Introduction
Plasma cell myeloma (PCM) is a neoplastic
proliferation of plasma cells producing monoclonal immunoglobulins.
t(11;14) translocation is one of the most common chromosomal
translocations in PCM (1). In a
cohort of 351 PCM patients in the Eastern Cooperative Oncology
Group phase III clinical trial E9486, 16% were detected to have
t(11;14)(q13;q32) (2). The initial
diagnostic process may be challenging, and the prognostic
significance of t(11;14) translocation remains debatable. The
present clinical case evaluated various characteristics associated
with the t(11;14) translocation.
Case report
A 40-year old Hong Kong Chinese male patient with a
past history of interstitial lung disease, presented in April 2014
with incidental findings of rouleaux on the blood film. Physical
examination did not reveal any lymphadenopathy. Serum protein
electrophoresis indicated 27 g/l immunoglobin (Ig) G paraprotein
and the immunoglobulin pattern revealed elevated IgG at 29.3 g/l.
The IgA and IgM levels were 1.36 and 0.31 g/l, respectively. The
patients hemoglobin, renal function and calcium levels were normal,
and a skeletal survey did not reveal any osteolytic lesions.
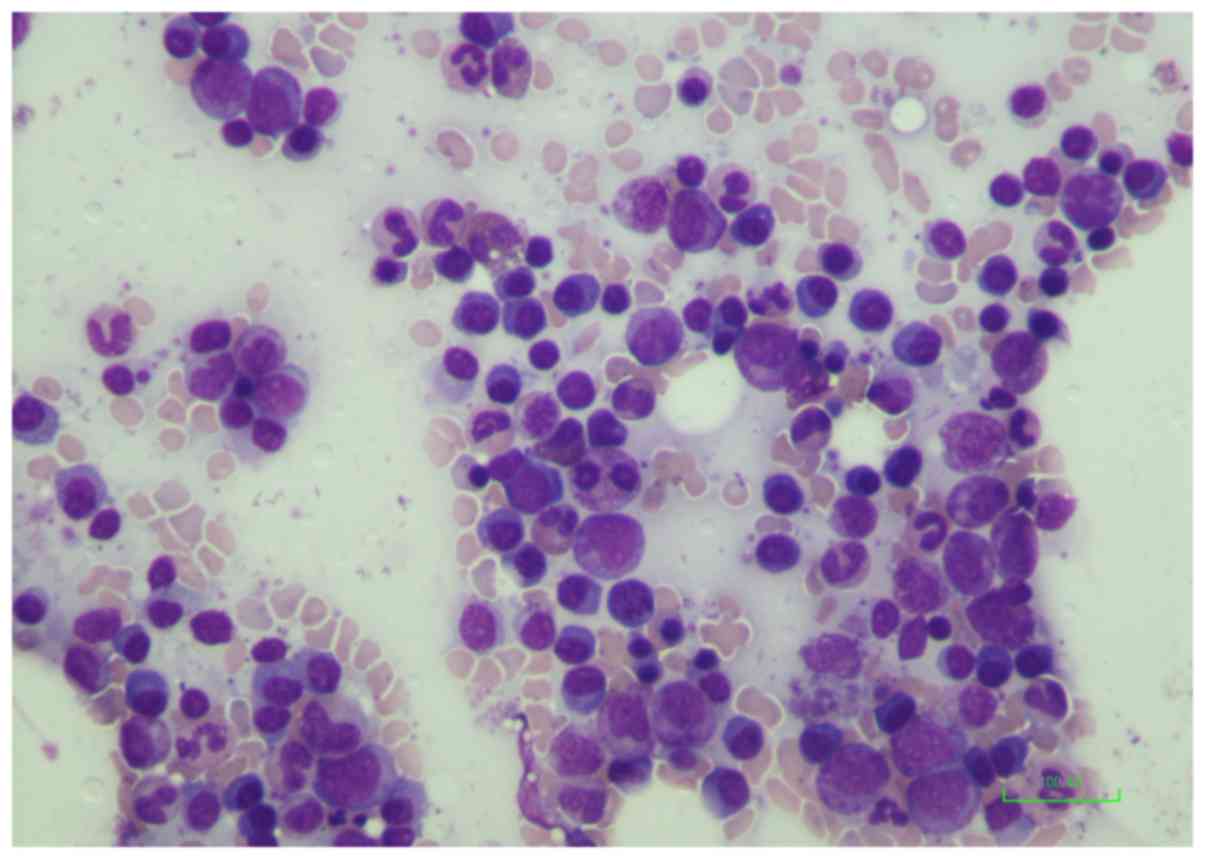
The bone marrow aspirate demonstrated 27% small to
medium plasmacytoid cells (Fig. 1)
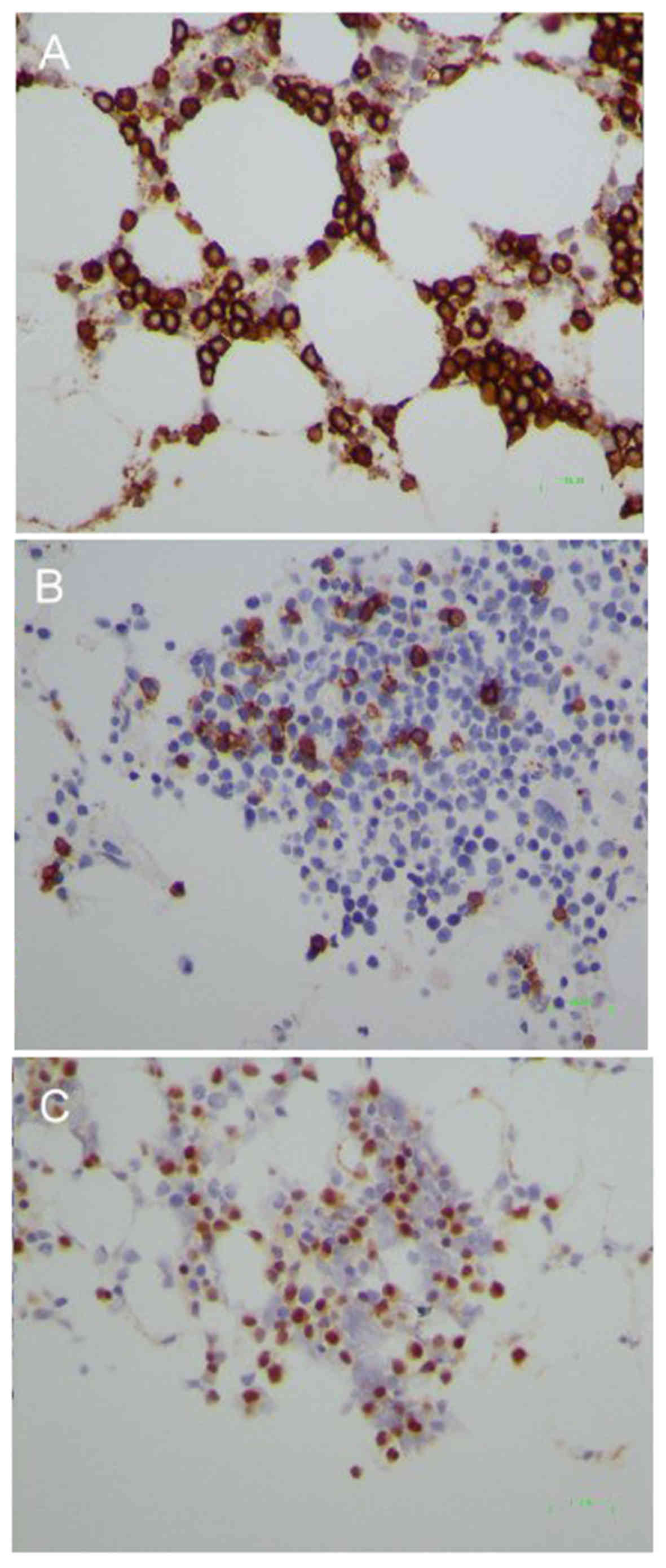
which were positive for cluster of differentiation (CD)138
(Fig. 2A), CD20 (Fig. 2B), CD19, CD38 and CD45, and negative
for CD5. They also exhibited strong surface l light chain
restriction. The initial diagnosis was of involvement of B cell
non-Hodgkin lymphoma with extensive plasmacytic
differentiation.
Marrow clotting and trephine biopsy, however, did
not reveal any obvious lymphoid aggregates, however CD138-positive
lymphoplasmacytoid cells were diffusely increased with partial
Cyclin D1 expression (Fig. 2C). The
final diagnosis was revised to be plasma cell myeloma.
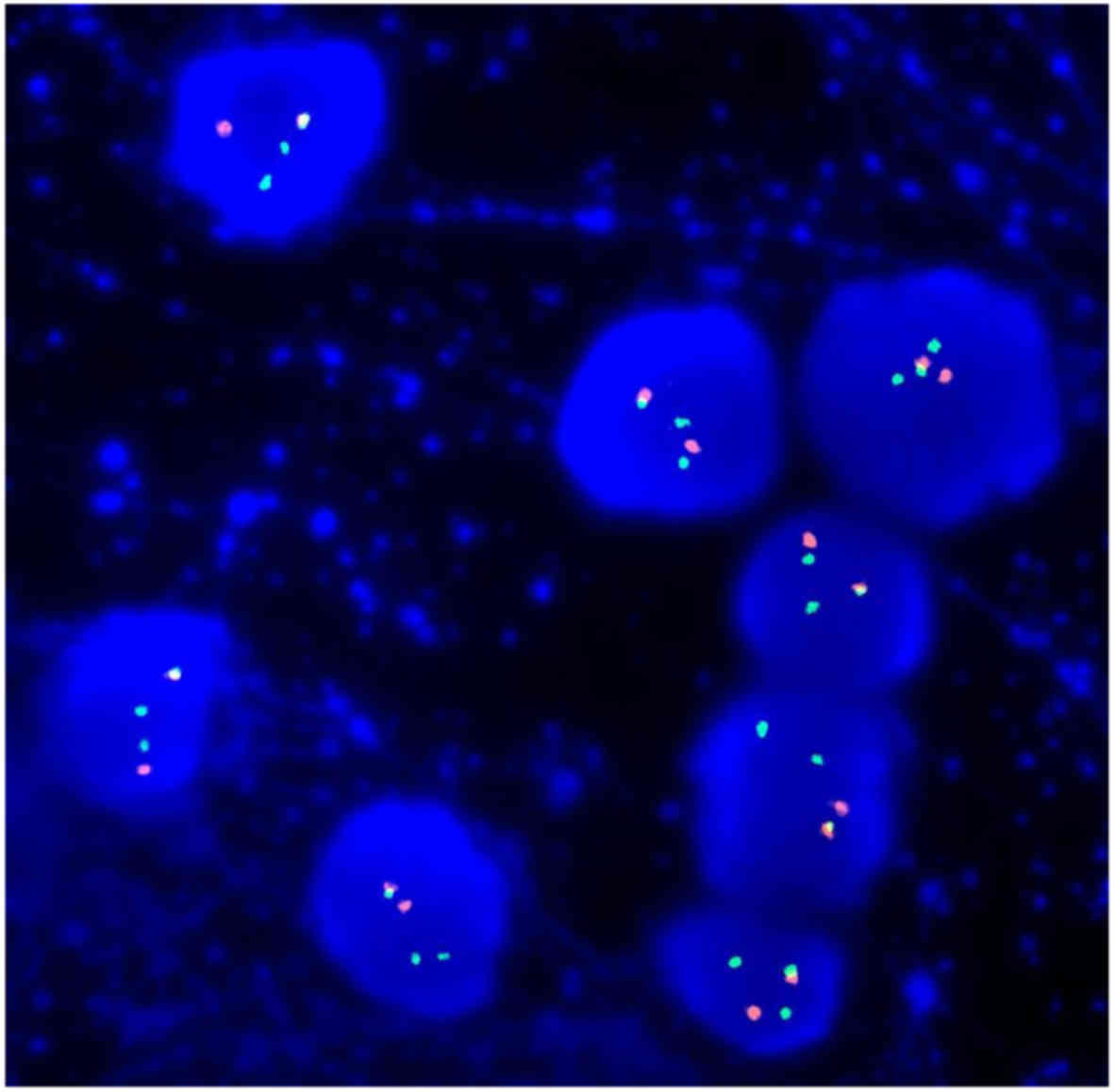
Fluorescent in situ hybridization identified
t(11;14)(q13;q32) (Fig. 3) and no
17p or 13q deletion, 1q amplification, t(4;14) or t(14;16). The
Myeloid Differentiation Primary Response 88 (MYD88) L265P mutation
was not detected.
The patient's myeloma was closely monitored for 2
years and he remained asymptomatic. From February 2017, his
paraprotein increased to 68 g/l with mild anaemia, despite a normal
serum creatinine level. A skeletal survey indicated multiple new
osteolytic lesions of the skull. Repeated bone marrow analysis
revealed 80% plasma cells.
The patient was administered two cycles of
bortezomib, thalidomide and dexamethasone, however the paraprotein
did not improve. Serum creatinine levels also increased to 235
µmol/l in June 2017. Treatment was then altered to bortezomib,
cyclophosphamide and prednisolone, however there was no improvement
in serum creatinine. Therefore, vincrisitine, doxorubicin and
dexamethasone (VAD) treatment was administered. The paraprotein
decreased to 38 g/l following VAD, however the patient's condition
was complicated with pulmonary tuberculosis and Escherichia coli
septicemia with multi-organ failure, despite intensive care.
Discussion
PCM with t(11;14)(q13;q32) is associated with
lymphoplasmacytoid morphology, and ~80% of PCM cases with
t(11;14)(q13;q32) are positive for Cyclin D1 (3). A total of 66% of the PCM patients with
t(11;14) translocation express CD20 (4). This often leads to a misdiagnosis of
lymphoplasmacytic lymphoma, mantle cell lymphoma or other B-cell
lymphomas. MYD88 L265P somatic mutation is highly prevalent in
lymphoplasmacytic lymphoma (5) and
was not detected in the current case. It is important to
corroborate the clinical and pathological findings with a multitude
of tests, including flow cytometry, immunohistochemistry and
mutation testing, and to obtain histological proof of any
lymphadenopathy in a case of PCM with lymphoplasmacytoid
morphology.
The prognosis of t(11;14) translocation is also
controversial. Moreau et al (6) suggested that PCM with t(11;14) is
associated with a superior overall survival compared with either
t(4;14) or no translocation, however the study group were treated
with older drug regimens (VAD). With novel agents, including
lenalidomide, PCM with t(11;14) is associated with inferior overall
survival (7).
Anti-CD20 reagents, including rituximab, have not
been demonstrated to be beneficial as singularly administered
reagents in CD20-positive PCM (8).
In a prospective phase II study of 14 patients, only one patient
had a minor response. Disease progression, or lack of response to
rituximab, was postulated to be due to the lack of B-cell
involvement in continued propagation in PCM patients (9).
No large clinical trials have verified the benefits
of combination therapy of rituximab and conventional agents in
CD20-positive PCM. Bergua et al (10) reported a case of relapsed
CD20-positive PCM which was heavily pretreated with chemotherapies,
including bortezomib, and the relapse was responsive to rituximab,
vincristine, adriamycin and dexamethasone. The toxicity of VAD,
however, should not go unnoticed. In a prospective multicenter
study of 139 PCM patients receiving VAD, 27% developed an infection
of WHO grade 2 or above, of which pulmonary infections were the
most common (11). This observation
could be attributed to the presence of a central line catheter and
the high-dose steroid. Previously, use of venetoclax, a B cell
lymphoma-2 inhibitor as a monotherapy, has demonstrated promising
anti-myeloma activity in PCM with t(11;14) translocation, with an
acceptable safety profile (12).
In conclusion, the initial diagnosis of PCM with
t(11;14) may be difficult based on the morphology and flow
cytometry. The treatment options and prognosis are also variable
and further studies should be performed.
Acknowledgements
The authors would like to thank Dr Ma Shiu Kwan
Edmond (Hong Kong Sanatorium and Hospital, Hong Kong, SAR, China)
for his supervision.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable as no datasets were
generated or analyzed during the current study.
Authors' contributions
SYK, WKL and SFY interpreted laboratory data,
reviewed slides, performed critical appraisal of the results and
wrote the manuscript. SYK, KSL, KPY, CYH, HKL, HNC and YMY and SFY
were involved in diagnostic workup procedures, treatment planning
and decisions, patient care and critical appraisal of the
manuscript. All authors gave final approval of the version to be
published.
Ethics approval and consent to
participate
Written informed consent was obtained the patient's
father.
Consent for publication
Written informed consent was obtained the patient's
father for the publication of any data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chesi M and Bergsagel PL: Molecular
pathogenesis of multiple myeloma: Basic and clinical updates. Int J
Hematol. 97:313–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fonseca R, Blood EA, Oken MM, Kyle RA,
Dewald GW, Bailey RJ, Van Wier SA, Henderson KJ, Hoyer JD,
Harrington D, et al: Myeloma and the t(11;14)(q13;q32); evidence
for a biologically defined unique subset of patients. Blood.
99:3735–3741. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoyer JD, Hanson CA, Fonseca R, Greipp PR,
Dewald GW and Kurtin PJ: The (11;14)(q13;q32) translocation in
multiple myeloma. A morphologic and immunohistochemical study. Am J
Clin Pathol. 113:831–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robillard N, Avet-Loiseau H, Garand R,
Moreau P, Pineau D, Rapp MJ, Harousseau JL and Bataille R: CD20 is
associated with a small mature plasma cell morphology and t(11;14)
in multiple myeloma. Blood. 102:1070–1071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamadeh F, MacNamara SP, Aguilera NS,
Swerdlow SH and Cook JR: MYD88 L265P mutation analysis helps define
nodal lymphoplasmacytic lymphoma. Mod Pathol. 28:564–574. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreau P, Facon T, Leleu X, Morineau N,
Huyghe P, Harousseau JL, Bataille R and Avet-Loiseau H: Intergroupe
Francophone du Myélome: Recurrent 14q32 translocations determine
the prognosis of multiple myeloma, especially in patients receiving
intensive chemotherapy. Blood. 100:1579–1583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman GP, Gertz MA, Dispenzieri A, Lacy
MQ, Buadi FK, Dingli D, Hayman SR, Kapoor P, Lust JA, Russell S, et
al: Impact of cytogenetic classification on outcomes following
early high-dose therapy in multiple myeloma. Leukemia. 30:633–639.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreau P, Voillat L, Benboukher L, Mathiot
C, Dumontet C, Robillard N, Hérault O, Garnache F, Garand R,
Varoqueaux N, et al: IFM group: Rituximab in CD20 positive multiple
myeloma. Leukemia. 21:835–836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Treon SP, Pilarski LM, Belch AR, Kelliher
A, Preffer FI, Shima Y, Mitsiades CS, Mitsiades NS, Szczepek AJ,
Ellman L, et al: CD20-directed serotherapy in patients with
multiple myeloma: Biologic considerations and therapeutic
applications. J Immunother. 25:72–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergua JM, Cabrera C, Arteta EG and Prieto
J: Rituximab in CD20 positive multiple myeloma. Leukemia.
22:1082–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segeren CM, Sonneveld P, van der Holt B,
Baars JW, Biesma DH, Cornellissen JJ, Croockewit AJ, Dekker AW,
Fibbe WE, Löwenberg B, et al: Vincristine, doxorubicin and
dexamethasone (VAD) administered as rapid intravenous infusion for
first-line treatment in untreated multiple myeloma. Br J Haematol.
105:127–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar S, Kaufman J, Gasparetto C, Mikhael
J, Vij R, Pegourie B, et al: Efficacy of venetoclax as targeted
therapy for relapsed/refractory t(11;14) multiple myeloma. Blood.
2017.blood-2017-06-788786. View Article : Google Scholar
|