Introduction
Adenoid cystic carcinoma (ACC) usually occurs in
salivary glands, but it has also been described in other organs,
including the breast, trachea, uterine cervix, larynx and
Bartholin's glands (1–5). However, ACC of the breast is rare
(6); thus, there are only a few
published studies describing the findings of multiple imaging
techniques, and non-specific imaging characteristics have been
found on mammography and ultrasonography (7,8). The aim
of this study was to present a rare case of proven ACC of the
breast and describe its imaging characteristics.
Case report
In April 2016, a 66-year-old woman was admitted to
the Sun Yat-sen Memorial Hospital (Guangzhou, China) with a mass in
the left breast that had been present for 4 years. The patient
reported gradual enlargement of this mass over the last 2 months.
Breast examination revealed a palpable mass in the subareolar
region measuring 5.0×5.0 cm, with nipple retraction. There was no
abnormal nipple discharge or skin redness. Axillary and
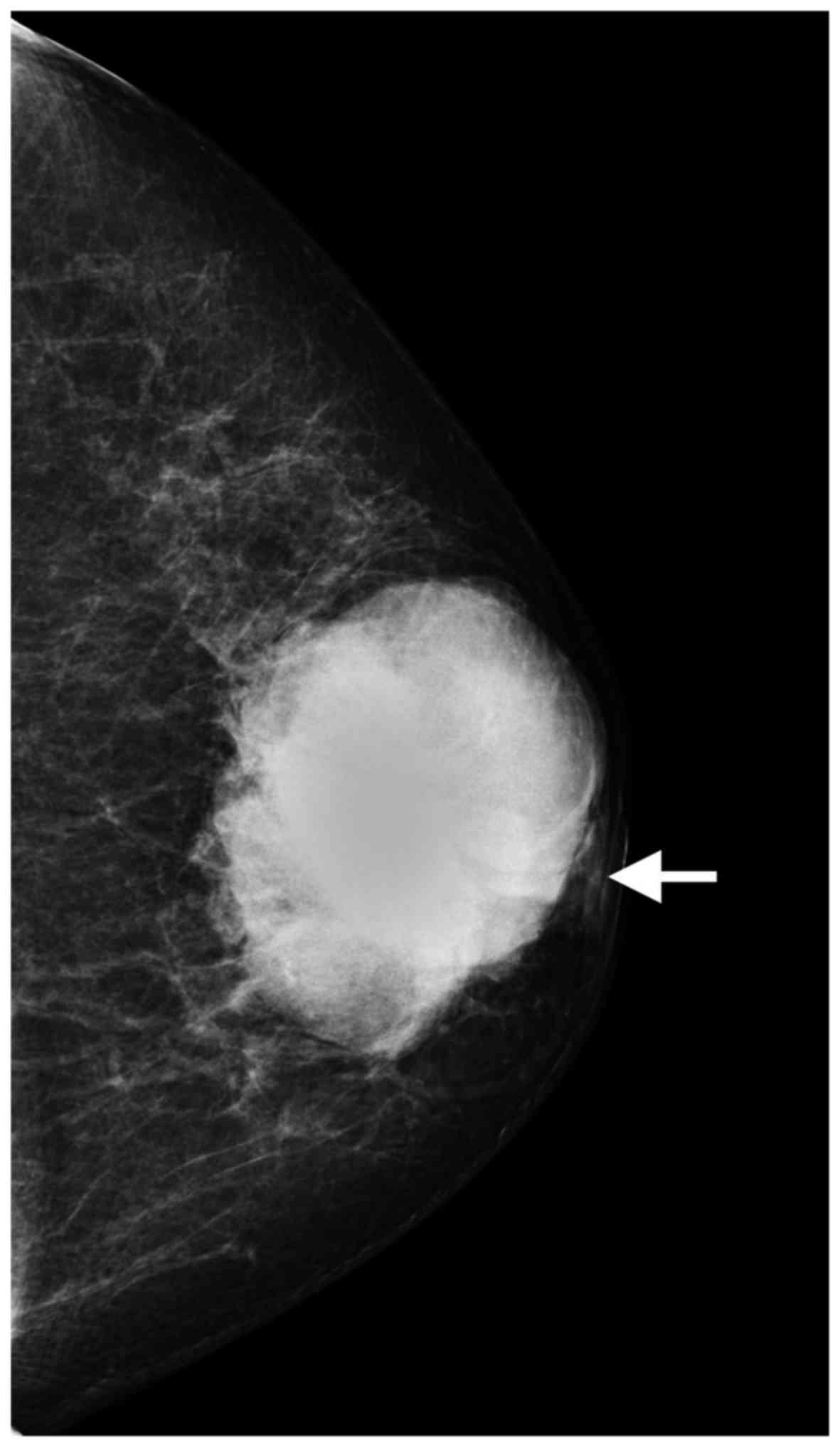
subclavicular lymph nodes were not palpable. Mammography identified
a 6.8×5.0 cm, high-density, irregular mass, with nipple retraction
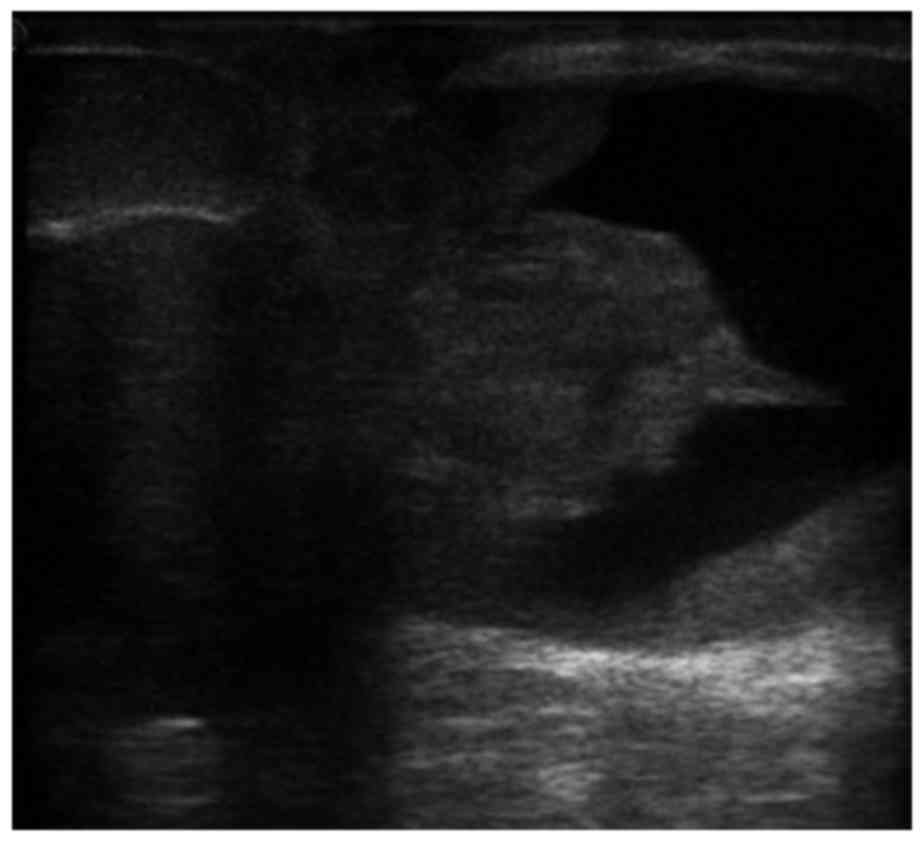
(Fig. 1). Ultrasonography revealed
an irregular mass sized 5.0×4.3×5.0 cm, without a clearly
circumscribed margin, in the subareolar region. The internal echo
of the mass was mixed cystic and solid, with partial enhancement of
the posterior echo (Fig. 2). On
Doppler examination, internal vascularity was seen. The patient
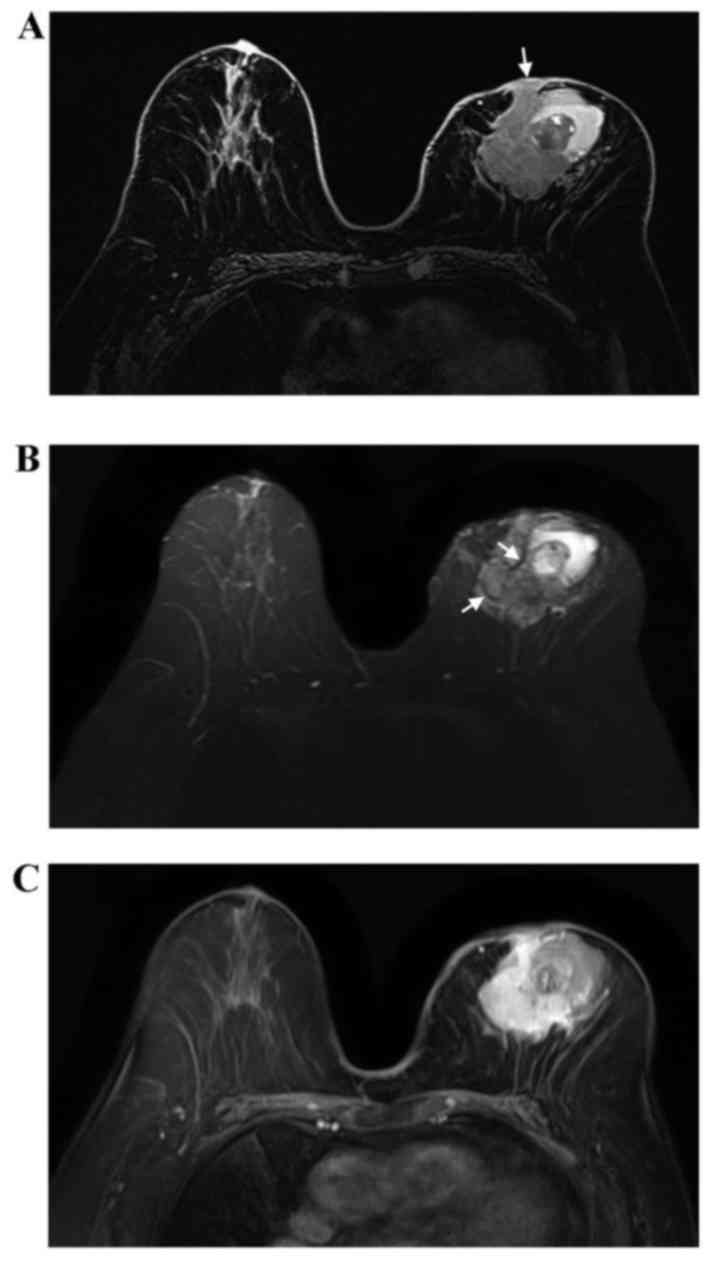
underwent a contrast-enhanced bilateral breast magnetic resonance
imaging (MRI) examination in the prone position. The MRI revealed a
left breast mass sized 6.3×4.6×6.2 cm. The mass appeared to have an
irregular shape and irregular margins. The lesion was mixed cystic
and solid. Compared with normal glandular tissue, the solid part of
the lesion appeared heterogeneously isointense on T1-weighted
imaging (WI; Fig. 3A), with a
slightly high signal on T2WI (Fig.
3B). Furthermore, the solid part of the lesion included
internal septations with a low signal on T2WI (Fig. 3B). Following injection of contrast
agent, the enhancement of the solid component was rapid and
heterogeneous (Fig. 3C). The cystic
parts of lesion appeared as high-signal on T1WI and T2WI, and two
layers were observed in the largest cystic part. The tumor
infiltrated the left nipple and adjacent skin.
Ultrasound-guided 14-gauge core biopsy was performed
with a BARD biopsy instrument. On microscopic examination, the
tumor cells were arranged in a tubular or cribriform pattern, and
exhibited consistent size, small nuclei and nuclear fission. The
findings of the histopathological evaluation of the specimens
conformed to those of invasive carcinoma, and suggested the
possibility of ACC. On immunohistochemistry, the tumor cells were
negative for progesterone receptor (PR) and human epidermal growth
factor receptor 2 (HER2), but positive for estrogen receptor (ER)
(focally, 1% positive) and CD117. Due to the large size of the
mass, the patient underwent left modified radical mastectomy with
axillary lymph node dissection. The breast specimen measured
17×14×5.0 cm and contained a mixed cystic and solid, poorly defined
mass measuring 4.5×4.5×3.0 cm. The solid part of the lesion was
grey-white and grey-red. Grey-red papillary projections and a
hematoma were observed in the cystic cavity. The pathological
examination of the specimen identified the tumor as ACC of the left
breast. The resected lymph nodes were negative for tumor
metastasis. Since the ER was 1% positive, treatment with tamoxifen
(20 mg/day) for 5 years was decided upon, but was discontinued
after 2 months, as the patient was unable to afford treatment. No
chemotherapy or radiotherapy have been performed to date. At the
last follow-up visit (May 29, 2017), the patient remained free of
locoregional recurrence and distant metastases. The patient
provided a signed informed consent regarding the publication of the
case details and associated images.
Discussion
ACC was first described by Billroth in 1856 and was
characterized as ‘cylindroma’ (9).
Geschickter and Copeland referred to this tumor as ‘adenocystic
basal cell cancer of the breast’ in 1945, acknowledging its eccrine
gland origin and slow growth (10).
ACC of the breast is a particularly rare tumor, comprising <0.1%
of all breast malignancies (6), and
it occurs mainly in women during their fifth and sixth decades of
life (11). Consistent with the
current literature, our patient was 66-year-old. The most common
clinical manifestation of ACC of the breast is a soft mass,
commonly located in the subareolar area (6), as was the case in our patient. Multiple
cytoarchitectural patterns have been reported (cribriform, tubular,
trabecular and solid), and a mixture of different growth patterns
is generally observed (12). In the
present case, the tumor cells exhibited tubular or cribriform
arrangement. ACC of the breast is frequently negative for ER and
PR, as well as HER2 gene amplification (triple-negative) (6). However, ER-positive and PR-positive ACC
has also been reported (13). In the
present study, the patient was PR- and HER2-negative, but focally
(1%) positive for ER. The significance of the positive hormone
receptor status is not known, and it may be associated with a
non-pure ACC or an invasive cribriform carcinoma with a
significantly worse prognosis. The tumor in the present case
exhibited diffuse CD117 positivity. CD117 is typically positive in
ACC, and it is used to differentiate ACC from conventional breast
carcinomas (14). ACC has been
reported to have an excellent survival rate, despite its malignant
nature, with distant metastases and involvement of the axillary
nodes being exceedingly rare (15).
On mammography, the majority of the ACCs of the
breast present as high-density masses with irregular or lobulated
shape and indistinct margins, which have been described as
malignant-appearing (8,16–19);
these characteristics were consistent with our findings. The
sonographic appearance of ACC has been described as a hypoechoic or
heterogeneous mass with irregular, lobulated or oval contours and
unclear margin (7,8,18). In
the present case, the shape and margins of the mass were consistent
with previously reported cases, while the echo of the mass was
different. The internal echo of the mass in the present case was
mixed cystic and solid. One previous case also reported small cysts
within in a rounded nodule (18).
The MRI appearance of ACC of the breast remains
controversial. Glazebrook et al (8) found that, on MRI, the masses appeared
to have a lobulated or irregular shape, with spiculated margins.
Two larger masses exhibited extensive hyperintensity on T2WI, with
rapid and heterogeneous enhancement, which was consistent with our
case. However, Tsuboi et al (20) and Tang et al (7) found that the majority of the cases had
a benign appearance. In addition, Tang et al (7) found that all masses had internal
septations with low signal on T2WI, and that the internal
septations in the larger masses were enhanced in the delayed phase.
Internal septations on T2WI were also observed in the present case,
but exhibited heterogeneous enhancement. Tang et al
(7) hypothesized that the internal
septations observed on T2WI may correspond to stroma being embedded
within the tumor islands. The internal septations may help to
distinguish ACC of the breast from infiltrating ductal carcinoma
and fibroadenoma of the breast. Some fibroadenomas display
non-enhancing internal septations, while the internal septations of
ACC have been described as delayed-enhanced, particularly in the
larger lesions. Furthermore, the mass in the present study was
mixed cystic and solid, and the cystic parts of lesion contained
hematomas, which has not been previously reported.
In conclusion, ACC of the breast is a rare type of
breast cancer, which may be difficult to distinguish from other
types of breast lesions on mammography. The following signs may
suggest the diagnosis: mixed cystic and solid mass with unclear
margin on ultrasonography and MRI, and internal septations on T2WI
that exhibit delayed enhancement on MRI (7). A core biopsy may be required to confirm
the diagnosis.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adenoid cystic carcinoma
|
|
MRI
|
magnetic resonance imaging
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
PR
|
progesterone receptor
|
|
T1WI
|
T1-weighted imaging
|
|
T2WI
|
T2-weighted imaging
|
References
|
1
|
Cavanzo FJ and Taylor HB: Adenoid cystic
carcinoma of the breast. An analysis of 21 cases. Cancer.
24:740–745. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cleveland RH, Nice CM Jr and Ziskind J:
Primary adenoid cystic carcinoma (cylindroma) of the trachea.
Radiology. 122:597–600. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prempree T, Villasanta U and Tang CK:
Management of adenoid cystic carcinoma of the uterine cervix
(cylindroma): Report of six cases and reappraisal of all cases
reported in the medical literature. Cancer. 46:1631–1635. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olofsson J and van Nostrand AW: Adenoid
cystic carcinoma of the larynx: A report of four cases and a review
of the literature. Cancer. 40:1307–1313. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Addison A and Parker RT: Adenoid cystic
carcinoma of Bartholin's gland: A review of the literature and
report of a patient. Gynecol Oncol. 5:196–201. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boujelbene N, Khabir A, Boujelbene N,
Sozzi W Jeanneret, Mirimanoff RO and Khanfir K: Clinical
review-breast adenoid cystic carcinoma. Breast. 21:124–127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang W, Peng WJ, Gu YJ, Zhu H, Jiang TT
and Li C: Imaging manifestation of adenoid cystic carcinoma of the
breast. J Comput Assist Tomogr. 39:523–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glazebrook KN, Reynolds C, Smith RL,
Gimenez EI and Boughey JC: Adenoid cystic carcinoma of the breast.
AJR Am J Roentgenol. 194:1391–1396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Billroth T: Studies on the Development of
the Blood Vessels Combined with Observations from the Royal
Surgical University Clinic of Berlin. Georg Reimer, Berlin. 55–69.
1856.(In German).
|
|
10
|
Geschickter CF and Copeland MM: Diseases
of the Breast: Diagnosis, Pathology, Treatment. 2nd. J B
Lippincott; Philadelphia: pp. 421–422. 1945
|
|
11
|
Ghabach B, Anderson WF, Curtis RE, Huycke
MM, Lavigne JA and Dores GM: Adenoid cystic carcinoma of the breast
in the United States (1977 to 2006): A population-based cohort
study. Breast Cancer Res. 12:R542010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Da Silva L, Buck L, Simpson PT, Reid L,
McCallum N, Madigan BJ and Lakhani SR: Molecular and morphological
analysis of adenoid cystic carcinoma of the breast with synchronous
tubular adenosis. Virchows Arch. 454:107–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arpino G, Clark GM, Mohsin S, Bardou VJ
and Elledge RM: Adenoid cystic carcinoma of the breast: Molecular
markers, treatment, and clinical outcome. Cancer. 94:2119–2127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mastropasqua MG, Maiorano E, Pruneri G,
Orvieto E, Mazzarol G, Vento AR and Viale G: Immunoreactivity for
c-kit and p63 as an adjunct in the diagnosis of adenoid cystic
carcinoma of the breast. Mod Pathol. 18:1277–1282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peters GN and Wolff M: Adenoid cystic
carcinoma of the breast. Report of 11 new cases: Review of the
literature and discussion of biological behavior. Cancer.
52:680–686. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youk JH, Kim MJ, Kim EK, Lee JY, Oh KK and
Park BW: Recurrence of adenoid cystic carcinoma in the breast after
lumpectomy and adjuvant therapy. J Ultrasound Med. 25:921–924.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ichikawa K, Mizukami Y, Takayama T,
Takemura A, Miyati T and Taniya T: A case of adenoid cystic
carcinoma of the breast. J Med Ultrason (2001). 34:193–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Luis E, Apesteguía L, Noguera JJ, Pina
L, Martínez-Regueira F, Miguel C and Sáenz J: Adenoid cystic
carcinoma of the breast. Radiologia. 48:235–240. 2006.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santamaría G, Velasco M, Zanón G, Farrús
B, Molina R, Solé M and Fernández PL: Adenoid cystic carcinoma of
the breast: Mammographic appearance and pathologic correlation. AJR
Am J Roentgenol. 171:1679–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuboi N, Ogawa Y, Inomata T, Nishioka A,
Yoshida D, Yoshida S and Moriki T: Dynamic MR appearance of adenoid
cystic carcinoma of the breast in a 67-year-old female. Radiat Med.
16:225–228. 1998.PubMed/NCBI
|