Introduction
Small-cell lung cancer (SCLC), the most aggressive
subtype of pulmonary carcinoma, accounts for up to 15% of all newly
diagnosed lung cancers, with an overall 5-year survival rate of
only 6% (1,2). The initial response to chemotherapy may
be favourable, but relapse is common (3). Paraneoplastic neurological syndromes
(PNS) represent a wide spectrum of rare autoimmune diseases that
may often be the first manifestation of an underlying SCLC
(4). Among cancer patients, <1%
overall develop PNS, but it occurs in up to 3–5% of those with SCLC
(5). These severe, often rapidly
progressive and debilitating neuropathies are frequently associated
with type 1 antineuronal nuclear antibodies (ANNA-1), previously
referred to as anti-Hu antibodies (6).
The Hu antigens are intracellular proteins, normally
expressed throughout the central nervous system (CNS) and
peripheral nervous system (7). In
healthy adults, anti-Hu antibodies are not detected in the serum,
as the blood-brain barrier sequesters the developing CNS from the
immune system (5). Interestingly,
all SCLCs also express the Hu antigen (specifically HuD) (8). Detectable levels of circulating anti-Hu
antibodies may be found in ~20% of SCLC patients, although not all
will develop PNS (5,9). It remains unclear why only some SCLC
patients develop PNS (10). It has
been hypothesized that these ectopic neuronal antigens are
recognized by the immune system as foreign, triggering the
production of ANNA-1 with consequential paraneoplastic effects
manifesting clinically with various degrees of severity and
neurological symptoms (7,8).
When detected in the serum or cerebrospinal fluid
(CSF), anti-Hu antibodies have been associated with a wide spectrum
of neurological and neuro-ophthalmological disorders presenting as
PNS (11). Adie's syndrome (AS),
namely tonic pupil with areflexia, has been previously reported as
a rare neuro-ophthalmic presentation of anti-Hu paraneoplastic
syndrome associated with SCLC (11).
It is hypothesized that concurrent loss of parasympathetic ciliary
ganglia and dorsal root ganglionic cells may be responsible for AS
(12,13). We herein present a unique case of
concomitant AS and rapidly progressive PNS associated with ANNA-1,
with complete response of chemotherapy-treated SCLC and subsequent
partial recovery of the neurological deficit following multimodal
therapy, with long-term stability.
Case report
In December 2012, a 55-year-old woman, former heavy
smoker (53 pack-years), was admitted to the Clinic for Respiratory
Diseases ‘Jordanovac’, University Hospital Centre Zagreb (Zagreb,
Croatia) due to hemoptysis and evaluation of a pulmonary nodule in
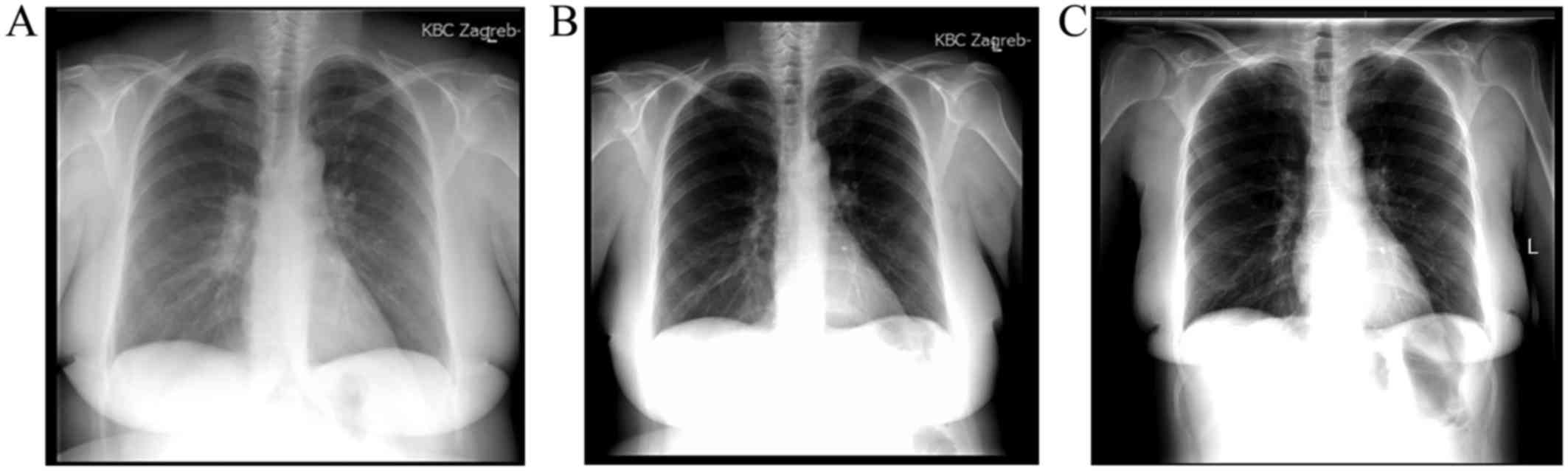
the right lung field juxtaposed to the right hilum on chest X-ray
(Fig. 1A). The patient's past
medical history included hypercholesterolemia, for which she was
being treated with simvastatin. A computed tomography (CT) scan of
the thorax revealed a proliferative nodule (1.7 cm in diameter) in
the lateral basal bronchopulmonary segment of the right lower lobe.
Following fiberbronchoscopy, the clinical stage was determined to
be IIIA (T1aN2M0) or limited-stage (localised disease) SCLC. A
chemotherapy regimen with cisplatin and etoposide was immediately
initiated. By the third cycle of chemotherapy (February 2013), the
patient began to experience paresthesia and weakness of the right
hand. The head CT and magnetic resonance imaging (MRI) scans were
unremarkable. Physical examination detected anisocoria. The patient
was promptly referred to the staff ophthalmologist and
neurologist.
Ocular examination revealed a right mydriatic pupil,
without direct or consensual pupillary constriction to light, and
no constriction on attempted accommodation. A neuro-ophthalmologist
was consulted. Following local instillation of one drop of diluted
pilocarpine (0.1%) in each eye, the affected pupil showed
cholinergic supersensitivity by constricting, a finding consistent
with unilateral Adie's pupil. The nerve conduction study pointed to
a suspected peripheral neuropathy congruent with the presenting
clinical characteristics. The etiology was hypothesized to be
associated with the underlying malignancy. The treating neurologist
recommended immunological work-up, particularly serological tests
for antinuclear antibodies, to evaluate the possibility of a PNS.
Autoantibody screening was positive for anti-Hu antibody at a low
titre.
By the sixth cycle of chemotherapy, the patient was
exhibiting symptoms of further peripheral sensory and motor
neurological decline. A repeat brain MRI scan was again
unremarkable. Follow-up electromyoneurography indicated profound
neurological and electromyographic progression. Nerve conduction
studies showed severe axonal sensorimotor polyneuropathy with
sensory predominance, and signs of cerebrospinal toxic-like
lesions. Based on the results of the electrophysiological study,
neurological examination and earlier positive serum ANNA-1, it was
concluded that the patient was suffering from a PNS, more
specifically peripheral neuropathy with cerebellar symptomatology.
A planned positron emission tomography-CT scan and radiotherapy
were not performed due to the patient's poor performance
status.
Within 2 months, the patient was bedridden, no
longer able to tolerate sitting due to vertigo, and experiencing
uncontrolled movements of her right hand and head tremor; her
speech was laboured with discrete dyslalia and she exhibited
horizontal nystagmus on left and right gaze. The muscle tone was
decreased overall, most prominently in the right extremities.
Areflexia of both the upper and lower extremities was observed. Due
to worsening of her clinical condition, the patient underwent
plasmapheresis treatments (five in total) through a central venous
catheter every second day. Each treatment consisted of 3,500 ml of
intravenous 5% albumin and concomitant oral methylprednisolone at
32 mg. The patient tolerated the therapy well and exhibited a minor
improvement of the neurological status. A higher dose of oral
methylprednisolone (48 mg daily) was recommended at discharge.
By September 2013, the patient's condition had
declined further. Given that the plasmapheresis treatments had not
led to any significant improvement in neurological status,
cyclophosphamide at one 50-mg tablet daily was added to her
treatment, along with valproate and clonazepam. The myoclonus
improved during in-hospital treatment. The patient was re-evaluated
in March and October 2014. Further neurological recovery was
observed. The motor coordination of her right hand improved and she
was able to independently sit and walk a few steps with assistance.
In addition to the medication, continuous and intense physical
rehabilitation at home and in-hospital proved key to the patient's
improvement. She continued to exhibit a slow but steady recovery of
neurological functioning over the next year. In November 2014,
serum ANNA-1 remained positive at a low titre. At the clinical
visit (late November 2015), the patient's neurological status and
overall health were stable. The latest telephone update 1 year
later (early November 2016) was promising, as the patient reported
her condition to be stable.
Partial tumour regression was observed on chest
X-ray after the first cycle of chemotherapy. Subsequent follow-up
radiological examinations remained unchanged, until complete
response was noted 6 months later in May 2013 (Fig. 1B), as well as on subsequent visits.
The latest follow-up radiological examinations [thoracic CT scan in
May 2015 and chest X-ray in November 2015 (Fig. 1C)] revealed no tumour recurrence.
Discussion
Anti-Hu antibodies are a polyclonal IgG type of
antibody that bind to intracellular antigens found in neural as
well as tumour cells, thus leading to immune cross-reactivity
(9,14). This simultaneous attack of neurons
and malignant cells may cause PNS symptoms and slow down tumour
growth (14). In 88% of the cases,
the presence of anti-Hu antibodies is associated with an underlying
neoplasm; of those, 81% are SCLCs (15). SCLC is reported to account for
>90% of cases of PNS related to ANNA-1 (5). The present case highlights the
importance of considering rare associations as possible
diagnoses.
Chemotherapy is generally not considered to be
effective in SCLC, as recurrence is inevitable in the majority of
patients, regardless of the extent of the disease (3,16).
However, the presence of ANNA-1 appears to be protective (17), and has been shown in several reports
to be associated with increased tumour chemosensitivity, indolent
tumour growth, complete response to therapy, and prolonged survival
(6,15,16,18–22).
Spontaneous tumour regression associated with ANNA-1 in patients
with histologically proven SCLC, without any active treatment, has
also been described in 2 cases in the literature (7,23), and
in 1 rare case of non-SCLC (24).
The present study adds further evidence to support the theory that
ANNA-1 in the serum may confer antitumour activity, causing
regression of lung neoplasms (7).
In the case presented herein, unilateral Adie's
pupil and peripheral sensorimotor neuropathy with cerebellar
symptomatology developed during chemotherapy for limited-stage
SCLC. Anti-Hu antibodies were detected in the patient's serum at a
low titre, which is a highly uncommon finding, as the patient also
had Hashimoto's thyroiditis. Low antibody titres are usually
present in SCLC patients without autoimmune diseases (25). Those with SCLC and no clinical PNS
have detectable anti-Hu antibodies in their serum in ~16–25% of the
cases (25–27). When SCLC and PNS are found
concurrently, the latter is usually mediated by a high titre of
anti-Hu antibodies (28).
According to the limited number of cases reported in
the literature, a multimodality approach to the treatment of PNS,
consisting of immunosuppressive therapy with a combination of
intravenous immunoglobulin, methylprednisolone and
cyclophosphamide, may transiently stabilize the disorder (5). However, to the best of our knowledge,
there are currently no studies reporting long-term improvement in
PNS (5). It appears that a vigorous
immunosuppressive treatment combining high doses of steroids,
cyclophosphamide and intravenous immunoglobulins in
anti-Hu-associated PNS (regardless of the type) in severely
disabled patients at the onset of treatment is not beneficial
(29). It has been reported that 65%
of patients with SCLC and paraneoplastic encephalomyelitis with
detectable serum ANNA-1 succumb to neurological complications
rather than tumour progression (6,16). The
prognosis is poor, with the median survival for anti-Hu patients
following the onset of treatment reported to be 6 months (range,
3–35 months) (29). The recent
discovery that ANNA-1 may cause neuronal cell death may account for
the irreversible nature of paraneoplastic neurological deficits in
patients with these autoantibodies (30).
Although the exact underlying pathological mechanism
has not been fully elucidated, it is likely that humoral immunity
may not be solely responsible for the incidence of PNS. Several
types of T cells may in fact be present in different PNS patients,
and these variants may explain the spectrum of clinical outcomes
that have been described in the literature thus far with regards to
tumour response and PNS severity (31). A number of experimental studies have
suggested that cytotoxic T cells may be the main effectors of the
immune response (32). This T-cell
mediated response may result in neuronal cell death and axonal
degeneration that are extremely difficult to treat (32,33). It
remains unclear, however, why only some SCLC patients develop PNS,
given that the HuD antigen is constantly expressed by these tumour
cells (29).
A few exceptional reports of neurological recovery
have been described in the literature, both spontaneous and
following immunosuppressor treatment alone (29). In the present case, the patient was
already bedridden at the time of initiation of multimodal
immunosuppressive therapy for anti-Hu-associated PNS. The positive
outcome described herein may be attributed to the addition of
plasmapheresis to the treatment regimen, which may have exerted a
synergistic effect. Interestingly, however, it has been
demonstrated that plasmapheresis reduces anti-Hu antibody levels in
the serum, but not in the CSF (10).
Unlike the majority of previous reports of imminent death from
progressive PNS, the patient described herein has achieved steady
recovery of the neurological status over the course of the past 45
months since the first presentation of neurological deficit, and
has remained stable.
In general, PNS should be highly suspected in a SCLC
patient who develops a progressive neurological and/or
neuro-ophthalmic disorder in a subacute course after all other
possible causes have been excluded (5). The presence of anti-neural antibodies,
such as anti-Hu, should be investigated in the patient's serum. A
multidisciplinary team approach cannot be underestimated, as it
entails a comprehensive investigation of the presenting symptoms.
We recommend that both a neurologist and ophthalmologist be
included in the initial diagnostic process and subsequent regular
follow-ups. Similarly, in patients with neurological symptoms, but
without known lung cancer diagnosis, a search for anti-Hu may
reveal an underlying tumour, such as SCLC. Given that there is
currently no screening tool for early SCLC detection, ANNA-1 and
other SCLC-related autoantibodies may prove useful as
antibody-based early cancer detection biomarkers. Discovering SCLC
when the malignancy is at an early stage and undetectable by
available radiological methods should lead to better prognosis and
prolong survival. Further research using animal-based models to
study immunological events that occur during SCLC growth may
provide insight into the process that drives the development of
paraneoplastic disease in humans. Translating these results into
clinical practice may enable initiating treatment during a window
of better functional state, leading to improved clinical
outcomes.
Written informed consent was obtained from the
patient for publication of this manuscript and any accompanying
radiological images.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013. November. 2015,
National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/based
on SEER data submission, posted to the SEER web site. April
25–2016
|
|
2
|
Worden FP and Kalemkerian GP: Therapeutic
advances in small cell lung cancer. Expert Opin Investig Drugs.
9:565–579. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalemkerian GP: Advances in
pharmacotherapy of small cell lung cancer. Expert Opin
Pharmacother. 15:2385–2396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Darnell RB and Posner JB: Paraneoplastic
syndromes involving the nervous system. N Engl J Med.
349:1543–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanaji N, Watanabe N, Kita N, Bandoh S,
Tadokoro A, Ishii T, Dobashi H and Matsunaga T: Paraneoplastic
syndromes associated with lung cancer. World J Clin Oncol.
5:197–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalmau J, Graus F, Rosenblum MK and Posner
JB: Anti-Hu-associated paraneoplastic encephalomyelitis/sensory
neuronopathy. A clinical study of 71 patients. Medicine
(Baltimore). 71:59–72. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mawhinney E, Gray OM, McVerry F and
McDonnell GV: Paraneoplastic sensorimotor neuropathy associated
with regression of small cell lung carcinoma. BMJ Case Reports.
2010:pii: bcr0120091486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts WK, Deluca IJ, Thomas A, Fak J,
Williams T, Buckley N, Dousmanis AG, Posner JB and Darnell RB:
Patients with lung cancer and paraneoplastic Hu syndrome harbour
HuD-specific type 2 CD8+ T cells. J Clin Invest. 119:2042–2051.
2009.PubMed/NCBI
|
|
9
|
Gozzard P and Maddison P: Which antibody
and which cancer in which paraneoplastic syndromes? Pract Neurol.
10:260–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pignolet BS, Gebauer CM and Liblau RS:
Immunopathogenesis of paraneoplastic neurological syndromes
associated with anti-Hu antibodies: A beneficial antitumor immune
response going awry. Oncoimmunology. 2:e273842013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruno MK, Winterkorn JM, Edgar MA, Kamal A
and Stübgen JP: Unilateral Adie pupil as sole ophthalmic sign of
anti-Hu paraneoplastic syndrome. J Neuroophthalmol. 20:248–249.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harriman DG and Garland H: The pathology
of Adie's syndrome. Brain. 91:401–418. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hersh B, Dalmau J, Dangond F, Gultekin S,
Geller E and Wen PY: Paraneoplastic opsoclonus-myoclonus associated
with anti-Hu antibody. Neurology. 44:1754–1755. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin L, Qu H and Chen Q: Proliferative
response of peripheral blood mononuclear cells in anti-Hu
antibody-associated patients with paraneoplastic neurological
syndrome and their depressant effect on small cell lung cancer
cells. Mol Med Rep. 11:1595–1600. 2015.PubMed/NCBI
|
|
15
|
Graus F, Dalmou J, Reñé R, Tora M, Malats
N, Verschuuren JJ, Cardenal F, Viñolas N, del Muro J Garcia, Vadell
C, et al: Anti-Hu antibodies in patients with small-cell lung
cancer: Association with complete response to therapy and improved
survival. J Clin Oncol. 15:2866–2872. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Douglas CA and Ellershaw J: Anti-Hu
antibodies may indicate a positive response to chemotherapy in
paraneoplastic syndrome secondary to small cell lung cancer.
Palliat Med. 17:638–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kazarian M, Calbo J, Proost N, Carpenter
CL, Berns A and Laird-Offringa IA: Immune response in lung cancer
mouse model mimics human anti-Hu reactivity. J Neuroimmunol.
217:38–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dalmau J, Graus F, Cheung NK, Rosenblum
MK, Ho A, Cañete A, Delattre JY, Thompson SJ and Posner JB: Major
histocompatibility proteins, anti-Hu antibodies and paraneoplastic
encephalomyelitis in neuroblastoma and small cell lung cancer.
Cancer. 75:99–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smitt P Sillevis, Grefkens J, de Leeuw B,
van den Bent M, van Putten W, Hooijkaas H and Vecht C: Survival and
outcome in 73 anti-Hu positive patients with paraneoplastic
encephalomyelitis/sensory neuronopathy. J Neurol. 249:745–753.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aguirre-Cruz L, Charuel JL, Carpentier AF,
Benyahia B, Delattre JY and Musset L: Clinical relevance of
non-neuronal auto-antibodies in patients with anti-Hu or anti-Yo
paraneoplastic diseases. J Neurooncol. 71:39–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maddison P and Lang B: Paraneoplastic
neurological autoimmunity and survival in small-cell lung cancer. J
Neuroimmunol. 201–202. 159–162. 2008.
|
|
22
|
Tsou JA, Kazarian M, Patel A, Galler JS,
Laird-Offringa IA, Carpenter CL and London SJ: Low level anti-Hu
reactivity: A risk marker for small cell lung cancer? Cancer Detect
Prev. 32:292–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gill S, Murray N, Dalmau J and Thiessen B:
Paraneoplastic sensory neuronopathy and spontaneous regression of
small cell lung cancer. Can J Neurol Sci. 30:269–271. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pujol JL, Godard AL, Jacot W and Labauge
P: Spontaneous complete remission of a non-small cell lung cancer
associated with anti-Hu antibody syndrome. J Thorac Oncol.
2:168–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kazarian M and Laird-Offringa IA:
Small-cell lung cancer-associated autoantibodies: Potential
applications to cancer diagnosis, early detection, and therapy. Mol
Cancer. 10:332011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Voltz RD, Posner JB, Dalmau J and Graus F:
Paraneoplastic encephalomyelitis: An update of the effects of the
anti-Hu immune response on the nervous system and tumour. J Neurol
Neurosurg Psychiatry. 63:133–136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Graus F, Keime-Guibert F, Reñe R, Benyahia
B, Ribalta T, Ascaso C, Escaramis G and Delattre JY:
Anti-Hu-associated paraneoplastic encephalomyelitis: Analysis of
200 patients. Brain. 124:1138–1148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
List M, Jamous F, Gupta A and Huntington
M: Anti-Hu positive antibodies and small cell carcinoma: A single
center review. S D Med. 68:251–255. 2015.PubMed/NCBI
|
|
29
|
Keime-Guibert F, Graus F, Fleury A, René
R, Honnorat J, Broet P and Delattre JA: Treatment of paraneoplastic
neurological syndromes with antineuronal antibodies (Anti-Hu,
Anti-Yo) with a combination of immunoglobulins, cyclophosphamide
and methylprednisolone. J Neurol Neurosurg Psychiatry. 68:479–482.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greenlee JE, Clawson SA, Hill KE, Wood B,
Clardy SL, Tsunoda I, Jaskowski TD and Carlson NG: Neuronal uptake
of anti-Hu antibody, but not anti-Ri antibody, leads to cell death
in brain slice cultures. J Neuroinflammation. 11:1602014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roberts WK, Deluca IJ, Thomas A, Fak J,
Williams T, Buckley N, Dousmanis AG, Posner JB and Darnell RB:
Patients with lung cancer and paraneoplastic Hu syndrome harbor
HuD-specific type 2 CD8+T cells. J Clin Invest. 119:2042–2051.
2009.PubMed/NCBI
|
|
32
|
Antoine JC: Peripheral neuropathies
associated with antibodies directed to intracellular neural
antigens. Rev Neurol (Paris). 170:570–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pignolet BS, Gebauer CM and Liblau RS:
Immunopathogenesis of paraneoplastic neurological syndromes
associated with anti-Hu antibodies: A beneficial antitumor immune
response going awry. Oncoimmunology. 2:e273842013. View Article : Google Scholar : PubMed/NCBI
|