Introduction
Uterine cervical carcinoma is the second most common
cancer in women and the fifth most common type of cancer worldwide
(1), and it is a leading cause of
cancer-related mortality among Japanese women.
Recent studies have identified the pre-treatment
neutrophil-to-lymphocyte ratio (NLR) as an independent prognostic
indicator in several types of malignancies, including colorectal
cancer (2,3), hepatocellular carcinoma (4), pancreatic cancer (5) and non-small-cell lung cancer (6). The pre-treatment NLR was also found to
be an independent prognostic factor in patients with uterine
cervical carcinoma who have undergone radiation therapy or
concurrent chemoradiation therapy (7). Although the pre-treatment
platelet-to-lymphocyte ratio (PLR) may be used to predict the
prognosis of colorectal (8), ovarian
(9), prostate (10) and oral (11) cancers, its prognostic role in uterine
cervical carcinoma remains unclear. The aim of the present study
was to evaluate the prognostic significance of pre-treatment NLR
and PLR, as well as that of other clinicopathological
characteristics, in patients with uterine cervical carcinoma. The
associations between NLR and PLR and these factors were also
investigated.
Patients and methods
Patients
A total of 98 non-surgically treated patients with
uterine cervical carcinoma at Shimane University School of Medicine
(Izumo, Japan) between January 1997 and July 2013 were enrolled in
this study. Patient data (e.g., baseline characteristics,
laboratory findings and pathology reports) were collected from
electronic medical records. Uterine cervical carcinoma was
diagnosed via conventional morphological examination of hematoxylin
and eosin-stained sections. Tumor type and stage were classified
according to the classification systems of the World Health
Organization (12) and the
International Federation of Gynecology and Obstetrics (FIGO)
(13), respectively.
The study protocol was approved by the Ethics
Committee of Shimane University School of Medicine (approval no.:
960) and all the patients provided written informed consent. The
research was conducted in accordance with the Declaration of
Helsinki and Title 45, US Code of Federal Regulations, Part 46,
Protection of Human Subjects, effective December 13, 2001.
Definition of NLR and PLR
All laboratory data used in the present study were
obtained at least 4 weeks prior to treatment initiation. The NLR
was defined as the absolute neutrophil count divided by the
absolute lymphocyte count. The mean pre-treatment NLR was 3.5,
according to which patients were divided into high NLR (≥3.5) and
low NLR (<3.5) groups. The PLR was defined as the absolute
platelet count divided by the absolute lymphocyte count. The mean
pre-treatment PLR was 212, according to which patients were divided
into high PLR (≥212) and low PLR (<212) groups.
Statistical analysis
Univariate analysis was performed using binomial
logistic regression for ordered categorical variables. The
following patient clinicopathological characteristics were included
in this analysis: Age at diagnosis (<60 vs. ≥60 years), FIGO
classification (stage I/II vs. III/IV), histological type [squamous
cell carcinoma (SCC) vs. other], pre-treatment plasma D-dimer level
(<1.0 vs. ≥1.0 µg/ml), plasma fibrinogen level (<450
vs. ≥450 mg/dl), white blood cell (WBC) count (<8.6 vs. ≥8.6×
103/µl), platelet count (<35 vs.
≥35×104/µl), NLR (<3.5 vs. ≥3.5), PLR (<212
vs. ≥212), C-reactive protein (CRP) level (<0.2 vs. ≥0.2 mg/dl),
SCC antigen level (<1.5 vs. ≥1.5 U/ml) and carcinoembryonic
antigen (CEA) level (<5.0 vs. ≥5.0 ng/ml). The endpoints of the
analysis were overall survival (OS) and progression-free survival
(PFS). OS was defined as the time interval between the date of
diagnosis and the date of death. Patients who were alive at the
last follow-up were censored. PFS was defined as the time interval
between the date of diagnosis and the date of recurrence. Patients
without recurrence at the last follow-up were censored. OS and PFS
were calculated using the Kaplan-Meier method and statistical
significance was determined using the log-rank test.
To identify significant prognostic factors, a
multivariate analysis was performed using a Cox proportional
hazards model. Variables shown to be significant (P<0.05) in the
univariate analysis were entered into the multivariate analysis.
All statistical analyses were conducted using Statistical Package
for the Social Sciences for Windows, software version 19.0 (IBM
Corp., Armonk, NY, USA). A two-sided P<0.05 was considered
statistically significant.
Results
Patient clinicopathological
characteristics
This study analyzed data from 98 patients with
uterine cervical carcinoma. The median age at diagnosis was 65
years (range, 32–86 years), with 37.6% of the patients aged <60
and 63.3% ≥60 years. The majority of patients (78.6%) were
diagnosed with SCC; 33 patients (33.7%) had FIGO stage I/II disease
and 65 (66.3%) had FIGO stage III/IV disease.
A total of 68 patients (69.4%) were included in the
high pre-treatment NLR group (≥3.5) and 30 (30.6%) were in the low
pre-treatment NLR group (<3.5). A total of 34 patients (34.7%)
were included in the high pre-treatment PLR group (≥212) and 64
(65.3%) were in the low pre-treatment PLR group (<212). The
number (percentage) of patients with plasma D-dimer levels ≥1.0
µg/ml, plasma fibrinogen levels ≥450 mg/dl, WBC count
≥8.6×103/µl, platelet count
≥35×104/µl, CRP levels ≥0.2 mg/dl, SCC antigen
levels ≥1.5 U/ml and CEA levels ≥5.0 ng/ml were 72 (73.5%), 46
(46.9%), 32 (32.7%), 24 (24.5%), 82 (83.7%), 77 (78.6%) and 59
(60.2%), respectively.
Pre-treatment NLR and patient
clinicopathological characteristics
The correlations between the pre-treatment NLR
(<3.5 vs. ≥3.5) and patient clinicopathological characteristics
were evaluated via binomial logistic regression analysis. Only the
pre-treatment platelet count was found to be correlated with the
pre-treatment NLR, and this correlation was positive. There was no
significant association between age at diagnosis, FIGO stage or
histological type and the NLR (Table
I).
 | Table I.Associations between the pre-treatment
NLR and the clinicopathological characteristics of patients with
uterine cervical carcinoma (n=98). |
Table I.
Associations between the pre-treatment
NLR and the clinicopathological characteristics of patients with
uterine cervical carcinoma (n=98).
| Clinicopathological
characteristics | NLR <3.5
(n=68) | NLR ≥3.5 (n=30) | P-value |
|---|
| Age at diagnosis
(years), n (%) |
|
| 0.369 |
|
<60 | 23 (23.5) | 13 (13.3) |
|
| ≥60 | 45 (45.9) | 17 (17.3) |
|
| Histological type, n
(%) |
|
| 0.399 |
| SCC | 52 (53.1) | 25 (25.5) |
|
|
Other | 16 (16.3) | 5 (5.1) |
|
| FIGO stage, n
(%) |
|
| 0.950 |
| I/II | 23 (23.5) | 10 (10.2) |
|
|
III/IV | 45 (45.9) | 20 (20.4) |
|
| Platelet count (/µl),
n (%) |
|
| 0.002a |
|
<35×104 | 57 (58.2) | 17 (17.3) |
|
|
≥35×104 | 11 (11.2) | 13 (13.3) |
|
| CRP level (mg/dl), n
(%) |
|
| 0.460 |
|
<0.2 | 15 (15.3) | 1 (1.0) |
|
| ≥0.2 | 53 (54.1) | 29 (29.6) |
|
Pre-treatment PLR and patient
clinicopathological characteristics
The correlations between the pre-treatment PLR
(<212 vs. ≥212) and patient clinicopathological characteristics
were also assessed via binomial regression analysis. Only the
pre-treatment platelet count was found to be associated with the
pre-treatment PLR (Table II).
 | Table II.Associations between the pre-treatment
(PLR) and the clinicopathological characteristics of patients with
uterine cervical carcinoma (n=98). |
Table II.
Associations between the pre-treatment
(PLR) and the clinicopathological characteristics of patients with
uterine cervical carcinoma (n=98).
| Clinicopathological
characteristic | PLR <212
(n=64) | PLR ≥212 (n=34) | P-value |
|---|
| Age at diagnosis
(years), n (%) |
|
| 0.136 |
|
<60 | 20 (20.4) | 16 (16.3) |
|
| ≥60 | 44 (44.9) | 18 (18.4) |
|
| Histological type, n
(%) |
|
| 0.932 |
| SCC | 50 (51.0) | 27 (27.6) |
|
|
Other | 14 (14.3) | 7 (7.1) |
|
| FIGO stage, n
(%) |
|
| 0.115 |
|
I/II | 25 (25.5) | 8 (8.2) |
|
|
III/IV | 39 (39.8) | 26 (26.5) |
|
| Platelet count
(/µl), n (%) |
|
|
<0.001a |
|
<35×104 | 58 (59.2) | 16 (16.3) |
|
|
≥35×104 | 6 (6.1) | 18 (18.4) |
|
| CRP level (mg/dl),
n (%) |
|
| 0.460 |
|
<0.2 | 15 (15.3) | 1 (1.0) |
|
|
≥0.2 | 53 (54.1) | 29 (29.6) |
|
Univariate and multivariate analysis
of pre-treatment prognostic factors for uterine cervical
carcinoma
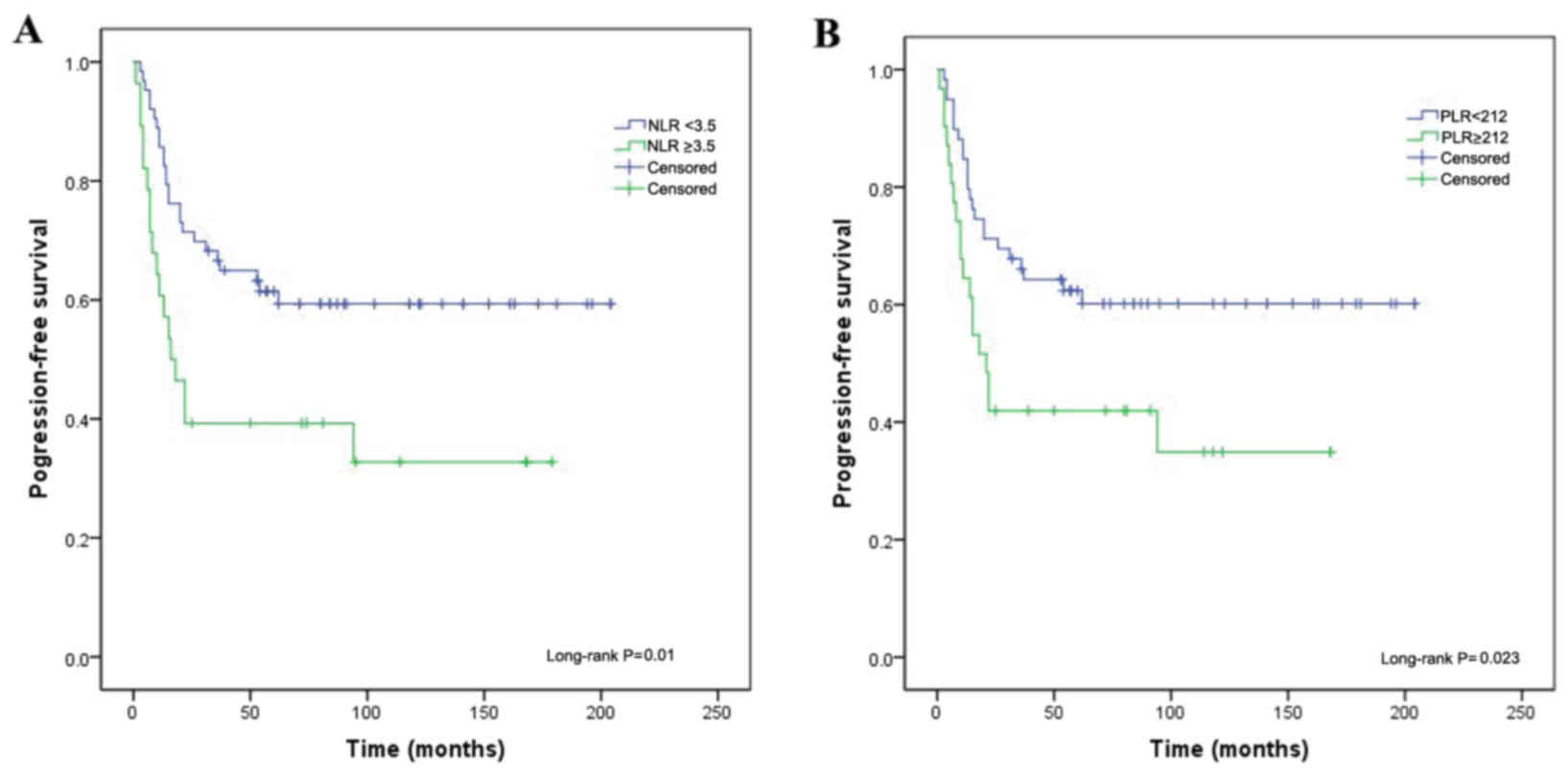
In the univariate analysis, the NLR (P=0.010,
Fig. 1A), PLR (P=0.023, Fig. 1B), platelet count (P=0.009) and CEA
level (P=0.012) were significantly associated with PFS in patients
with uterine cervical carcinoma. In the multivariate analysis, only
the NLR [hazard ratio (HR)=0.317, 95.0% confidence interval (CI):
0.167–0.602; P<0.001] and CEA level (HR=0.337, 95.0% CI:
0.168–0.676; P=0.002) were independently correlated with PFS
(Table III).
 | Table III.Univariate and multivariate analysis
of progression-free survival using a Cox proportional hazards model
in patients with uterine cervical carcinoma (n=98). |
Table III.
Univariate and multivariate analysis
of progression-free survival using a Cox proportional hazards model
in patients with uterine cervical carcinoma (n=98).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value |
|---|
| Age at diagnosis
(years) | Ref. |
|
|
|
|
|
|
| 0.885 | 0.472–1.650 | 0.702 | – | – | – |
|
| Ref. |
|
|
|
|
|
|
| 0.641 | 0.323–1.270 | 0.204 | – | – | – |
|
| Ref. |
|
|
|
|
|
|
| 0.525 | 0.259–1.066 | 0.075 | – | – | − |
|
| Ref. |
|
|
|
|
|
|
| 0.449 | 0.245–0.826 | 0.010a | 0.317 | 0.167–0.602 |
<0.001a |
|
| Ref. |
|
|
|
|
|
|
| 0.494 | 0.268–0.908 | 0.023a | N/A | N/A | N/A |
| Plasma D-dimer
level (µg/ml) | Ref. |
|
|
|
|
|
|
| 0.849 | 0.397–1.815 | 0.674 | – | – | – |
| Plasma fibrinogen
level (mg/dl) | Ref. |
|
|
|
|
|
|
| 0.636 | 0.327–1.238 | 0.183 | – | – | – |
|
| Ref. |
|
|
|
|
|
|
| 0.55 | 0.301–1.004 | 0.550 | – | – | – |
| Platelet count
(/µl) | Ref. |
|
|
|
|
|
|
| 0.432 | 0.229–0.713 | 0.009a | N/A | N/A | N/A |
|
| Ref. |
|
|
|
|
|
|
| 0.537 | 0.210–1.372 | 0.194 | – | – | – |
| SCC antigen level
(ng/ml) | Ref. |
|
|
|
|
|
|
| 0.938 | 0.445–1.977 | 0.866 | – | – | – |
|
| Ref. |
|
|
|
|
|
|
| 0.422 | 0.215–0.830 | 0.012a | 0.337 | 0.168–0.676 | 0.002a |
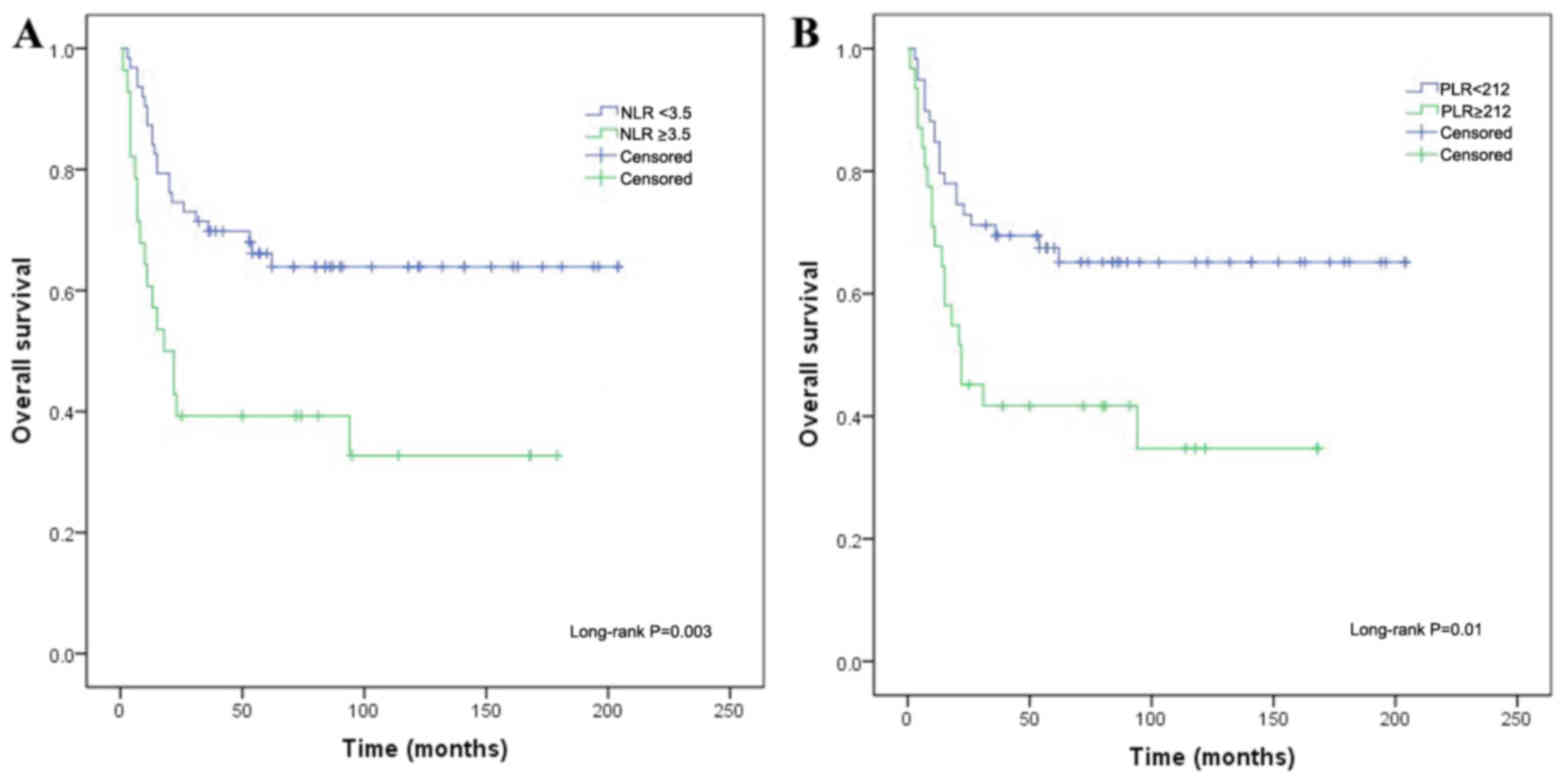
The factors significantly associated with OS in the
univariate analysis included the WBC count (P=0.042), NLR (P=0.003,
Fig. 2A), PLR (P=0.01, Fig. 2B), platelet count (P=0.009) and CEA
level (P=0.019). Of these, and similar to the results for PFS, only
the NLR (HR=0.274, 95.0% CI: 0.141–0.530; P<0.001) and CEA level
(HR=0.334, 95.0% CI: 0.162–0.689; P=0.003) were independently
correlated with OS in the multivariate analysis (Table IV).
 | Table IV.Univariate and multivariate analysis
of overall survival using a Cox proportional hazards model in
patients with uterine cervical carcinoma (n=98). |
Table IV.
Univariate and multivariate analysis
of overall survival using a Cox proportional hazards model in
patients with uterine cervical carcinoma (n=98).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients (n) | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value |
|---|
| Age at diagnosis
(years) |
|
|
|
|
|
|
|
|
<60 | 36 | Ref. |
|
|
|
|
|
|
≥60 | 62 | 0.882 | 0.460–1.689 | 0.704 | – | – | – |
| Histological
type |
|
|
|
|
|
|
|
|
SCC | 77 | Ref. |
|
|
|
|
|
|
Other | 21 | 0.575 | 0.287–1.152 | 0.119 | – | – | – |
| FIGO stage |
|
|
|
|
|
|
|
|
I/II | 33 | Ref. |
|
|
|
|
|
|
III/IV | 65 | 0.565 | 0.276–1.157 | 0.119 | – | – | – |
| NLR |
|
|
|
|
|
|
|
|
<3.5 | 68 | Ref. |
|
|
|
|
|
|
≥3.5 | 30 | 0.391 | 0.209–0.732 | 0.003a | 0.274 | 0.141–0.530 |
<0.001a |
| PLR |
|
|
|
|
|
|
|
|
<212 | 64 | Ref. |
|
|
|
|
|
|
≥212 | 34 | 0.438 | 0.233–0.820 | 0.010a | N/A | N/A | N/A |
| Plasma D-dimer
level (µg/ml) |
|
|
|
|
|
|
|
|
<1.0 | 26 | Ref. |
|
|
|
|
|
|
≥1.0 | 72 | 0.832 | 0.372–1.858 | 0.654 | – | – | – |
| Plasma fibrinogen
level (mg/dl) |
|
|
|
|
|
|
|
|
<450 | 52 | Ref. |
|
|
|
|
|
|
≥450 | 46 | 0.693 | 0.347–1.383 | 0.298 | – | – | – |
| WBC count
(/µl) |
|
|
|
|
|
|
|
|
<8.6×103 | 66 | Ref. |
|
|
|
|
|
|
≥8.6×103 | 32 | 0.523 | 0.280–0.977 | 0.042a | N/A | N/A | N/A |
| Platelet count
(/µl) |
|
|
|
|
|
|
|
|
<35×104 | 74 | Ref. |
|
|
|
|
|
|
≥35×104 | 24 | 0.419 | 0.217–0.808 | 0.009a | N/A | N/A | N/A |
| CRP level
(mg/dl) |
|
|
|
|
|
|
|
|
<0.2 | 16 | Ref. |
|
|
|
|
|
|
≥0.2 | 82 | 0.521 | 0.203–1.339 | 0.176 | – | – | – |
| SCC antigen level
(ng/ml) |
|
|
|
|
|
|
|
|
<1.5 | 21 | Ref. |
|
|
|
|
|
|
≥1.5 | 77 | 1.06 | 0.498–2.256 | 0.880 | – | – | – |
| CEA level
(ng/ml) |
|
|
|
|
|
|
|
|
<5.0 | 39 | Ref. |
|
|
|
|
|
|
≥5.0 | 59 | 0.429 | 0.212–0.868 | 0.019* | 0.334 | 0.162–0.689 | 0.003* |
Discussion
A high pre-treatment NLR was recently recognized as
a valid indicator of a poor prognosis in patients with various
types of malignancies, including colorectal cancer (2,3),
hepatocellular carcinoma (4),
pancreatic cancer (5) and
non-small-cell lung cancer (6). In
the present study, a high pre-treatment NLR was correlated with a
poor prognosis in patients with uterine cervical carcinoma, which
is consistent with the findings of previous cancer studies
(2–6).
The mechanism through which a high NLR adversely
affects prognosis remains unclear. There are two possible
explanations: First, the NLR is a measure of systemic inflammation,
and the correlation between inflammation and cancer has been
established, with inflammation considered to promote angiogenesis,
lymphangiogenesis, invasion and tumor metastasis (14). Second, neutrophils release a number
of angiogenic factors, such as vascular endothelial growth factor
and matrix metalloproteinase-9. Matrix metalloproteinase-9 acts as
an angiogenic switch that is critical for tumor progression
(15). Lymphocytes, on the other
hand, secrete interleukin-2, which inhibits tumor cell
proliferation by activating and stimulating the proliferation of
cytotoxic lymphocytes (16).
Although pre-treatment neutrophil and lymphocyte counts are
associated with systemic inflammation, there was no observed
association between the pre-treatment CRP level and the NLR in this
study. However, the NLR was associated with survival prognosis.
In a previous study (17), the pre-treatment PLR was an important
predictor of prognosis in patients with recurrent cervical cancer
following concurrent chemoradiation therapy. There is a potential
explanation as to why this was the case: Tumors promote the
activation and aggregation of platelets in their vasculature by
inducing the expression of angiogenesis regulatory factors and
their release from platelets (18).
In the present study, the pre-treatment PLR was a prognostic factor
in the univariate analysis, but not in the multivariate analysis.
As there was a significant association between the NLR and platelet
count (Table I), it is possible that
the NLR may have affected the PLR. This may explain, at least in
part, why the pre-treatment PLR was not found to be correlated with
prognosis in this study.
In addition, a high pre-treatment CEA level was
found to be a significant prognostic factor in uterine cervical
carcinoma in the present study, whereas a high pre-treatment SCC
antigen level was not. However, in two previous studies (19,20),
both factors were useful for predicting prognosis in patients with
uterine cervical carcinoma. It is well known that CEA levels are
higher in patients with adenocarcinoma compared with those in
patients with SCC, and a previous study (19) reported that the CEA level was a
prognostic marker in patients with cervical SCC.
The limitations of the present study include its
single-institution retrospective design.
In conclusion, the findings of the present study
demonstrated that a high NLR, but not a high PLR, predicts a poor
prognosis in non-surgically treated patients with uterine cervical
carcinoma; they also suggested that pre-treatment CEA levels may
independently predict OS and PFS. These findings may prove
valuable, as both the NLR and CEA levels may be measured easily and
cost-effectively via blood testing.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KoN and NT drafted the manuscript and carried out
the statistical analysis. MI, TM, TI, KO, HY, RO, HS, SR and SKa
carried out the treatment. KeN participated in the design of the
study. SKy conceived the study, and participated in its design and
coordination and helped to draft the manuscript. All the authors
have read and approved the final version of this manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shimane University School of Medicine (Izumo, Japan)
(approval no.: 960). All patients provided written informed
consent. The research was conducted in accordance with the
Declaration of Helsinki and Title 45, US Code of Federal
Regulations, Part 46, Protection of Human Subjects, effective
December 13, 2001.
Patient consent for publication
All patients provided written informed consent for
the publication of data in this study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CEA
|
carcinoembryonic antigen
|
|
CI
|
confidence interval
|
|
CRP
|
C-reactive protein
|
|
FIGO
|
International Federation of Obstetrics
and Gynecology
|
|
HR
|
hazard ratio
|
|
NLP
|
neutrophil-to-lymphocyte ratio
|
|
OS
|
overall survival
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
PFS
|
progression-free survival
|
|
SCC
|
squamous cell carcinoma
|
|
WBC
|
white blood cell
|
References
|
1
|
Jones SB: Cancer in the developing world:
A call to action. BMJ. 319:505–508. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walsh SR, Cook EJ, Goulder F, Justin TA
and Keeling NJ: Neutrophil-lymphocyte ratio as a prognostic factor
in colorectal cancer. J Surg Oncol. 91:181–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JH, Lee JY, Kim HK, Lee JW, Jung SG,
Jung K, Kim SE, Moon W, Park MI and Park SJ: Prognostic
significance of the neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in patients with stage III and IV
colorectal cancer. World J Gastroenterol. 23:505–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halazun KJ, Hardy MA, Rana AA, Woodland DC
IV, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr and
Emond JC: Negative impact of neutrophil-lymphocyte ratio on outcome
after liver transplantation for hepatocellular carcinoma. Ann Surg.
250:141–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatti I, Peacock O, Lloyd G, Larvin M and
Hall RI: Preoperative hematologic markers as independent predictors
of prognosis in resected pancreatic ductal adenocarcinoma:
Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg.
200:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizunuma M, Yokoyama Y, Futagami M, Aoki
M, Takai Y and Mizunuma H: The pretreatment
neutrophil-to-lymphocyte ratio predicts therapeutic response to
radiation therapy and concurrent chemoradiation therapy in uterine
cervical cancer. Int J Clin Oncol. 20:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan D, Fu Y, Su Q and Wang H: Prognostic
role of platelet-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
95:e38372016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao Y, Yan Q, Li S, Li B and Feng Y:
Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are
predictive of chemotherapeutic response and prognosis in epithelial
ovarian cancer patients treated with platinum-based chemotherapy.
Cancer Biomark. 17:33–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Xu F, Pan J, Zhu Y, Shao X, Sha J,
Wang Z, Cai Y, Liu Q, Dong B, et al: Platelet to lymphocyte ratio
as an independent prognostic indicator for prostate cancer patients
receiving androgen deprivation therapy. BMC Cancer. 16:3292016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Guo J, Feng C, Ke Z, Chen L and
Pan Y: The preoperative platelet-lymphocyte ratio versus
neutrophil-lymphocyte ratio: Which is better as a prognostic factor
in oral squamous cell carcinoma? Ther Adv Med Oncol. 8:160–167.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumours of female reproductive
organs. 4th edition. Lyon; IARC Press; 2014
|
|
13
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ardi VC, Kupriyanova TA, Deryugina EI and
Quigley JP: Human neutrophils uniquely release TIMP-free MMP-9 to
provide a potent catalytic stimulator of angiogenesis. Proc Natl
Acad Sci USA. 104:20262–20267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valle-Mendiola A, Gutiérrez-Hoya A,
Lagunas-Cruz Mdel C, Weiss-Steider B and Soto-Cruz I: Pleiotropic
Effects of IL-2 on Cancer: Its role in cervical cancer. Mediators
Inflamm. 2016:28495232016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura K, Nishida T, Haruma T, Haraga J,
Omichi C, Ogawa C, Kusumoto T, Seki N, Masuyama H and Hiramatsu Y:
Pretreatment platelet-lymphocyte ratio is an independent predictor
of cervical cancer recurrence following concurrent chemoradiation
therapy. Mol Clin Oncol. 3:1001–1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sabrkhany S, Griffioen AW and Oude Egbrink
MG: The role of blood platelets in tumor angiogenesis. Biochim
Biophys Acta. 1815:189–196. 2011.PubMed/NCBI
|
|
19
|
Huang EY, Hsu HC, Sun LM, Chanchien CC,
Lin H, Chen HC, Tseng CW, Ou YC, Chang HY, Fang FM, et al:
Prognostic value of pretreatment carcinoembryonic antigen after
definitive radiotherapy with or without concurrent chemotherapy for
squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol
Biol Phys. 81:1105–1113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Cui T, Du L, Xu X, Tian B, Sun T,
Han C, Zhao X and Jing J: The correlation between the serum
squamous carcinoma antigen and the prognosis of recurrent cervical
squamous carcinoma. J Clin Lab Anal. 31:2017. View Article : Google Scholar
|