Introduction
The treatment of unresectable head and neck cancer
(HNC) has improved with the use of modern chemotherapy and
radiotherapy (1,2). However, locoregional failure remains a
major concern, preventing complete cure. Although salvage surgery
has the highest disease-eradicating potential, only one-third of
patients are eligible (3). After
surgery, chemotherapy is a frequently preferred option; however,
the resulting median survival time is <9 months (4). With the advancement of modern radiation
techniques, re-irradiation using advanced technologies, including
intensity-modulated radiation therapy and/or stereotactic
radiotherapy, has become a promising therapeutic option. The
image-guided stereotactic radiotherapy system CyberKnife® enables
precise dose delivery over short treatment periods (5–9). Several
institutions, including ours, have reported on the outcome and
toxicity of re-irradiation using CyberKnife® hypofractionated
stereotactic body radiation therapy (SBRT) (5–10).
Lethal carotid blowout syndrome (CBOS) was
previously investigated in patients with HNC (7,9), and the
findings prompted the subsequent investigation of predisposing
factors for CBOS (10). The presence
of ulceration and lymph node irradiation were found to be risk
factors for CBOS, and the CBOS index, including carotid invasion of
>180°, was found to be useful for risk factor classification and
determination of indications for re-irradiation (10). As an increased frequency of AN was
observed among CBOS cases in an initial single-institution study by
our group (7), an assessment of
multi-institutional records of patients with HNC was conducted,
focusing on AN. The aim of the present study was to investigate the
role of AN in tumor control and toxicity following re-irradiation
using CyberKnife® SBRT in HNC patients.
Patients and methods
Patients
The medical records of patients who underwent
CyberKnife® SBRT (Accuray; Sunnyvale, CA, USA) in four hospitals
[Soseikai General Hospital (Kyoto, Japan), Osaka University
Hospital (Osaka, Japan), Fujimoto Hayasuzu Hospital (Miyakonojo,
Japan) and Okayama Kyokuto Hospital (Okayama, Japan)] between
August 2000 and July 2010 were reviewed for inclusion in the
present study. Among the patients with HNC who received
re-irradiation up to the prescribed dose for residual or recurrent
tumors within the irradiated area, only those who satisfied the
following criteria were included: Patients who had undergone
imaging analysis prior to SBRT to confirm the presence or absence
of AN and had completed a course of radical treatment, including
previous radiotherapy at ≥40 Gy [biological equivalent 2-Gy
fractions (EQD2) described in detail below], with or without
chemotherapy and surgery. Previous radiotherapy consisted of
40–74.8 Gy/20–62 fractions (1.2–2 Gy fractionation), with estimated
EQD2 of 40–75.1 Gy (α/β=10).
A total of 67 patients were considered eligible for
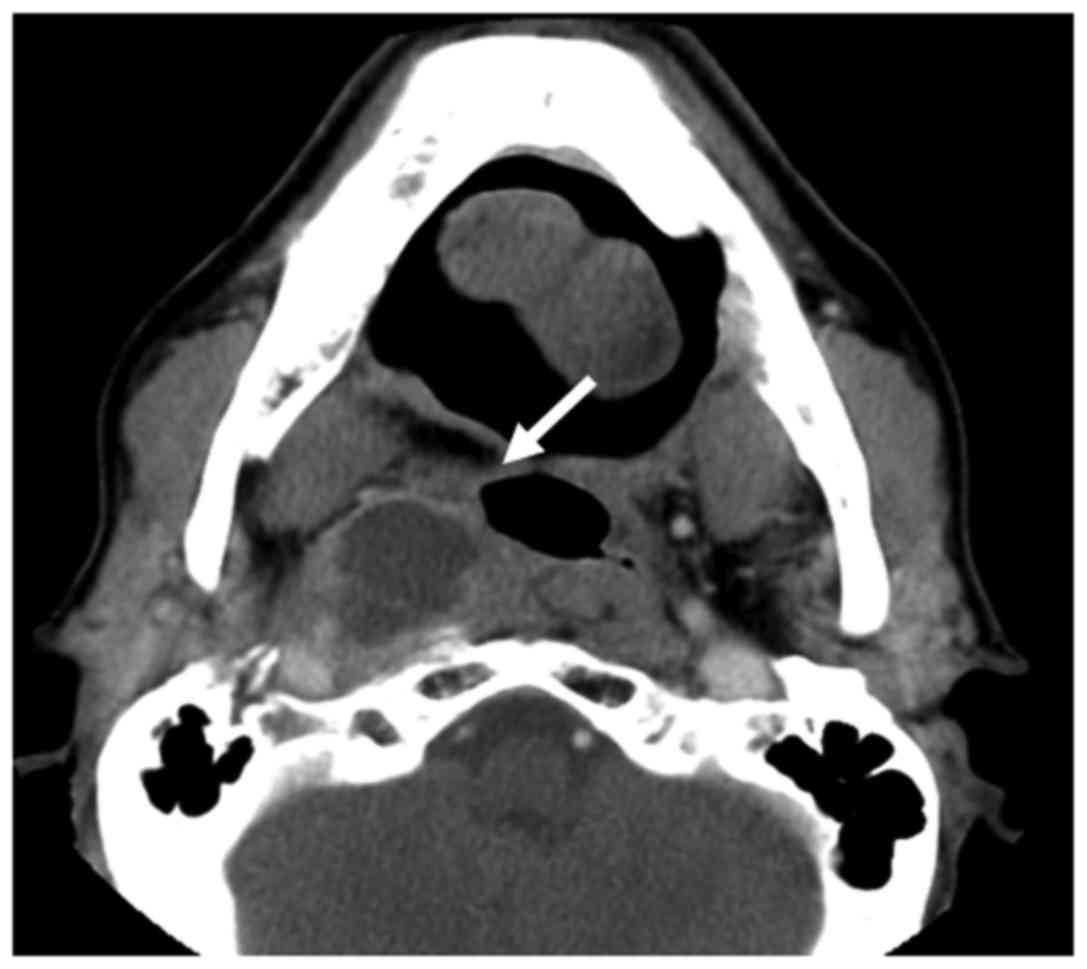
assessment. The patient characteristics are listed in Table I nd a representative case of a
patient with intratumoral AN is presented in Fig. 1. The conventional technique using a
linear accelerator was used during the first course of
radiotherapy. SBRT re-irradiation was performed using the
CyberKnife® system. The patients received a median dose of 30 Gy
(range, 15–39 Gy) over a median of five daily fractions (range, 1–8
fractions) that were prescribed at D90, D95, or a marginal dose.
D90 (D95) was defined as a minimum dose covering 90% (95%) of the
planning target volume (PTV). The marginal dose prescription was
defined as the percentage (maximum dose, 100%) of the isodose curve
covering the PTV. None of the patients received chemotherapy. All
irradiated lesions were located inside areas previously subjected
to high-dose irradiation. Prior to SBRT, the presence of AN was
confirmed by imaging analysis, such as computed tomography (CT)
and/or magnetic resonance imaging (MRI), contrast-enhanced if
required. AN was identified as a focal area of low density with a
surrounding rim of high density and/or enhancement on CT, or a
focal area of high signal intensity on T2-weighted images, or a
focal area of low signal intensity on T1-weighted images with a
surrounding rim of enhancement on MRI (11). These interpretations were made by at
least one diagnostic radiologist and one radiation oncologist. The
presence of mucosal ulceration of the upper aerodigestive tract was
determined by visual inspection (fibroscopy if required) and/or
imaging analysis (CT and/or MRI). EQD2 was calculated using the
linear quadratic model as follows: EQD2=prescription dose ×
(α/β+dose per fraction)/(α/β+2), where α/β = 10 for tumors and 3
for organs at risk. In principle, follow-up by physical examination
was performed at intervals of at least 1 month for the first year
and at intervals of 3–6 months thereafter. Examination with imaging
methods, such as CT and/or MRI and/or ultrasonography, was
performed after 3 and 6 months, 1, 1.5 and 2 years, and at 1-year
intervals thereafter, or when local or lymph node recurrence was
suspected. Initial response was assessed using the Response
Evaluation Criteria in Solid Tumors version 4.0 (http://www.jcog.jp/doctor/tool/ctcaev4.html). Written
informed consent was obtained from the patients for the publication
of their data and accompanying images.
 | Table I.Characteristics and treatment factors
of patients. |
Table I.
Characteristics and treatment factors
of patients.
|
| Abscess/necrosis (−)
(n=49) | Abscess/necrosis (+)
(n=18) |
|
|---|
|
|
|
|
|---|
| Variables | No. of patients or
median (range) | (%) | No. of patients or
median (range) | (%) | P-value |
|---|
| Age (years) | 63 (45–83) |
| 60 (44–66) |
| 0.008 |
| Gender |
|
|
|
|
|
|
Female | 11 | (22) | 4 | (22) | 0.690 |
| Male | 38 | (78) | 14 | (78) |
|
| Disease |
|
|
|
|
|
|
Nasopharyngeal cancer | 32 | (65) | 7 | (39) | 0.070 |
|
Oropharyngeal cancer | 9 | (18) | 8 | (44) |
|
|
Hypopharyngeal cancer | 8 | (16) | 2 | (11) |
|
| Oral
cancer | 0 | (0) | 1 | (6) |
|
| Irradiated area |
|
|
|
|
|
| Primary
site | 39 | (80) | 11 | (61) | 0.110 |
| Lymph
node | 10 | (20) | 7 | (39) |
|
| Lymph
node alone | 4 | (40) | 2 | (29) |
|
| Primary
and lymph node | 6 | (60) | 5 | (71) |
|
| Ulceration |
|
|
|
|
|
| No | 41 | (84) | 9 | (50) | 0.006 |
| Yes | 8 | (16) | 9 | (50) |
|
| Surgical history |
|
|
|
|
|
| No | 36 | (73) | 8 | (44) | 0.02 |
| Yes | 13 | (27) | 10 | (56) |
|
| Planning target
volume (cm3) | 13.5 (1–339) |
| 53 (5.2–241) |
| 0.003 |
| Treatment interval
(months) | 17.6 (3.1–122) |
| 24 (8.3–86.2) |
| 0.770 |
| Responsea | 15/16 | (64) | 3/3 | (33) | 0.040 |
Statistical analysis
All statistical analyses were performed using
Statview 5.0 statistical software (SAS Institute, Inc., Cary, NC,
USA). The percentage values were analyzed using the χ2
test, and values were compared using Mann-Whitney U test.
Cumulative incidences were estimated by the Kaplan-Meier method.
The durations were calculated from the first day of CyberKnife®
SBRT. Variables that had P-values <0.05 were further tested by
multivariate analysis using a Cox proportional hazards model. The
cut-off value was set at the average or median value of each
variable if not otherwise stated. All analyses used a significance
level of P<0.05.
Results
AN is associated with poor prognosis
of patients with recurrent HNC following SBRT
The median follow-up time for the surviving patients
after SBRT was 17 months (range, 1–122 months). As shown in
Table I, the frequency of AN was
significantly increased in patients who received surgery, and those
who had large, ulcerative tumors; furthermore, the median age in
the AN+ group was significantly lower compared with that
in the AN− group. Thus, younger, postoperative patients
with large ulcerative tumors tended to exhibit AN. In particular,
AN exhibited a strong correlation with ulceration (P=0.001;
Table I). The AN− group
exhibited a better initial response rate (15 complete responses +
16 partial responses = 64%) compared with the AN+ group
(3 complete responses + 3 partial responses = 33%) (P=0.04). The
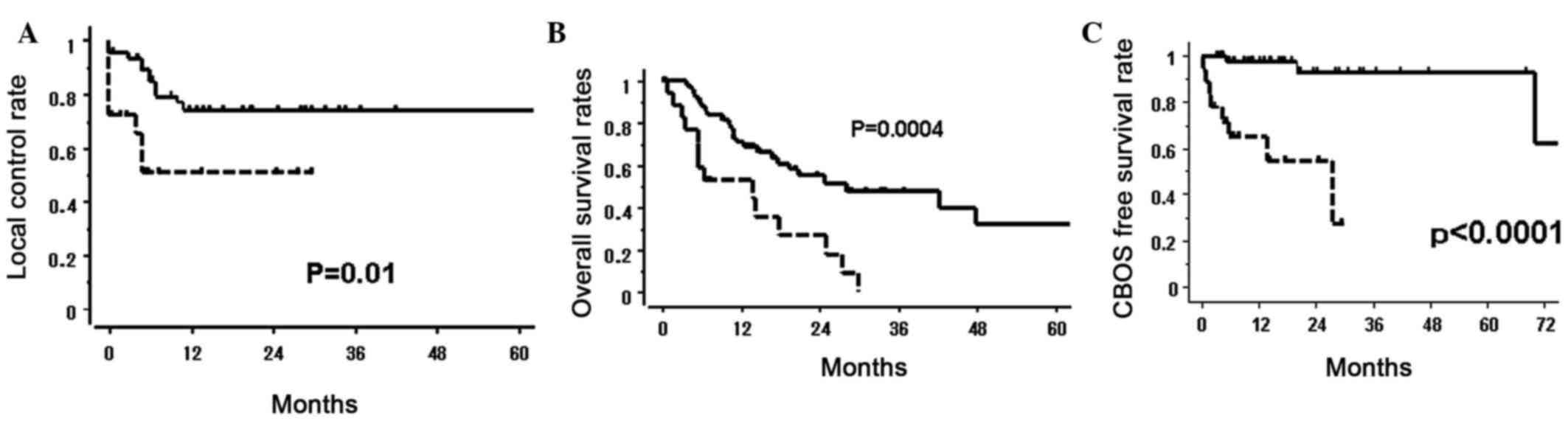
local control (LC) rate in the AN+ group was 51%, which
was significantly lower compared with that in the AN−
group (75%; P=0.01). The median survival time and 1-year survival
rates for the AN+ and AN− groups were 13.6
vs. 28.2 months (P<0.001) and 53 vs. 71% (P=0.0004),
respectively (Table II and Fig. 2). PTV, ulceration, primary site
(nasopharynx or other) and prescribed dose were statistically
significant predisposing factors for reduced overall survival (OS)
according to the univariate analysis (Table II). There were statistically
significant differences in LC and OS rates between the
AN+ and AN− groups (Table II, Fig.
2), indicating poor prognosis for patients with AN.
 | Table II.Analysis of prognostic factors. |
Table II.
Analysis of prognostic factors.
| Variables | No. of
patients | 1-y LC (%) | P-value | MST (months) | 1-y OS (%) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
<70 | 53 | 67 | 0.46 | 19.4 | 69 | 0.670 |
|
≥70 | 14 | 73 |
| 20.8 | 53 |
|
| Sex |
|
|
|
|
|
|
|
Male | 52 | 70 | 0.89 | 17.8 | 67 | 0.280 |
|
Female | 15 | 64 |
| 48 | 62 |
|
| PTV
(cm3) |
|
|
|
|
|
|
|
≤40 | 44 | 70 | 0.54 | 24.9 | 76 | 0.020 |
|
>40 | 23 | 66 |
| 10.3 | 47 |
|
| Abscess/necrosis
(AN) |
|
|
|
|
|
|
|
Yes | 18 | 51 | 0.01 | 13.9 | 53 |
<0.001 |
| No | 49 | 75 |
| 28.2 | 71 |
|
| Ulceration |
|
|
|
|
|
|
|
Yes | 17 | 55 | 0.05 | 6.6 | 38 |
<0.001 |
| No | 50 | 74 |
| 27.5 | 76 |
|
| Primary cancer
type |
|
|
|
|
|
|
|
NPC | 39 | 77 | 0.06 | 42.3 | 75 |
<0.001 |
|
Others | 28 | 54 |
| 13.9 | 53 |
|
| Treatment
intervala (months) |
|
|
|
|
|
|
|
≤30 | 38 | 56 | 0.05 | 17.7 | 61 | 0.150 |
|
>30 | 29 | 82 |
| 39.9 | 72 |
|
| Prescribed dose
(EQD2), Gy |
|
|
|
|
|
|
|
≤40 | 34 | 60 | 0.14 | 14.8 | 60 | 0.010 |
|
>40 | 33 | 76 |
| 42.3 | 72 |
|
| Surgical
history |
|
|
|
|
|
|
|
Yes | 23 | 67 | 0.59 | 14.4 | 65 | 0.500 |
| No | 44 | 69 |
| 24.8 | 66 |
|
Toxicity
A total of 21 patients (31%) experienced grade ≥3
adverse effects. Among them, CBOS was found in 11 patients and
resulted in 8 deaths, whereas the 3 remaining patients recovered
following intervention. All fatal adverse effects were due to CBOS.
A total of 44% (8/18) of patients in the AN+ group and
6% (3/49) of patients in the AN− group developed CBOS
(P=0.001). The AN+ group exhibited a lower CBOS-free
survival ratio (65% at 1 year) compared with the AN−
group (98% at 1 year; P<0.0001; Fig.
2C). In addition, among patients with carotid invasion at
≤180°, only AN+ recurrent oral cancer patients exhibited
CBOS, whereas among patients with carotid invasion at >180°, 44%
of AN+ and 10% of AN− patients developed CBOS
(P<0.01; Table III). Other
grade ≥3 radiation-induced adverse effects included 2 cases of
mucositis requiring percutaneous endoscopic gastrostomy, 2 cases of
lateral lobe necrosis (grade 4 in 1 case), 5 cases of fistulas and
1 case each of bone necrosis, soft tissue necrosis, visual
disturbance and ulceration.
 | Table III.Risk factors for CBOS. |
Table III.
Risk factors for CBOS.
| Factors | No CBOS | CBOS | %a |
|---|
| Carotid invasion
≤180° |
|
|
|
|
AN− | 20 | – | (0) |
|
AN+ | 1 | 1 | (50) |
| Carotid invasion
>180° |
|
|
|
|
AN− | 26 | 3 | (10) |
|
AN+ | 9 | 7 | (44) |
Discussion
To the best of our knowledge, the present study was
the first to investigate AN as a prognostic factor in patients with
recurrent HNC following re-irradiation using SBRT. Low-density
areas on CT and/or water intensity areas on MRI, which may indicate
central necrosis in the lymph node and/or ring enhancement in
contrast-enhanced images (10,11), are
occasionally encountered in a routine clinical examination.
However, it remains elusive whether these findings affect the
outcome and/or adverse effects of SBRT in patients with recurrent
HNC. The presence of AN has been identified as a factor associated
with the inflammatory and/or infection process, which weakens the
arterial walls and may result in CBOS. In addition, a hypoxic tumor
environment indicates a radioresistant and infiltrative nature,
which may be associated with worse prognosis. Certain studies have
indicated that central necrosis in lymph nodes is indicative of
malignancy and poor prognosis with extracapsular extension
(12,13). However, patients with human papilloma
virus infection have been found to have a better prognosis compared
with patients without this infection, and their lymph node
metastases frequently display cystic changes (14). In the present study, the detailed
morphology of AN, such as wall thickness and smoothness, could not
be assessed due to the heterogeneous methods of image collection
(CT and MRI, with or without contrast enhancement, with the use of
different image acquisition techniques and conditions); however,
image interpretation for diagnostic purposes should be performed in
future studies and the results of the present study should be
interpretated with caution.
Patients with fatal CBOS who exhibited AN were
encountered in our previous study (7). CBOS is one of the most devastating
complications of HNC and mainly occurs fas a postoperative
complication, particularly in patients with a history of
radiotherapy and/or when the tumor compromises the vascular axis
(7–10,15–17).
McDonald et al (16) have
reported that CBOS following re-irradiation is a rare [41/1,554
(2.6%)] and often fatal (75%) event. Zoumalan et al
(12) reported that 15 of 33
treatment-related deaths (40%) were associated with CBOS in a
cohort of 166 patients (overall mortality rate, 9%). Similarly, we
also previously reported that CBOS occurred in 8.4% of cases among
381 HNC patients treated with 484 re-irradiation sessions at seven
Japanese CyberKnife® institutions, and 69% of the cases were fatal
(10). In addition, the presence of
ulceration in association with carotid invasion at >180° was an
important risk factor for CBOS (11). The present study identified AN as an
additional risk factor for CBOS in patients with recurrent HNC
after SBRT.
The present study had several limitations. Due to
the retrospective nature of the study and inclusion of only a small
number of patients with a short follow-up period, selection- and
physician-based biases may exist. Therefore, the results of the
present study should be confirmed in a prospective trial with a
larger number of patients with longer follow-up periods. In
addition, there were several confounding factors exhibiting a
correlation with AN, such as age, postoperative status, tumor
volume and ulceration. Therefore, although AN was not found to be
an independent risk factor, it should be taken into consideration
when determining a patient's eligibility for re-irradiation using
SBRT.
In conclusion, younger postoperative patients with
large and ulcerative tumors tended to exhibit AN. Thus, AN is an
important prognostic factor for HNC patients following
reirradiation using CyberKnife®, as well as a predictor of fatal
CBOS.
References
|
1
|
Mazeron R, Tao Y, Lusinchi A and Bourhis
J: Current concepts of management in radiotherapy for head and neck
squamous-cell cancer. Oral Oncol. 45:402–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vokes EE, Weichselbaum RR, Lippman SM and
Hong WK: Head and neck cancer. N Eng J Med. 328:184–194. 1993.
View Article : Google Scholar
|
|
3
|
Temam S, Pape E, Janot F, Wibault P,
Julieron M, Lusinchi A, Mamelle G, Marandas P, Luboinski B and
Bourhis J: Salvage surgery after failure of very accelerated
radiotherapy in advanced head-and-neck squamous cell carcinoma. Int
J Radiat Oncol Biol Phys. 62:1078–1083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong SJ, Machtay M and Li Y: Locally
recurrent, previously irradiated head and neck cancer: Concurrent
re-irradiation and chemotherapy, or chemotherapy alone? J Clin
Oncol. 24:2653–2658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lartigau EF, Tresch E, Thariat J, Graff P,
Coche-Dequeant B, Benezery K, Schiappacasse L, Degardin M, Bondiau
PY, Peiffert D, et al: Multi institutional phase II study of
concomitant stereotactic reirradiation and cetuximab for recurrent
head and neck cancer. Radiother Oncol. 109:281–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kress MA, Sen N, Unger KR, Lominska CE,
Deeken JF, Davidson BJ, Newkirk KA, Hwang J and Harter KW: Safety
and efficacy of hypofractionated stereotactic body reirradiation in
head and neck cancer: Long-term follow-up of a large series. Head
Neck. 37:1403–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kodani N, Yamazaki H, Tsubokura T, Shiomi
H, Kobayashi K and Nishimura T, Aibe N, Ikeno H and Nishimura T:
Stereotactic body radiation therapy for head and neck tumor:
Disease control and morbidity outcomes. J Radiat Res. 52:24–31.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cengiz M, Özyiğit G, Yazici G, Doğan A,
Yildiz F, Zorlu F, Gürkaynak M, Gullu IH, Hosal S and Akyol F:
Salvage reirradiaton with stereotactic body radiotherapy for
locally recurrent head-and-neck tumors. Int J Radiat Oncol Biol
Phys. 81:104–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamazaki H, Ogita M, Kodani N, Nakamura S,
Inoue H, Himei K, Kotsuma T, Yoshida K, Yoshioka Y, Yamashita K and
Udono H: Frequency, outcome and prognostic factors of carotid
blowout syndrome after hypofractionated re-irradiation of head and
neck cancer using CyberKnife®: A multi-institutional study.
Radiother Oncol. 107:305–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamazaki H, Ogita M, Himei K, Nakamura S,
Kotsuma T, Yoshida K and Yoshioka Y: Carotid blowout syndrome in
pharyngeal cancer patients treated by hypofractionated stereotactic
re-irradiation using CyberKnife®: A multi-institutional
matched-cohort analysis. Radiother Oncol. 115:67–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
King AD, Tse GM, Ahuja AT, Yuen EH,
Vlantis AC, To EW and van Hasselt AC: Necrosis in metastatic neck
nodes: Diagnostic accuracy of CT, MR imaging, and US. Radiology.
230:720–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zoumalan RA, Kleinberger AJ, Morris LG,
Ranade A, Yee H, DeLacure MD and Myssiorek D: Lymph node central
necrosis on computed tomography as predictor of extracapsular
spread in metastatic head and neck squamous cell carcinoma: Pilot
study. J Laryngol Otol. 124:1284–1288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding ZX, Liang BL, Shen J, Xie BK, Huang
SQ and Zhang B: Magnetic resonance imaging diagnosis of cervical
lymph node metastasis from lingual squamous cell carcinoma. Chin J
Cancer. 24:199–203. 2005.(In Chinese).
|
|
14
|
Goldenberg D, Begum S, Westra WH, Khan Z,
Sciubba J, Pai SI, Califano JA, Tufano RP and Koch WM: Cystic lymph
node metastasis in patients with head and neck cancer: An
HPV-associated phenomenon. Head Neck. 30:898–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esteller E, León X, de Juan M and Quer M:
Delayed carotid blow-out syndrome: A new complication of
chemoradiotherapy treatment in pharyngolaryngeal carcinoma. J
Laryngol Otol. 126:1189–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McDonald MW, Moore MG and Johnstone PA:
Risk of carotid blowout after reirradiation of the head and neck: A
systematic review. Int J Radiat Oncol Biol Phys. 82:1083–1089.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen KC, Yen TT, Hsieh YL, Chen HC, Jiang
RS, Chen WH and Liang KL: Postirradiated carotid blowout syndrome
in patients with nasopharyngeal carcinoma: A case-control study.
Head Neck. 37:794–799. 2015. View Article : Google Scholar : PubMed/NCBI
|