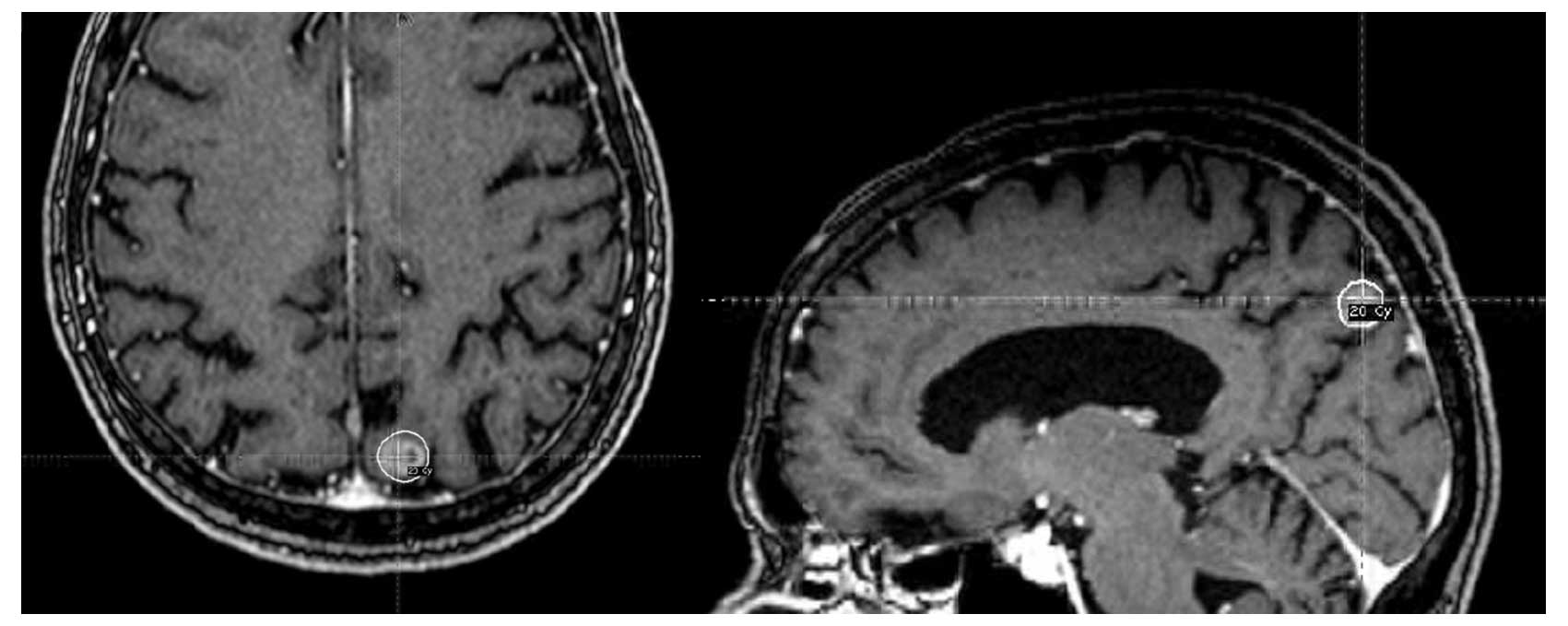
Knife SRS on May 13, 2011 (45 months after the third SRS treatment
and 71 months since being diagnosed with brain metastases) to the
one lesion that was known to be new and progressing in the right
parietal area; this was performed utilizing 2 isocenters with a
total of 20 Gy prescribed to the 50% isodose line (Fig. 2).
Notably, the patient's lung disease remained
controlled up to October, 2011; at that time, although she remained
asymptomatic, a follow-up CT demonstrated occlusion of the bronchus
of the remaining portion of the right lung and soft tissue density
suggestive of a recurrence. A subsequent PET/CT scan confirmed
prominent uptake within this region, with no other evidence of
metastatic disease. The patient received a course of palliative
radiation therapy of 30 Gy in 10 fractions to the progressive chest
disease, which was completed on December 14, 2011, followed by
initiation of targeted therapy with erlotinib.
The patient remained highly functional, without
significant neurological symptoms and with largely stable
intracranial disease until October, 2014 (>10 years since the
first diagnosis of brain metastasis), at which point she had five
growing brain lesions that were associated with vasogenic edema and
local mass effect. On October 23, 2014 (41 months after the fourth
SRS treatment and 112 months since being diagnosed with brain
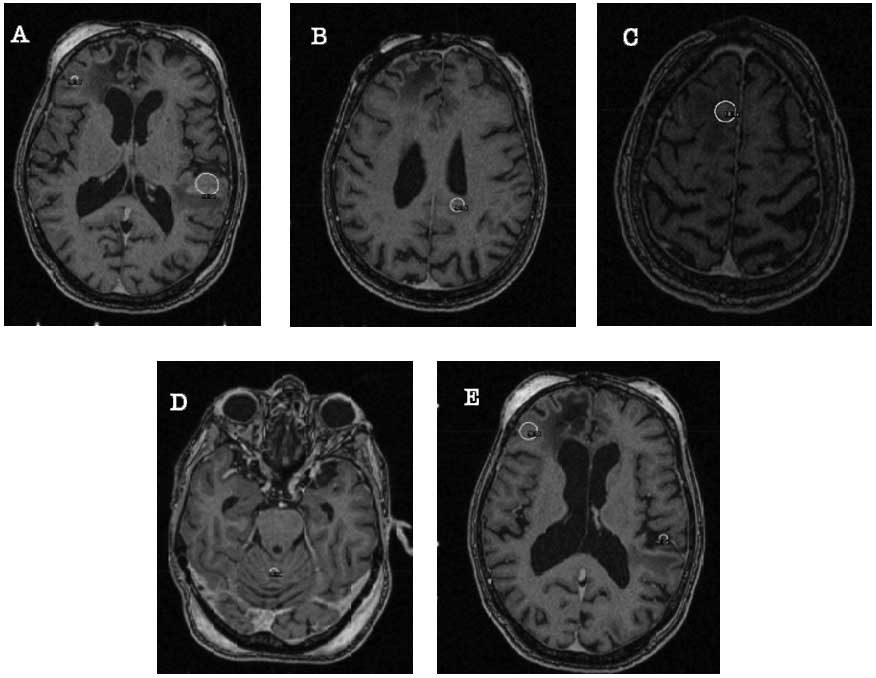
metastases), the patient, then aged 84 years, received her fifth
Gamma Knife SRS: The first lesion in the right frontal lobe (with a
total volume of 0.57 cm3) was targeted with 18 Gy
prescribed to the 50% isodose line, covering 100% of the target;
the second lesion in the left atrial region (with a total volume of
0.29 cm3) was targeted with 18 Gy prescribed to the 50%
isodose line, covering 100% of the target; the third lesion in the
right frontal lobe (with a total volume of 0.57 cm3) was
targeted with 18 Gy prescribed to the 50% isodose line, covering
100% of the target; the fourth lesion in the left temporal lobe
(with a total volume of 1.4 cm3) was targeted with 16 Gy
prescribed to the 50% isodose line, covering 100% of the target;
and the 5th lesion in the cerebellar vermis (with a total volume of
0.09 cm3) was targeted with a dose of 20 Gy prescribed
to the 50% isodose line, covering 100% of the target (Fig. 3). The patient again tolerated the
treatment well and without complications.
On the last follow-up on November 17, 2014, before
this case report was submitted, the patient remained highly
functional, with stable intracranial disease 10.5 years since first
developing brain metastases, and with stable lung disease. The
patient occasionally noted problems with her energy levels;
however, she had no other health complaints, no sleep disturbances
and no neurological symptoms, with the exception of occasional
headaches. The patient reported no dizziness, confusion, declining
mentation or any other neurological issues.
Discussion
The cumulative incidence of brain metastases in lung
cancer patients has been reported in two series: One from the
Metropolitan Detroit Cancer Surveillance System, reporting the
highest incidence in primary lung cancer patients (19.9%) compared
with melanoma (6.9%), renal (6.5%), breast (5.1%) and colorectal
(1.8%) primary cancers (9); and the
other from a Dutch series, reporting a 5-year cumulative incidence
of 7.4, 16.3, 9.8, 5.0 and 1.2% in patients with melanoma and lung,
renal, breast and colorectal carcinoma, respectively (10).
The Recursive Partitioning Analysis (RPA) developed
by the Radiation Therapy Oncology Group (RTOG) likely remains the
most reliable and widely used prognostic index for brain
metastases. The RPA divides patients with brain metastases into 3
classes as follows: Class I (best survival) are patients with a
Karnofsky performance status (KPS) of ≥70, aged <65 years, with
a controlled primary tumor, and no other systemic metastases; class
III (worst survival) includes patients with a KPS <70; these
patients have a median survival of only 2.3 months; finally class
II includes all patients intermediate between classes I and III,
with a median survival of 4.2 months (11). Based on the RPA, our patient belonged
to class II at presentation, with an expected median survival of
4.2 months had she received WBRT. The RPA did not account for the
primary diagnosis; instead, the diagnosis-specific graded
prognostic assessment (DS-GPA) was developed later, based on a
retrospective analysis of 4,259 patients with consideration of the
primary diagnosis (12). Based on
DS-GPA, our patient's score at presentation was 2.5, with an
estimated median survival of 9.4 months.
A number of RTOG studies have attempted to identify
the optimal dose and fractionation for WBRT in the setting of brain
metastases, including RTOG 6901, 7361 and 7601. The cumulative
experience obtained from these RTOG studies revealed that: i) 30 Gy
in 10 fractions is the most widely accepted, tolerable and
effective fractionation in this patient subset; ii) 20 Gy in 5
fractions, 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in
15 fractions, 40 Gy in 20 fractions and 50 Gy in 20 fractions did
not achieve superior results with respect to palliation of the
symptoms, median time to progression, cause of death or median
survival; and iii) ultra-short fractionation (10 Gy in 1 fraction
and 12 Gy in 2 fractions) is not as effective as standard
fractionation (20–30 or 40 Gy in 1–4 weeks) for palliation of
patients with brain metastases (3–6).
Surgery is now widely applied, particularly in
patients with 1–3 brain metastases, aiming primarily to provide
prompt symptomatic relief, obtain histological diagnosis and
possibly improve local control. With the application of modern
surgical techniques, the morbidity and mortality have been
significantly reduced and the option of resection has become
feasible for a larger number of patients, including those with
lesions in eloquent as well as non-eloquent regions of the brain
(7). An overall survival (OS)
advantage has also been reported in two randomized clinical trials
comparing surgery plus WBRT vs. WBRT alone. Patchell et al
(13) randomized 48 single-brain
metastasis patients to either WBRT or surgery followed by WBRT (25
in the surgical group and 23 in the radiation group). Patients who
had undergone surgical resection exhibited a significant OS
improvement compared with the WBRT alone patients (40 vs. 15 weeks,
respectively; P<0.01). Furthermore, surgical patients had fewer
recurrences in the brain, and a better quality of life compared
with WBRT alone patients. Noordijk et al (14) randomized 66 patients with a single
brain metastasis to surgery plus WBRT vs. WBRT alone. The patients
were stratified for lung cancer vs. other primary sites and for
progressive vs. stable systemic disease. The study reported that,
among 63 evaluable patients, surgery plus WBRT achieved a longer OS
(median, 10 vs. 6 months, respectively; P=0.04). The largest
difference between the two treatment arms was observed in patients
with inactive extracranial disease (median, 12 vs. 7 months,
respectively; P=0.02). Patients with active extracranial disease
had an equal median OS of 5 months, regardless of the treatment
modality. Patients who were randomized to surgery and WBRT remained
functionally independent for a longer period of time. In addition,
patients aged >60 years had a hazard ratio of 2.74 (P=0.003) for
death compared with younger patients. The majority of the patients
succumbed to to systemic disease progression (14).
Stereotactic radiosurgery (SRS) utilizes multiple
convergent beams to deliver high-precision radiation to a defined
target volume with a rapid dose fall-off, with the ultimate goal of
delivering a high dose per fraction to the target volume and
minimal radiation to the surrounding clinical structures; this
requires high precision for localization of the target volume, as
well as patient positioning during treatment. In our institution,
SRS is delivered by the Leksell Gamma Knife Perfexion system
(Elekta AB, Stockholm, Sweden) that uses 192 cobalt-60 gamma ray
sources spatially oriented so that all the beams converge at a
single point (isocenter). The Gamma Knife is able to provide a
target accuracy of 0.1–1 mm (15).
Other methods of delivering SRS include high-energy X-rays produced
by linear accelerators, or even charged particles, such as protons
produced by cyclotrons (16,17).
Initially, SRS was investigated as a boost after
WBRT in patients with 1–3 newly diagnosed brain metastases. RTOG
9508 demonstrated superior survival (6.5 vs. 4.9 months), a higher
response rate after 3 months of treatment and better local control
after 1 year of treatment (82 vs. 71%) for patients receiving a
radiosurgery boost (18). However, an
increasing number of studies (none within the context of randomized
clinical trials) reported that SRS may be as effective as surgery,
with comparable local control (19–22).
Yamamoto et al (23) recently
reported the results of a study which enrolled patients with 1–10
newly diagnosed brain metastases (the largest tumor was <10 ml
in volume and <3 cm in longest diameter; the total cumulative
volume was ≤15 ml) and a KPS score of ≥70 from 23 facilities in
Japan; the results of the study suggested that SRS without WBRT in
patients with 5–10 brain metastases is non-inferior to treatment in
patients with 2–4 brain metastases and may be a suitable
alternative for WBRT in patients with ≤10 brain metastases
(23). The EORTC 22952–26001 study
included 359 patients with 1–3 brain metastases who underwent SRS
or surgery, with 100 patients allocated to obsrvation and 99
allocated to adjuvant WBRT. While adjuvant WBRT reduced
intracranial relapses and neurological deaths, it failed to improve
the OS or the duration of functional independence (24).
The treatment approach to brain metastases has been
evolving over the last couple of decades, with advances in
diagnostic radiology, radiation oncology technology, surgical
techniques and systemic therapies, all leading to longer survival.
Therefore, several institutions are selecting SRS as a definitive
or postoperative approach over WBRT to minimize the risk of
cognitive decline (8). Asher et
al recently proposed a new treatment paradigm, namely
neoadjuvant SRS followed by surgical resection. A total of 47
patients with a total of 51 brain metastatic lesions received
neoadjuvant SRS followed by surgical resection. The 1-year OS and
local control rates were 60 and 85.6%, respectively. That study
concluded that neoadjuvant SRS is a safe and effective modality
meriting consideration in a multi-institutional trial (8).
SRS will likely continue to play a significant role
in the treatment of brain metastases in the future, possibly with
even more improved patient outcomes. Indeed, as our case
demonstrates, with appropriate use of this powerful modality in
carefully selected patients (possibly treated with a tailored
multidisciplinary care plan of targeted agents, surgery and
systemic therapy), there may be further improvement in the outcomes
of patients presenting with a clinical challenge such as metastatic
intracranial disease.
In conclusion, Gamma Knife SRS is a safe and
effective treatment modality for patients with recurrent
intracranial metastasis, with durable local control and minimal
cognitive impairment.
Acknowledgements
To the memory of Christopher C. Getch, MD
(1961–2012), the treating neurosurgeon for this case who performed
the patient's surgery, follow-up assessments and first four Gamma
Knife SRS treatments, and passed away unexpectedly on January 9,
2012. To the memory of Andrew Parsa, MD (1967–2015), Chairman of
Neurological Surgery at Northwestern University's Feinberg School
of Medicine, who passed away unexpectedly on April 13, 2015.
References
|
1
|
Wen PY and Loeffler JS: Management of
brain metastases. Oncology (Williston Park). 13:941–954, 957-61;
discussion 961–962. 1999.PubMed/NCBI
|
|
2
|
Johnson JD and Young B: Demographics of
brain metastasis. Neurosurg Clin N Am. 7:337–344. 1996.PubMed/NCBI
|
|
3
|
Borgelt B, Gelber R, Kramer S, Brady LW,
Chang CH, Davis LW, Perez CA and Hendrickson FR: The palliation of
brain metastases: Final results of the first two studies by the
Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys.
6:1–9. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurtz JM, Gelber R, Brady LW, Carella RJ
and Cooper JS: The palliation of brain metastases in a favorable
patient population: A randomized clinical trial by the Radiation
Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 7:891–895.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelber RD, Larson M, Borgelt BB and Kramer
S: Equivalence of radiation schedules for the palliative treatment
of brain metastases in patients with favorable prognosis. Cancer.
48:1749–1753. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borgelt B, Gelber R, Larson M, Hendrickson
F, Griffin T and Roth R: Ultra-rapid high dose irradiation
schedules for the palliation of brain metastases: Final results of
the first two studies by the Radiation Therapy Oncology Group. Int
J Radiat Oncol Biol Phys. 7:1633–1638. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawaya R, Hammoud M, Schoppa D, Hess KR,
Wu SZ, Shi WM and Wildrick DM: Neurosurgical outcomes in a modern
series of 400 craniotomies for treatment of parenchymal tumors.
Neurosurgery. 42:1044–55; discussion 1055–1056. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asher AL, Burri SH, Wiggins WF, Kelly RP,
Boltes MO, Mehrlich M, Norton HJ and Fraser RW: A new treatment
paradigm: Neoadjuvant radiosurgery before surgical resection of
brain metastases with analysis of local tumor recurrence. Int J
Radiat Oncol Biol Phys. 88:899–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and Sawaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the Metropolitan
Detroit Cancer Surveillance System. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schouten LJ, Rutten J, Huveneers HA and
Twijnstra A: Incidence of brain metastases in a cohort of patients
with carcinoma of the breast, colon, kidney and lung and melanoma.
Cancer. 94:2698–2705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
Radiation Therapy Oncology Group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh
J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, et al:
Diagnosis-specific prognostic factors, indexes and treatment
outcomes for patients with newly diagnosed brain metastases: A
multi-institutional analysis of 4,259 patients. Int J Radiat Oncol
Biol Phys. 77:655–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noordijk EM, Vecht CJ, Haaxma-Reiche H,
Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N,
Metsaars JA and Wattendorff AR: The choice of treatment of single
brain metastasis should be based on extracranial tumor activity and
age. Int J Radiat Oncol Biol Phys. 29:711–717. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwartz M: Stereotactic radiosurgery:
Comparing different technologies. CMAJ. 158:625–178.
1998.PubMed/NCBI
|
|
16
|
Kuo JS, Yu C, Petrovich Z and Apuzzo ML:
The CyberKnife stereotactic radiosurgery system: Description,
installation and an initial evaluation of use and functionality.
Neurosurgery. 53:1235–1239; discussion 1239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller DW: A review of proton beam
radiation therapy. Med Phys. 22:1943–1954. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andrews DW, Scott CB, Sperduto PW,
Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J,
Bahary JP, et al: Whole-brain radiation therapy with or without
stereotactic radiosurgery boost for patients with one to three
brain metastases: Phase III results of the RTOG 9508 randomised
trial. Lancet. 363:1665–1672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pirzkall A, Debus J, Lohr F, Fuss M, Rhein
B, Engenhart-Cabillic R and Wannenmacher M: Radiosurgery alone or
in combination with whole-brain radiotherapy for brain metastases.
J Clin Oncol. 16:3563–3569. 1998.PubMed/NCBI
|
|
20
|
Alexander E III, Moriarty TM and Loeffler
JS: Radiosurgery for metastases. J Neurooncol. 27:279–285. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alexander E III, Moriarty TM, Davis RB,
Wen PY, Fine HA, Black PM, Kooy HM and Loeffler JS: Stereotactic
radiosurgery for the definitive, noninvasive treatment of brain
metastases. J Natl Cancer Inst. 87:34–40. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flickinger JC, Kondziolka D, Lunsford LD,
Coffey RJ, Goodman ML, Shaw EG, Hudgins WR, Weiner R, Harsh GR IV
and Sneed PK: A multi-institutional experience with stereotactic
radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol
Phys. 28:797–802. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto M, Serizawa T, Shuto T, Akabane
A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et
al: Stereotactic radiosurgery for patients with multiple brain
metastases (JLGK0901): A multi-institutional prospective
observational study. Lancet Oncol. 15:387–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kocher M, Soffietti R, Abacioglu U, Villà
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: Adjuvant whole-brain radiotherapy versus
observation after radiosurgery or surgical resection of one to
three cerebral metastases: Results of the EORTC 22952–26001 study.
J Clin Oncol. 29:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|