Introduction
Primary duodenal cancer (PDC) is rare, accounting
for only a small proportion of gastrointestinal malignancies. The
most frequent symptoms of PDC include abdominal pain, vomiting and
weight loss. However, the clinical presentations of PDC are
non-specific. The most common type of PDC is adenocarcinoma and it
frequently occurs in the descending duodenum (1). Mucinous adenocarcinoma (MA) is derived
from the epithelium, is characterized by the production of copious
amounts of mucin, and is considered as a distinct pathological
entity with poor prognosis. MA has been described in the breast,
ovary, vulva, lung, pancreas, stomach, appendix and colorectum, but
rarely in the duodenum (2,3). As duodenal mucinous adenocarcinoma
(DMA) is rare, its typical clinical presentation has not been
well-documented. Gastroduodenoscopy and gastrointestinal barium
radiography are considered as effective auxiliary examinations in
the diagnosis of DMA. Currently, surgical resection is the standard
treatment of choice for DMA, and the patients may benefit markedly
from curative surgery with negative resection margins (1).
To the best of our knowledge, this is the first
report of DMA presenting as ileus, obstructive jaundice and massive
ascites simultaneously.
Case report
A 70-year-old female patient was admitted to the
First People's Hospital of Jingmen (Jingmen, China) in February
2016 due to gradually aggravated abdominal pain and distension
accompanied by jaundice for 10 days. The pain was localized to the
subxiphoid and right upper abdominal areas, with paroxysmal
exacerbations. There was no associated fever, nausea or vomiting.
The abdominal examination revealed positive shifting dullness and
prominent direct tenderness in the subxiphoid area and the right
upper quadrant, with no rebound tenderness or muscle spasm. The
bowel sounds were diminished. No subcutaneous varicose veins on the
abdominal wall or gastrointestinal peristaltic wave was observed.
The patient had undergone cholecystectomy and
choledochoduodenostomy 10 years prior, but further information was
unavailable. Hematological investigations revealed an elevated
white blood cell count of 13.5×109/l (normal range,
3.5–9.5×109/l), a hemoglobin level of 85 g/l (normal
range, 115–150 g/l) and a platelet count of 164×109/l
(normal range, 100–300×109/l). Hepatic function tests
revealed a total bilirubin concentration of 127.8 µmol/l (normal
range, 0–21 µmol/l), a direct bilirubin concentration of 112.2
µmol/l (normal range, 0–7 µmol/l), an albumin concentration of 23.8
g/l (normal range, 40–55 g/l), an alkaline phosphatase
concentration of 410.0 U/l (normal range, 35–125 µmol/l), and a
γ-glutamate transpeptidase concentration of 135.0 U/l (normal
range, 4–60 µmol/l). There was renal insufficiency, with elevated
creatinine concentration (140.9 µmol/l; normal range, 41–84
µmol/l). The level of the C-reactive protein was 148.5 mg/l (normal
range, 0–10 mg/l). The levels of carbohydrate antigen (CA) 125 and
CA199 were 418.0 U/ml (normal range, 0–35 U/ml) and 459.7 U/ml
(normal range, 0–27 U/ml), respectively. The α-fetoprotein (AFP),
CA153 and carcinoembryonic antigen (CEA) levels were normal.
Carcinoma cells were not found on exfoliative cytology of the
ascitic fluid. An abdominal X-ray revealed an air-fluid level.
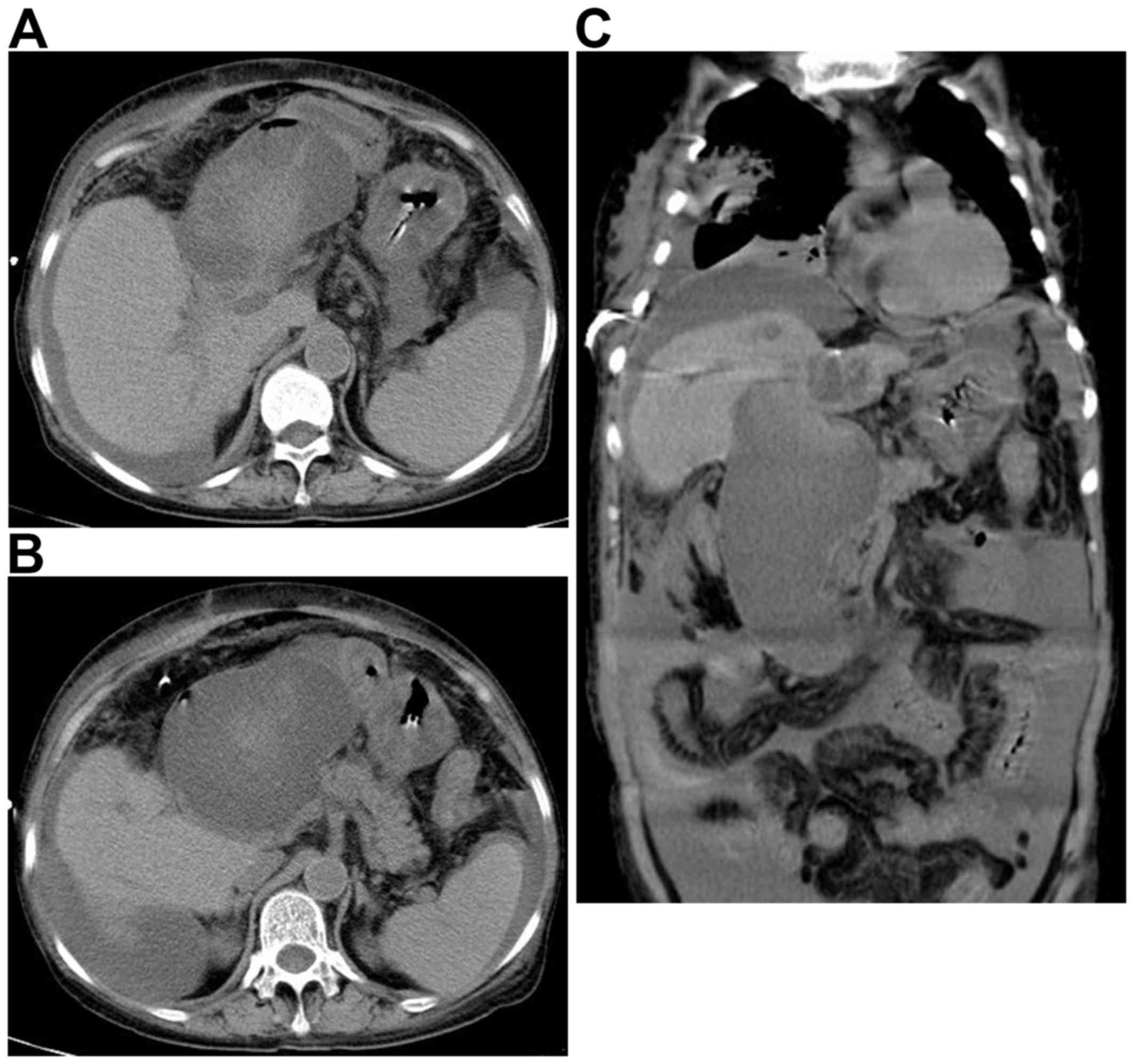
Computed tomography (CT) scans revealed calculi and dilation of
intrahepatic duct, choledochectasia, a possible choledochocyst and
a large fluid collection in the abdominal and pelvic cavity; no
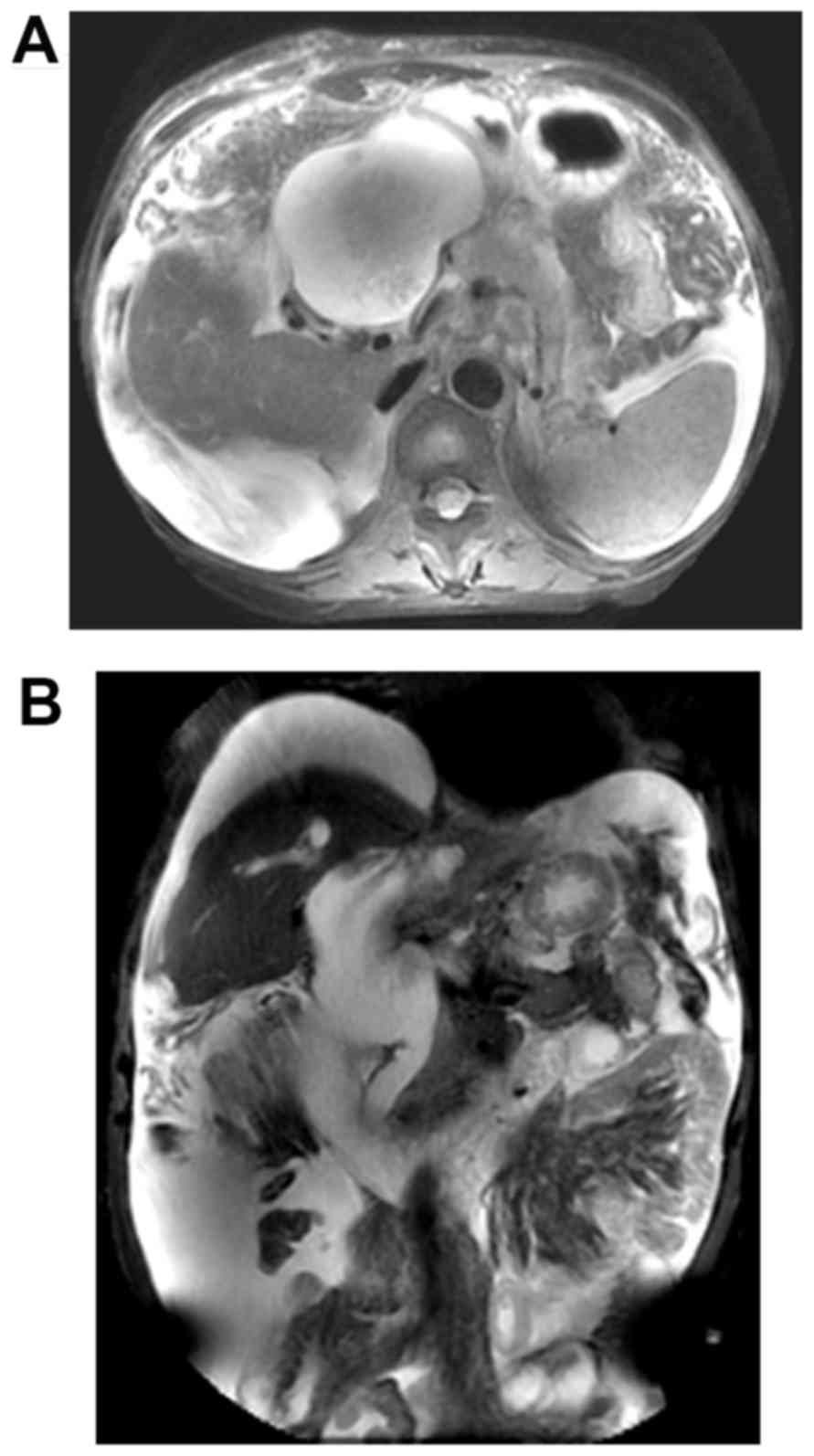
duodenal neoplasm or enlarged lymph nodes were identified (Fig. 1). The results of magnetic resonance
imaging (MRI) scans were similar to those of CT scans (Fig. 2). Due to the renal insufficiency, the
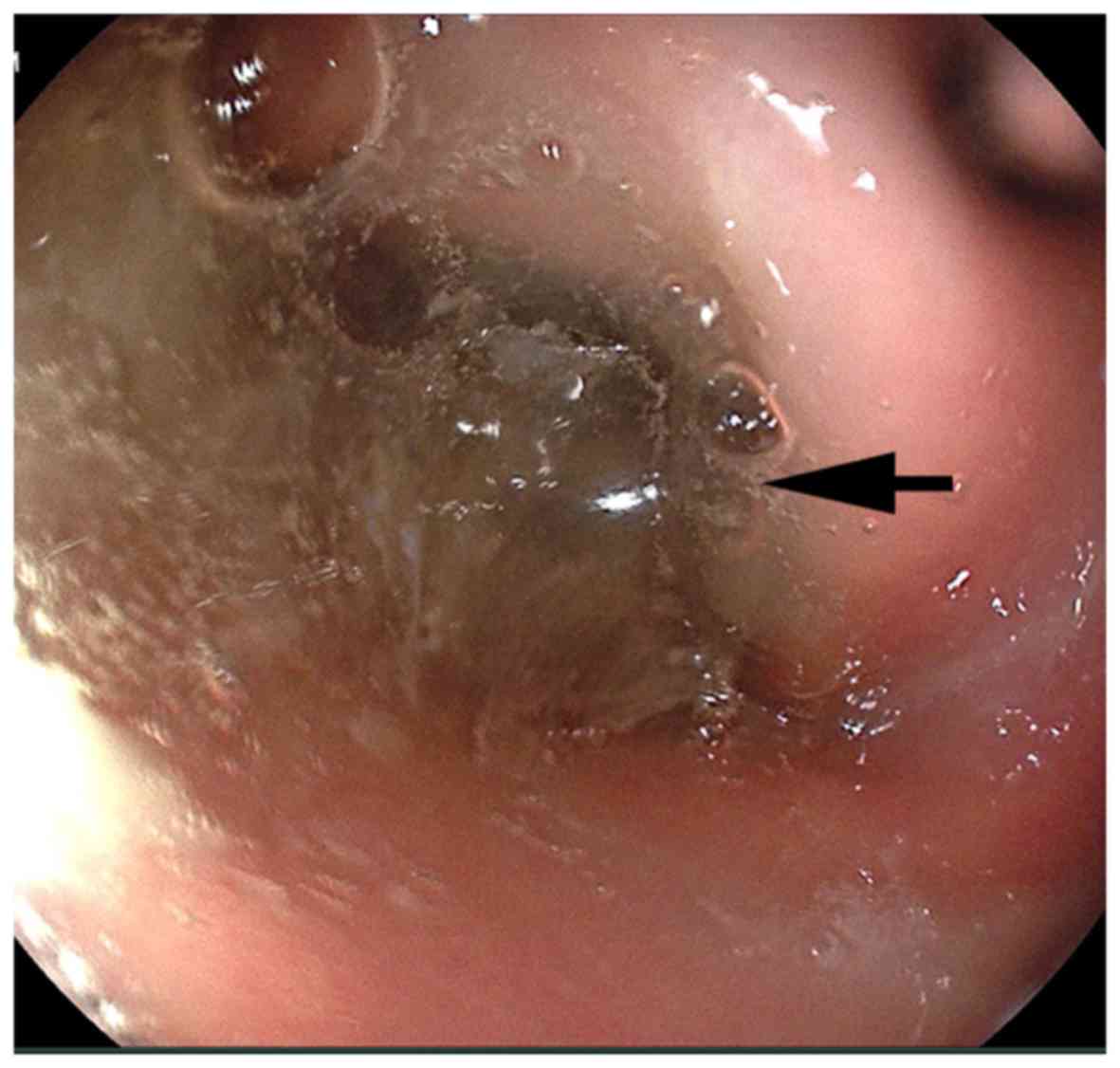
patient did not undergo enhanced CT or MRI. A gastroscopy revealed
a large amount of semi-transparent colloidal substance (Fig. 3). Symptomatic nutritional support
therapy was conducted after hospitalization and the patient was
actively investigated to determine the cause of the symptoms.
However, abdominal pain and distension gradually increased and were
accompanied by discontinuous nausea and vomiting. An etiology could
not be identified based on the symptoms, signs and auxiliary
examination. The possibility of a malignant neoplasm was
considered. However, the CT and MRI scans did not reveal any
lesions and cancer cells were not detected in exfoliative cytology
examination of the ascitic fluid. Therefore, an exploratory
laparotomy was conducted to investigate the status of the abdomen.
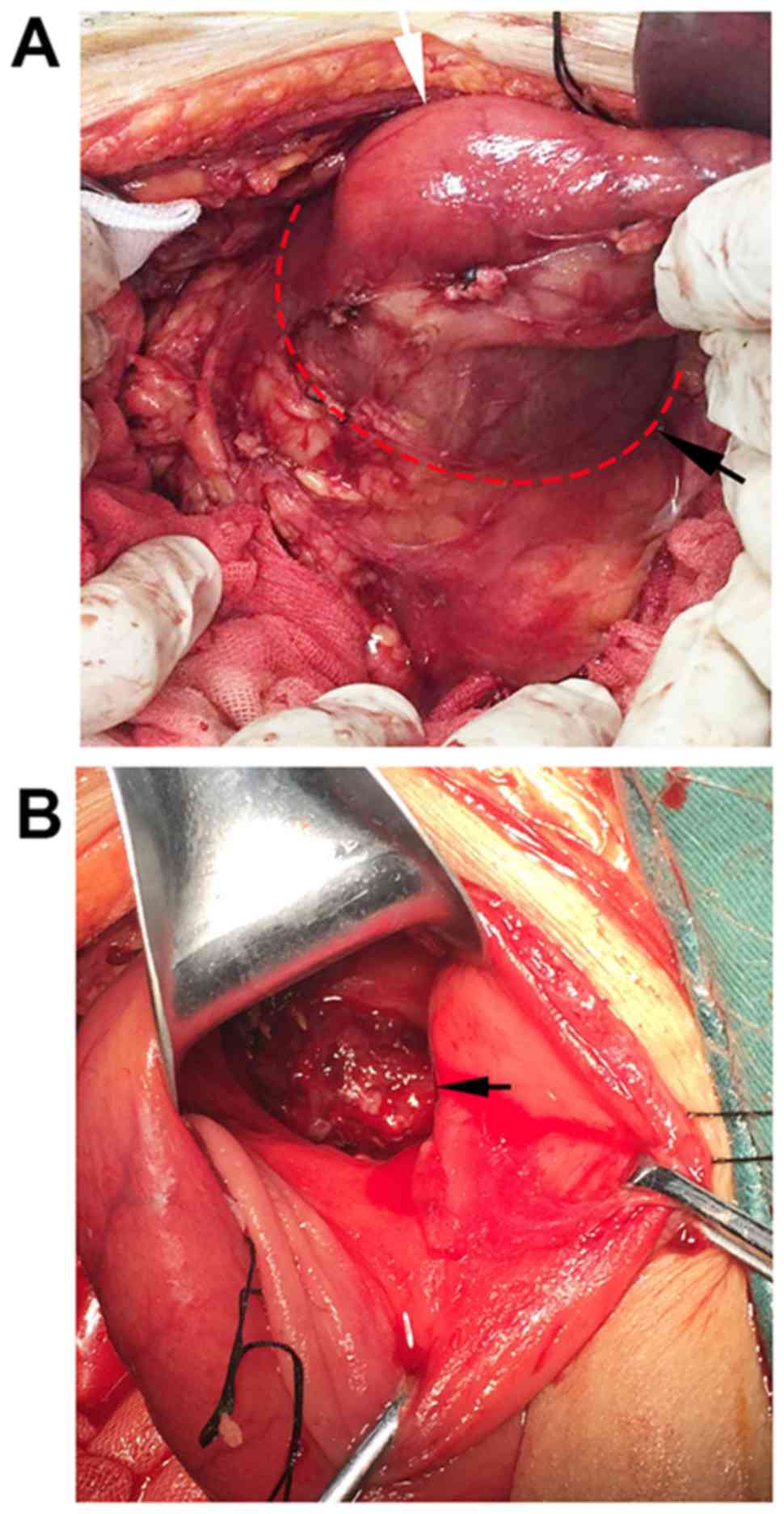
Peritoneal cancer foci were not observed. The duodenum was
obviously dilated, rather than a choledochocyst that was initially
suspected based on the CT findigs (Fig.
4A). The gastric antrum was then incised to explore the
duodenum and it was found to contain a copious amount of a
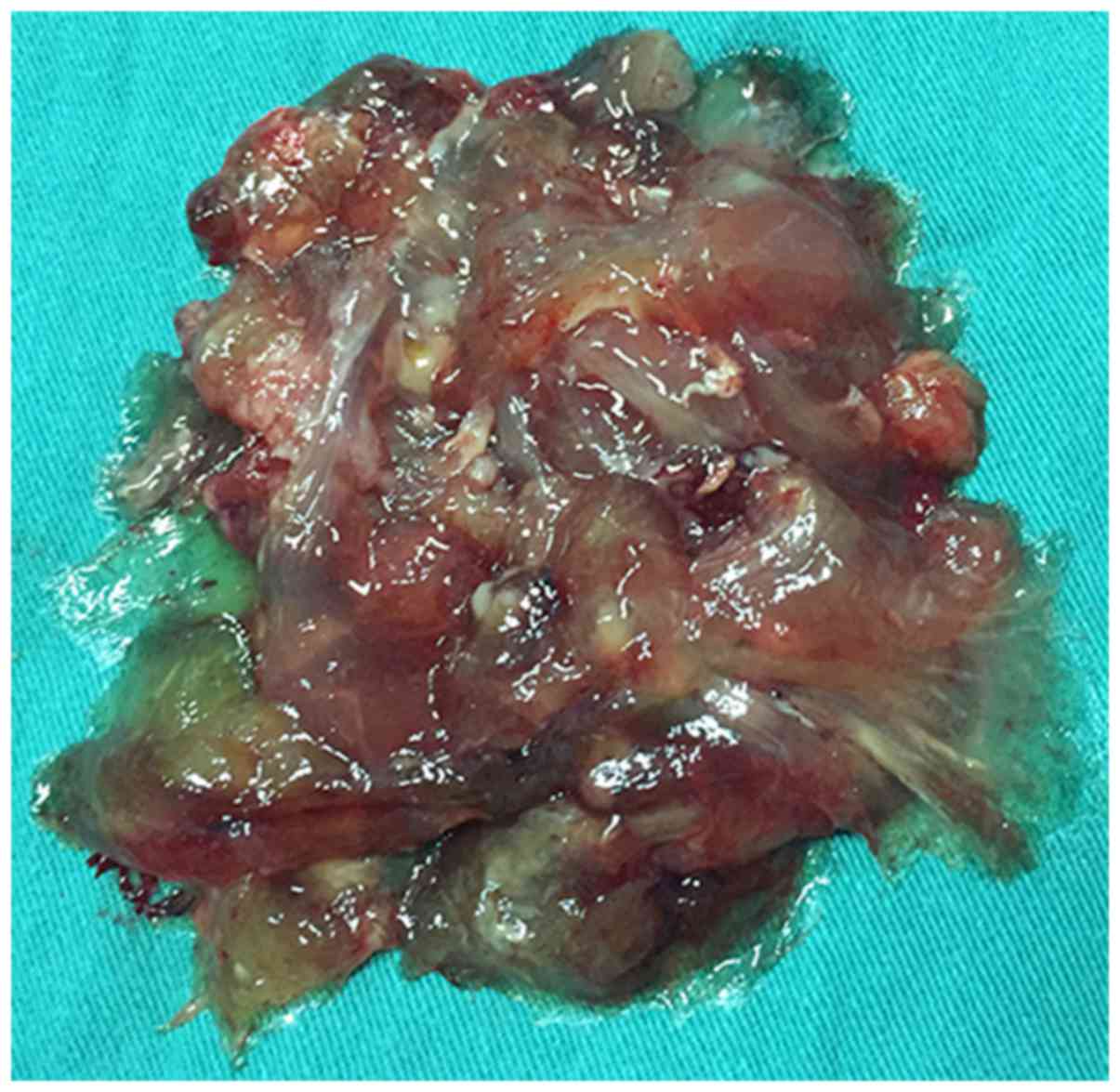
colloidal substance (Fig. 5). After
clearing this colloidal substance, a cancer lesion with an
irregular margin, 2.0 cm in diameter, was identified in the
antimesenteric border of the duodenal bulb (Fig. 4B), and DMA was considered as the
possible diagnosis. Based on the findings in the abdomen, and since
the patient was elderly and in poor general condition, a
gastrojejunostomy rather than radical resection was performed. The
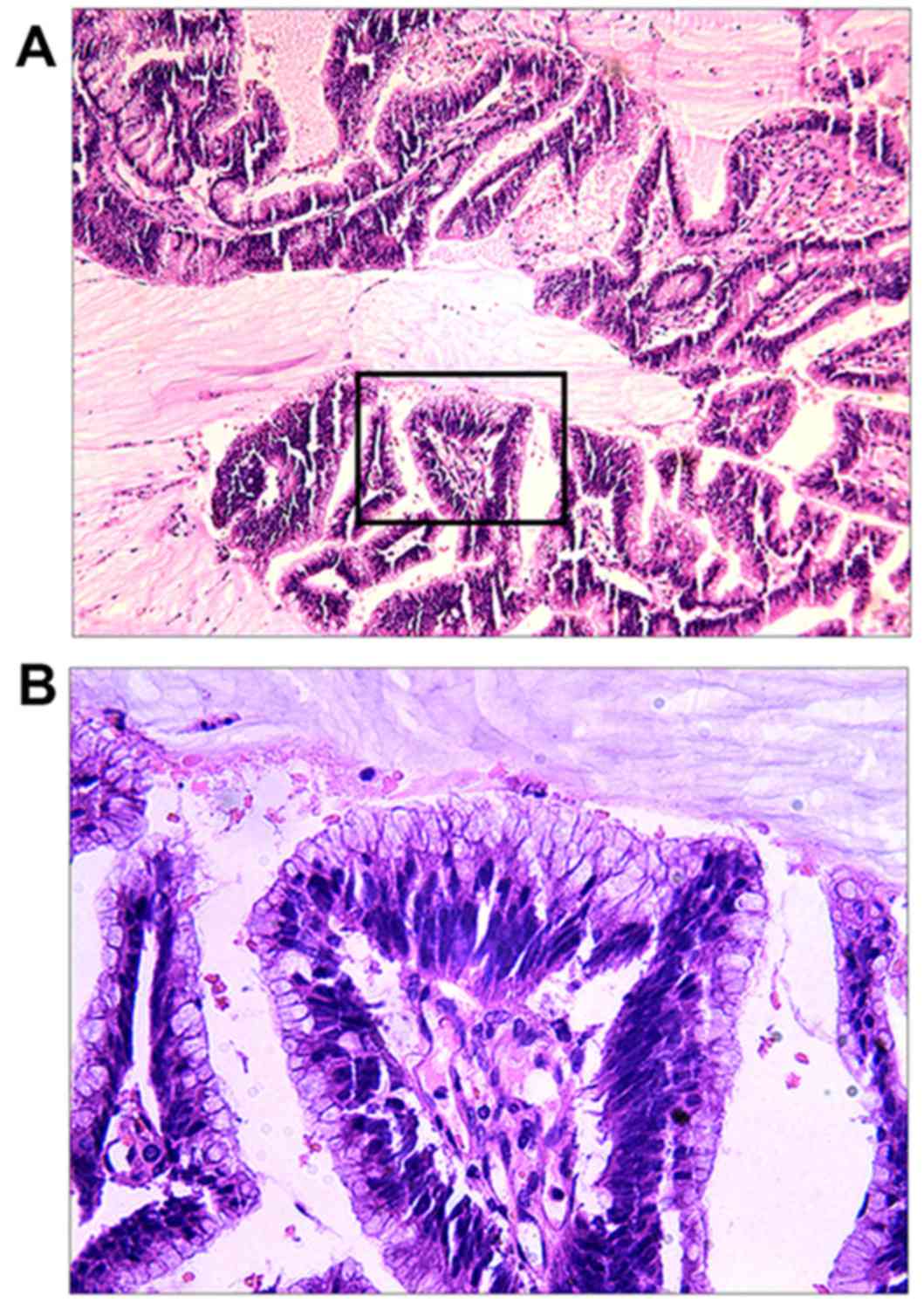
postoperative histopathological examination of the colloidal tumor
revealed MA (Fig. 6A and B). On
immunohistochemical examination, the cancer tissue was positive for
pan-cytokeratin, cytokeratin 19, CDX-2, CEA and Ki-67 (8%), and
negative for excision repair-1. The general condition of the
patient postoperatively was poor and adjuvant chemotherapy was not
administered. The patient succumbed to the disease 42 days after
surgery.
Consent was obtained from the patient and her family
regarding the publication of the case details and associated
images.
Discussion
Primary DMA is an exceedingly rare type of duodenal
cancer and little information on this subject is available in the
literature. At present, the pathogenesis of DMA has not been fully
elucidated. The low incidence rate and non-specific presentation
increase the difficulty of DMA diagnosis.
Mucinous adenocarcinoma (MA) is diagnosed when
>50% of the neoplasm comprises mucinous cells on histological
examination, according to the criteria of the World Health
Organization (4). MA may arise at
various sites of the gastrointestinal tract, more frequently the
stomach and colorectum, and the prognosis is unfavorable. Mucinous
gastric carcinoma (MGC) is usually diagnosed at an advanced stage,
and diagnosis of early MGC is extremely rare. Advanced-stage MGC is
associated with a dismal prognosis, as its biological behavior is
similar to that of other types of gastric cancer (5). Colorectal MA, a subtype of colorectal
cancer, is associated with a high risk of metastasis and a poorer
prognosis compared with non-mucinous subtypes (6). The College of American Pathologists
reported that the prognosis does not vary significantly among
different MA subtypes, but rather relies on stage and grade
(7). Generally, ~0.1–1.3% of
gastrointestinal malignancies are located in the small intestine,
and the duodenum accounts for >50% of those cases (8). Unfortunately, the majority of small
bowel adenocarcinomas are diagnosed at an advanced stage, with
metastasis in 35% of the cases (9).
Thus, early diagnosis of DMA is crucial.
The common symptoms of PDC include abdominal pain,
vomiting, weight loss, gastrointestinal bleeding and jaundice
(10). The cause of jaundice is
frequently a peripapillary carcinoma, resulting in obstructive
jaundice. In addition to abdominal pain, our patient presented with
ileus, obstructive jaundice and massive ascites. The symptoms were
attributed to the copious amount of colloidal substance
accumulating in the duodenum. Gastroduodenoscopy and
gastrointestinal barium radiography are commonly effective methods
for diagnosing PDC (1). However, in
the present case, only a large amount of colloidal substance was
observed and the tumor in the duodenum was not identified. The
presence of the colloidal substance was initially mistaken for a
recently ingested food of similar consistency and the possibility
of DMA was not considered. The role of CT in detecting PDC has not
been adequately addressed. Zhang et al reported a
sensitivity of 74.2% of CT scans in identifying the lesion in
patients with PDC (1). Other studies
reported that CT was effective for detecting cancerous lesions of
the duodenum as well as for tumor staging preoperatively and
postoperatively (11). However,
there is currently no information on the DMA characteristics on CT
and MRI. In the present case, CT and MRI scans revealed no signs of
DMA. It may be hypothesized that the copious amount of the
colloidal substance in the duodenum affected the findings on CT
imaging. Gastroduodenoscopy is a valuable method for detection of
duodenal cancers, and it has been reported that it may detect ~90%
of PDCs (12). Chae et al
reported a DMA presenting as a laterally spreading tumor at the
antimesenteric border of the second portion of the duodenum on
upper gastroendoscopy, with no extracellular mucin or exudate due
to the early stage (13). In the
present case, the gastric cavity was filled with copious amounts of
colloidal mucin on gastroendoscopy. However, the possibility of DMA
was not considered at the time. Compared with early DMA, a large
amount of mucin in the gastric cavity on gastroendoscopy may be a
warning sign for advanced DMA. The levels of five serological tumor
markers (CEA, CA199, CA125, CA724 and AFP) are valuable tools in
the diagnosis, evaluation of prognosis and monitoring of the
treatment response of several gastrointestinal cancers, although
their role in predicting DMA has not been elucidated. Serum CA125
is a relatively sensitive tool for the differentiation of malignant
ascites (14). In the present case,
the serum CA125 level was markedly elevated and accompanied by
massive ascites. The possibility of malignant ascites was
considered, despite the uncertain location of the neoplasm.
Therefore, increased serum tumor markers may be potential signals
for DMA.
Since radical resection with a negative margin may
prolong survival, curative surgery is considered as the best option
for duodenal cancer. Chae et al reported the case of an
asymptomatic patient with early DMA, and with the cancer only
invading the submucosa; subsequently, Roux-en-Y duodenojejunostomy
and jejunojejunostomy were performed (13), with no evidence of recurrence during
1 year of follow-up. Considering her poor condition, the patient in
the present case was unable to tolerate complete resection; thus, a
gastrojejunostomy was performed to relieve the obstruction.
Unfortunately, the patient's general condition was poor
postoperatively and adjuvant chemotherapy was not administered; she
finally succumbed to the disease 42 days after surgery.
In conclusion, DMA is a rare malignancy with no
specific manifestations. To the best of our knowledge, this is the
first case of DMA presenting as ileus, obstructive jaundice and
massive ascites simultaneously. Early detection and subsequent
radical resection appear to be an effective way for improving the
outcome. At present, preoperative diagnosis of DMA remains
difficult and surgeons should include this neoplasm in the
differential diagnosis when encountering the abovementioned
symptoms. Further studies are required to establish standard
protocols for the diagnosis and treatment of DMA.
Competing interests
The authors declare that they have no competing
interests.'
References
|
1
|
Zhang S, Cui Y, Zhong B, Xiao W, Gong X,
Chao K and Chen M: Clinicopathological characteristics and survival
analysis of primary duodenal cancers: A 14-year experience in a
tertiary centre in South China. Int J Colorectal Dis. 26:219–226.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sui Y, Zou J, Batchu N, Lv S, Sun C, DU J,
Wang Q, Song Q and Li Q: Primary mucinous adenocarcinoma of the
vulva: A case report and review of the literature. Mol Clin Oncol.
4:545–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan J, Yang S, Shen P, Sun H, Xiao J, Wang
Y, Wu B, Ji F, Yan J, Xue H and Zhou D: C-kit signaling promotes
proliferation and invasion of colorectal mucinous adenocarcinoma in
a murine model. Oncotarget. 6:27037–27048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jass JR and Sobin LH: Histologic Typing of
Intestinal Tumours. 2nd. Berlin: Springer Verlag; 1989, View Article : Google Scholar
|
|
5
|
Yasuda K, Adachi Y, Shiraishi N, Yamaguchi
K, Shiromizu A and Kitano S: Pathology and prognosis of mucinous
gastric carcinoma. J Surg Oncol. 76:272–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Symonds DA and Vickery AL: Mucinous
carcinoma of the colon and rectum. Cancer. 37:1891–1900. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Compton C, Fenoglio-Preiser CM, Pettigrew
N and Fielding LP: American joint committee on cancer prognostic
factors consensus conference: Colorectal working group. Cancer.
88:1739–1757. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson JM, Melvin DB, Gray GF and
Thorbjarnarson B: Primary malignancies of the small bowel: A report
of 96 cases and review of the literature. Ann Surg. 180:175–179.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bakaeen FG, Murr MM, Sarr MG, Thompson GB,
Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM,
Schleck CD and Donohue JH: What prognostic factors are important in
duodenal adenocarcinoma? Arch Surg. 135:635–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adedeji OA, Trescoli-Serrano C and
Garcia-Zarco M: Primary duodenal carcinoma. Postgrad Med J.
71:354–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han SL, Cheng J, Zhou HZ, Zeng QQ and Lan
SH: The surgical treatment and outcome for primary duodenal
adenocarcinoma. J Gastrointest Cancer. 40:33–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chae MJ, Baek IH, Oh YM, Lim JU, Jeon JW,
Shin HP, Joo KR and Lee JI: A Patient with duodenal mucinous
adenocarcinoma presenting as a laterally spreading tumor. Clin
Endosc. 48:336–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trapé J, Gurt G, Franquesa J, Montesinos
J, Arnau A, Sala M, Sant F, Casado E, Ordeig JM, Bergos C, et al:
Diagnostic accuracy of tumor markers CYFRA21-1 and CA125 in the
differential diagnosis of ascites. Anticancer Res. 35:5655–5660.
2015.PubMed/NCBI
|