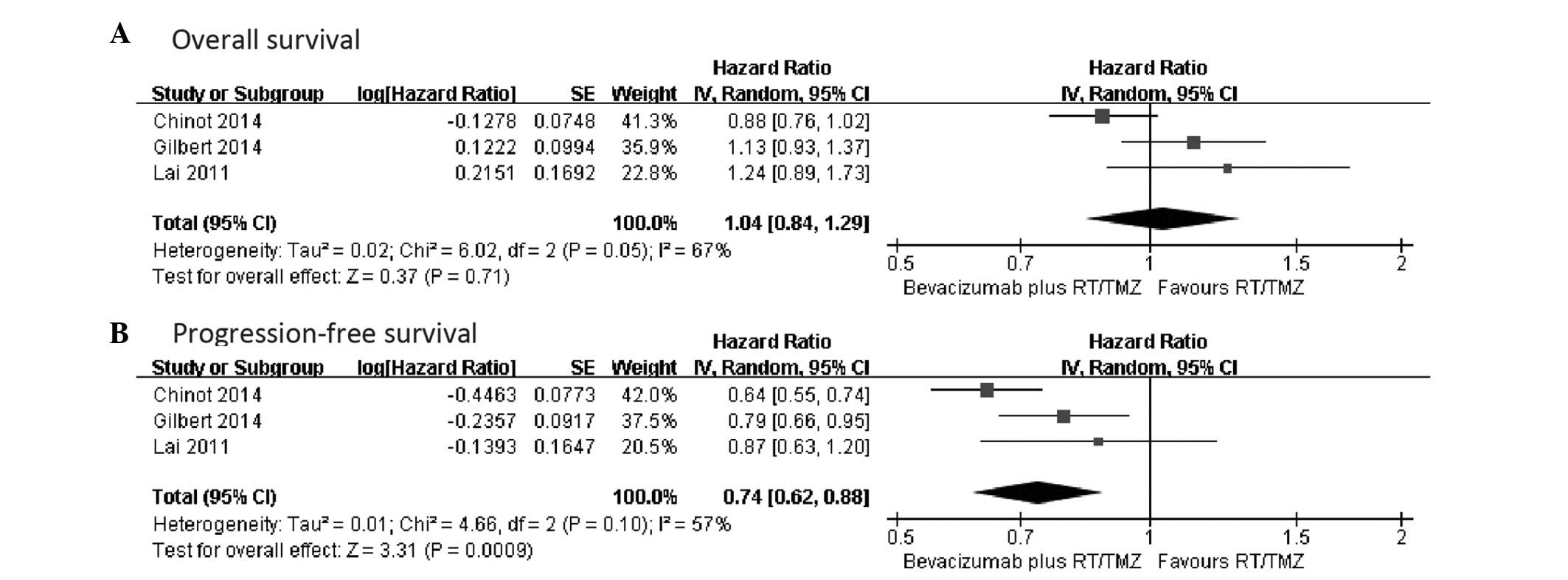
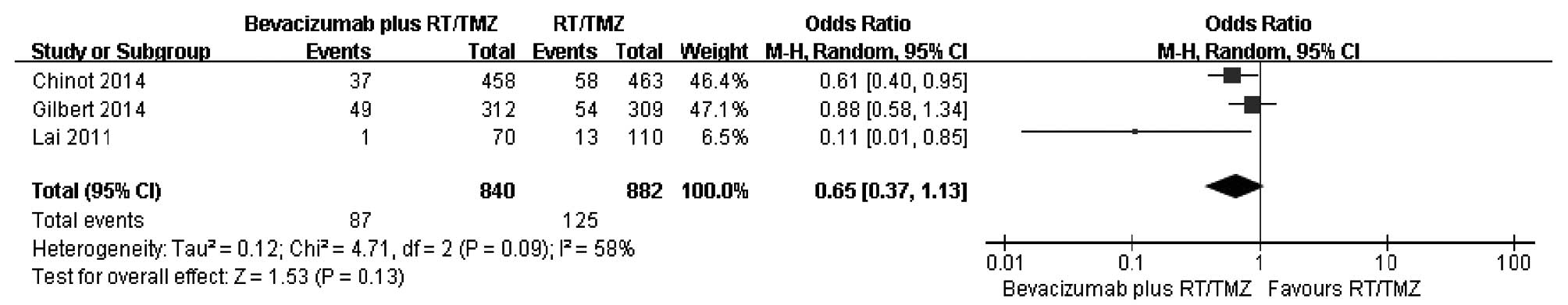
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain RK, di Tomaso E, Duda DG, Loeffler
JS, Sorensen AG and Batchelor TT: Angiogenesis in brain tumours.
Nat Rev Neurosci. 8:610–622. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burrell K, Singh S, Jalali S, Hill RP and
Zadeh G: VEGF regulates region-specific localization of
perivascular bone marrow-derived cells in glioblastoma. Cancer Res.
74:3727–3739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N, Hillan KJ and Novotny W:
Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody
for cancer therapy. Biochem Biophys Res Commun. 333:328–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irizarry Robles L, Hambardzumyan D, Nakano
I, Gladson CL and Ahluwalia MS: Therapeutic targeting of VEGF in
the treatment of glioblastoma. Expert Opin Ther Targets.
16:973–984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DR, Leeper HE and Uhm JH:
Glioblastoma survival in the United States improved after Food and
Drug Administration approval of bevacizumab: A population-based
analysis. Cancer. 119:3489–3495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahluwalia MS and Gladson CL: Progress on
antiangiogenic therapy for patients with malignant glioma. J Oncol.
2010:6890182010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narayana A, Kelly P, Golfinos J, Parker E,
Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, et
al: Antiangiogenic therapy using bevacizumab in recurrent
high-grade glioma: Impact on local control and patient survival. J
Neurosurg. 110:173–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khasraw M, Ameratunga MS, Grant R, Wheeler
H and Pavlakis N: Antiangiogenic therapy for high-grade glioma.
Cochrane Database Syst Rev. 9:CD0082182014.PubMed/NCBI
|
|
13
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narayana A, Gruber D, Kunnakkat S,
Golfinos JG, Parker E, Raza S, Zagzag D, Eagan P and Gruber ML: A
clinical trial of bevacizumab, temozolomide, and radiation for
newly diagnosed glioblastoma. J Neurosurg. 116:341–345. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai A, Tran A, Nghiemphu PL, Pope WB,
Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al:
Phase II study of bevacizumab plus temozolomide during and after
radiation therapy for patients with newly diagnosed glioblastoma
multiforme. J Clin Oncol. 29:142–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takano S, Ishikawa E, Nakai K, Matsuda M,
Masumoto T, Yamamoto T and Matsumura A: Bevacizumab in Japanese
patients with malignant glioma: From basic research to clinical
trial. Onco Targets Ther. 7:1551–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Odia Y, Shih JH, Kreisl TN and Fine HA:
Bevacizumab-related toxicities in the National Cancer Institute
malignant glioma trial cohort. J Neurooncol. 120:431–440. 2014.
View Article : Google Scholar : PubMed/NCBI
|