Introduction
Gastrointestinal stromal tumors (GISTs) are the most
common type of mesenchymal neoplasms that originate from the
interstitial cell of Cajal (1,2). Most of
these tumors originate from the stomach (60%), followed by the
small intestine (30%), and the colon (5%) (3). GISTs are classified as intramural,
exoluminal, endoluminal, or mixed types, according to tumor
location (4). GISTs are considered
to have malignant potential, and several risk classifications have
been proposed (5–7). Recently, Joensuu's classification
system, which includes tumor size, mitotic figures, organ of
origin, and presence of rupture, is often used (7). Typically, GISTs appear as solid masses
and rarely present with cystic formations (8–16). The
cystic formation is reportedly caused by hemorrhage or necrosis
(17). Most stomach GISTs with
cystic formations progress with an exoluminal or intramural pattern
and are frequently misdiagnosed as epigastric cystic tumors derived
from the pancreas or liver (13,15,16).
Cushion sign refers to the morphometric fluctuation of the
submucosal tumor by forceps compression (18), which is generally a characteristic of
very soft or cystic tumors, such as lipomas or lymphangiomas. We
report a rare case of a cushion sign-positive stomach GIST with
cystic formation, which mainly developed inside the stomach.
Case report
A 72-year-old male, with a past medical history of
gastric ulcer, was referred to our hospital for further examination
of an abnormality in the stomach that was found by an
esophagogastroduodenoscopy (EGD) performed for a health checkup. He
had no abdominal symptoms, and his blood tests were normal,
including levels of carcinoembryonic antigen and carbohydrate
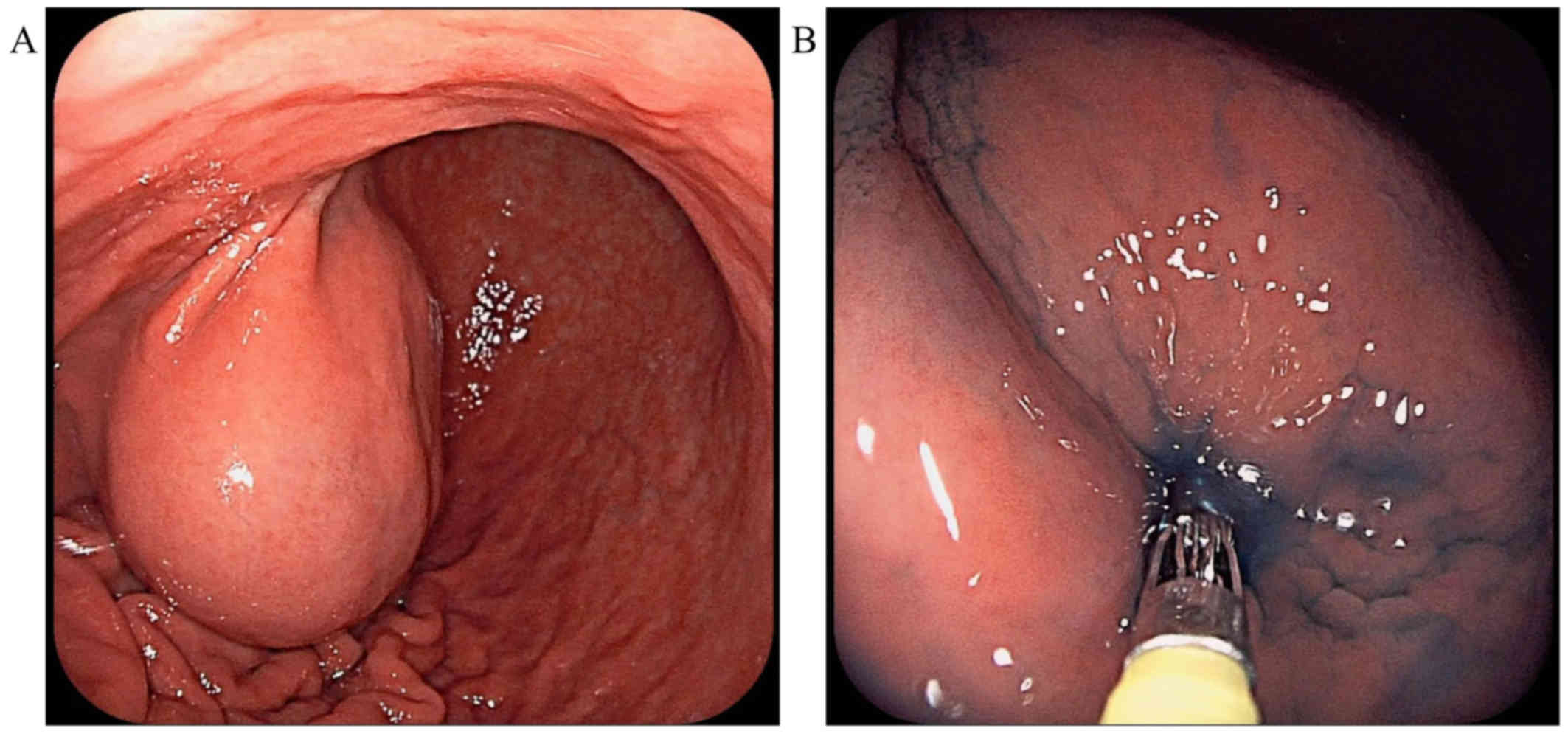
antigen 19-9 tumor markers. EGD revealed a mass covered by normal
mucosa with a bridging fold, approximately 50 mm in diameter, at
the anterior wall of the gastric angle (Fig. 1A). The submucosal tumor (SMT) was
round and smooth, without erosions or ulcers. The SMT was very
soft, with positive cushion sign when we used forceps to compress
the tumor (Fig. 1B). The mass was
thought to be a benign tumor, such as a lymphangioma, because the
inside of the tumor was considered to contain liquid. However,
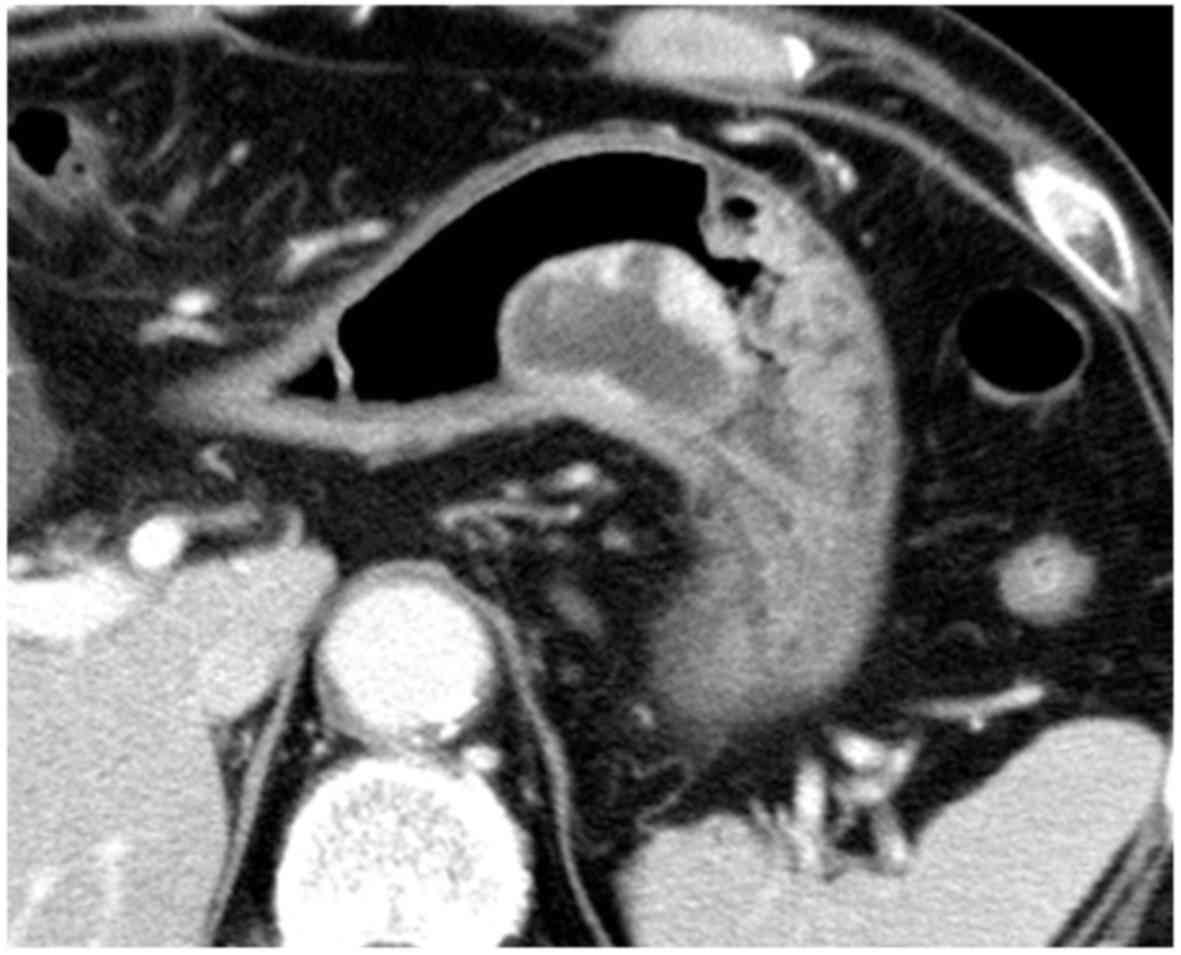
contrast-enhanced abdominal computed tomography (CT) showed a mass
containing both cystic and solid areas, mainly growing inside the
stomach, and revealed enhancement of the solid portion and
peripheral rim of the tumor (Fig.
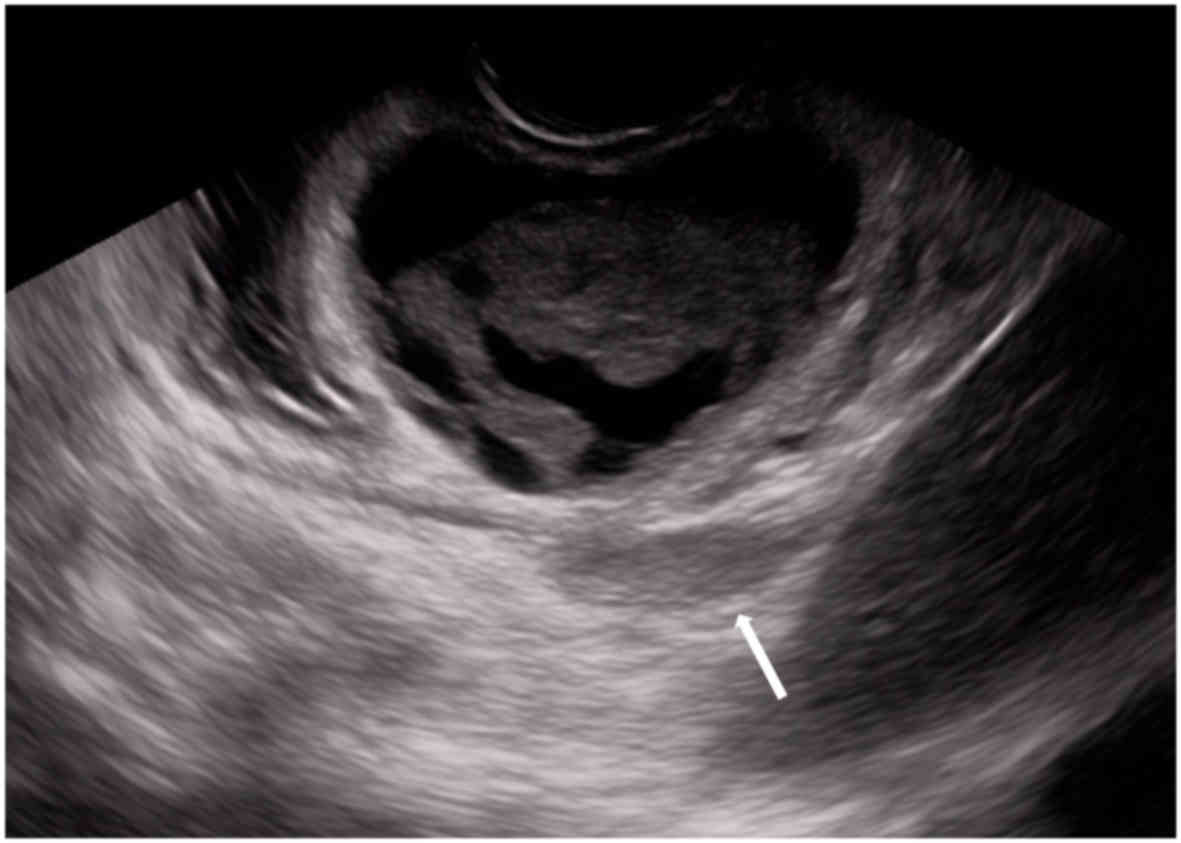
2). Furthermore, endoscopic ultrasound (EUS) using a
convex-type scope revealed a cystic tumor with solid component
located in the third to fourth layer of the stomach and part of the
solid component that had developed outside the stomach (Fig. 3). The cystic component appeared as
low-level echoes. Subsequently, EUS-fine needle aspiration
(EUS-FNA) of the solid component was performed for definitive
diagnosis. Histologically, specimens obtained via EUS-FNA included
spindle tumor cells. Immunohistochemical analysis of the specimens
revealed that they were diffusely positive for c-kit and CD34;
therefore, the tumor was diagnosed as GIST. However we considered
laparoscopic partial gastrectomy or laparoscopic and endoscopic
cooperative surgery (LECS) to treat the tumor, we employed LECS for
more reliable resection. The patient underwent LECS to remove the
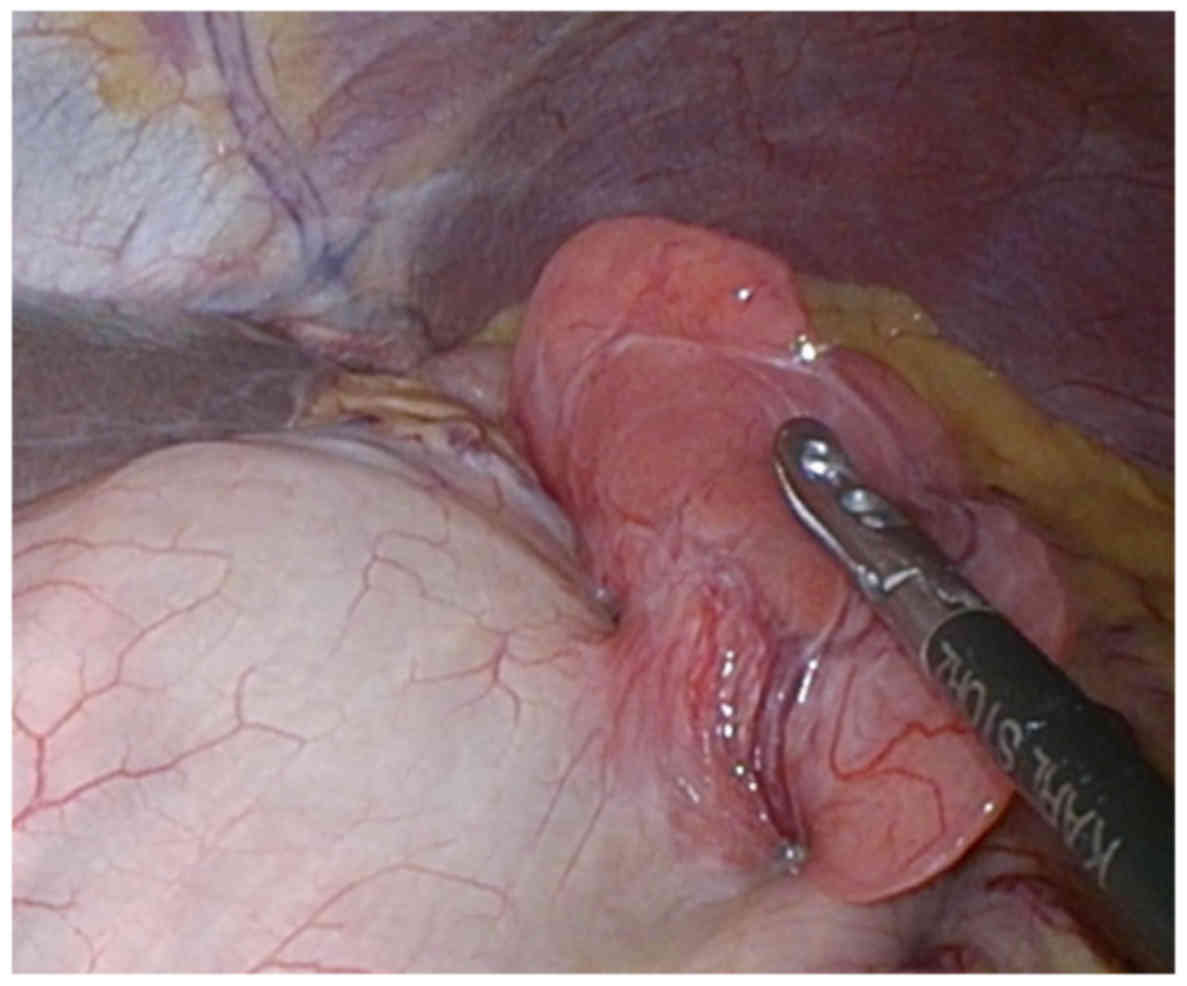
GIST. Intraoperative laparoscopic findings showed soft tissue
arising from the anterior wall of the stomach, freely mobile in the
peritoneal cavity (Fig. 4). Although
the lesion was located at the serosa side of the gastric SMT, there
were no findings of peritoneal dissemination. The tumor was
completely removed, and no intraoperative or postoperative
complications were observed. The patient was discharged from our
hospital within a week.
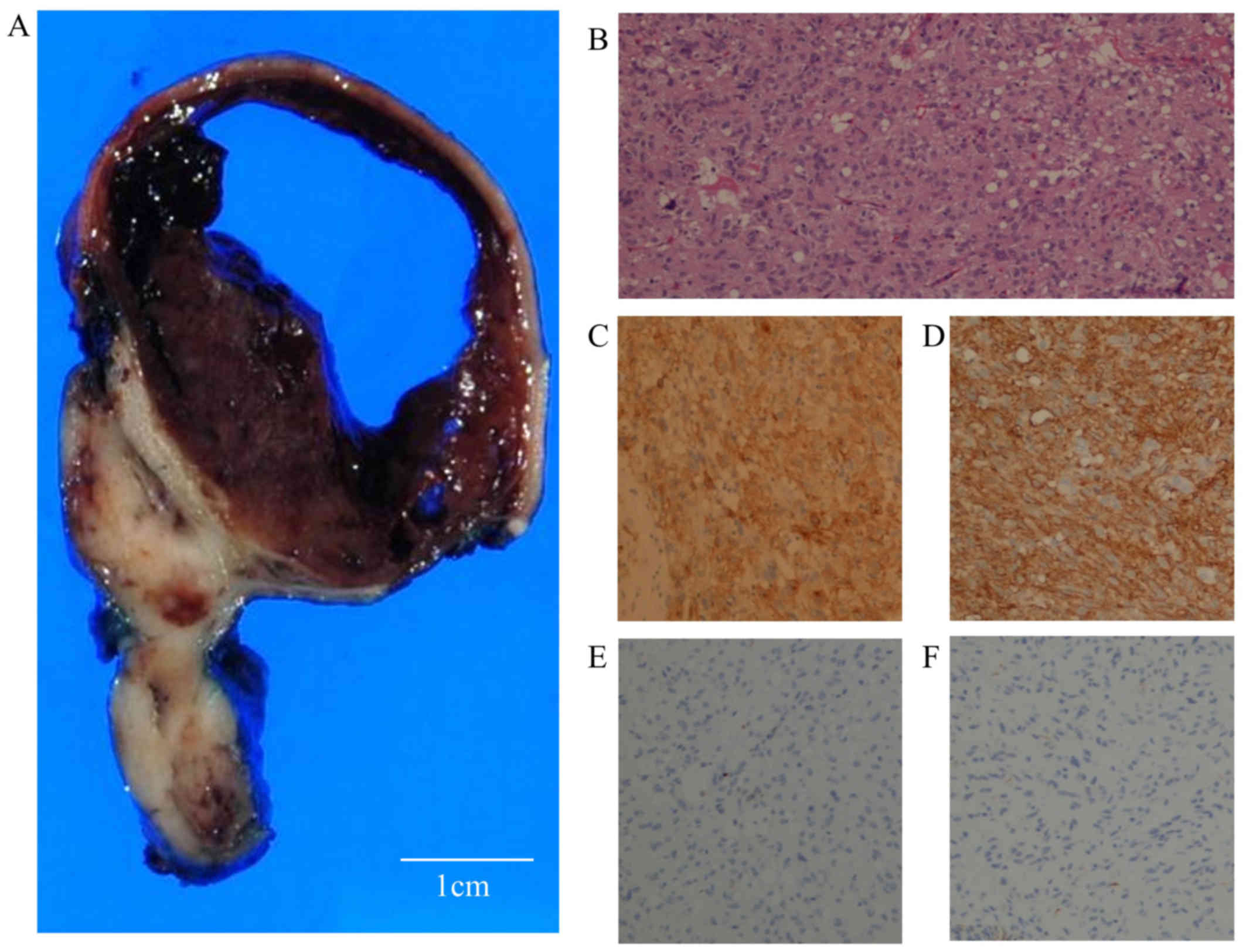
Macroscopically, the mass measured 57×51×13 mm,
comprising both solid and cystic regions (Fig. 5A). Most of the tumor seen on
endoscopy was the cystic component, which was filled with bloody
serous fluid. Histopathological findings revealed that the tumor
was located in the submucosal to muscularis layer of the stomach,
and the cystic component was located in the submucosal layer. The
cystic component showed hemorrhage, but no necrosis. Although part
of the tumor projected toward the serosa, forming the lesion as
seen on laparoscopy, there were no findings of serosal invasion.
Histopathological analysis of the tumor revealed the spindle cells
(Fig. 5B). The tumor cells were
immunostained with anti-c-kit (CA4502, 1:200 dilution; Dako Japan,
Tokyo, Japan), CD34 (NCL-L-END, 1:800 dilution), S-100
(NCL-L-S100p, 1:100 dilution), desmin (NCL-L-DES-DERII, 1:100
dilution), and smooth muscle actin (SMA) (NCL-L-SMA, 1:200
dilution) (all from Leica Biosystems, Newcastle, UK). These cells
were positive for c-kit and CD34 (Fig.
5C and D), and negative for S-100, desmin (Fig. 5E and F) and SMA, and the mitotic
count was 2/50 high-power fields. The tumor was diagnosed as a
mixed-type GIST with cystic formation, belonging to the
intermediate-risk group, based on Joensuu's classification system.
The patient received no adjuvant therapy and continues to do well
without recurrence for 40 months after LECS. Written informed
consent for this case report was obtained from the patient.
Discussion
Cysts are macroscopically identified in ~50% of
GISTs (19); however, stomach GISTs
are usually solid tumors and seldom exhibit predominant cystic
formations clinically. A few reported cases developed cystic
changes, and the majority of these were cases of large GISTs,
progressing as exoluminal or intramural patterns and misdiagnosed
as epigastric cystic tumors derived from other organs (Table I). In the present case, most of the
tumor developed inside the stomach, with positive cushion sign, and
presented unique form. To our best knowledge, this is the first
case to report a cushion sign-positive stomach GIST presented in an
atypical form. The cystic space of GIST is reportedly formed by
hemorrhage or necrosis (17).
Hypervascular tumors may lead to internal bleeding, and necrosis
can be caused by recurrent congestion, hemorrhage, or edema when
the tumors grow faster than the capacity of blood supply or vein
drainage (20). In the present case,
the cystic component contained bloody serous fluid, without
necrosis. This tumor might tend to have hemorrhage because of the
presence of numerous blood vessels pathologically.
 | Table I.Summary of cases of stomach GIST with
cystic formation. |
Table I.
Summary of cases of stomach GIST with
cystic formation.
| No. | Authors | Age (years) | Sex | Size (cm) | Growth pattern | Mitotic index
(HPFs) | Treatment | (Refs.) |
|---|
| 1 | Park et
al | 11 | F | 10 | Exoluminal | NA | Surgical resection
and chemotherapy | (8) |
| 2 | Osada et
al | 74 | M | 12 | Intramural | NA | Surgical resection
and chemotherapy | (9) |
| 3 | Cruz et
al | 37 | M | 32 | Exoluminal | 10/50 | Surgical resection
and chemotherapy | (10) |
| 4 | Yu et al | 81 | F | 6 | NA | 4/50 | Surgical
resection | (11) |
| 5 | Notani et
al | 85 | M | 22 | Exoluminal | 250-500/50 | Surgical resection
and chemotherapy | (12) |
| 6 | Zuh et al | 78 | M | 17 | Exoluminal | >10/50 | Surgical
resection | (13) |
| 7 | Okano et
al | 79 | M | 6 | Intramural | <5/50 | Surgical
resection | (14) |
| 8 | Hamza et
al | 74 | F | 6.6 | Exoluminal | 1/50 | Surgical resection
and chemotherapy | (15) |
| 9 | Sun et al | 75 | M | 13 | Exoluminal | <5/50 | Surgical resection
and chemotherapy | (16) |
| 10 | Present case | 72 | M | 5.7 | Mixed | 2/50 | LECS |
|
The Japanese guidelines for gastric SMT recommend
detailed examination with CT with contrast enhancement, EUS, and/or
EUS-FNA when SMTs are 2–5 cm in diameter (21). In addition, EUS is reported to be
useful modalities for diagnosing GISTs with cystic formation
(14). In the present case, we
initially thought the tumor was a lymphangioma, as the SMT was very
soft and cushion sign was positive. However, it was highly
important to conduct contrast-enhanced CT, EUS and EUS-FNA,
according to the guidelines. Recently, LECS has developed as a safe
and feasible procedure for the resection of gastric SMTs (22), and we selected tumor removal using
LECS. The laparoscopic findings showed that part of GIST was
arising from the gastric wall, which was the lesion outside the
stomach as seen on EUS. EUS was also a useful modality for
diagnosing the extension of GIST in this case.
In the present case, GIST belonged to the
intermediate-risk group due to the tumor size. However, some
reports have suggested that the real tumor volume may be smaller
than the imaging volume on cases of cystic GIST (13,23). It
is controversial that a component of cystic lesion is included in
the tumor size. Examination of additional cases is necessary for
accurate risk classification of GIST with cystic formation.
For high-risk group of GIST, administration of
imatinib for 3 years is recommended as adjuvant chemotherapy
(24), and the effect of imatinib
has been identified to be related to c-kit and PDGFR mutations
(25). Because this case belonged to
the intermediate-risk group, we did not search those mutations.
However, if this case recurs in the future, it is important to
examine these mutations.
In conclusion, we reported a rare case of a cushion
sign-positive stomach GIST with cystic formation. This case
suggests that the possibility of cystic formation of malignant
tumor, such as GIST, should be kept in mind when the tumor is large
and has a solid component, even if it appears as a cushion
sign-positive SMT.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YOk, TS and HK conceived of the study and
participated in its design and coordination and analyzed and
interpreted the data. YOk, TS and HK wrote the manuscript. TS, HI,
SO, KT, MY, RF, TM, KM, MH performed technical work. YOy performed
histological examination. YK, SK and SO performed the surgery.
Ethics approval and consent to
participate
Written informed consent for this case report was
obtained from the patient.
Consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nowain A, Bhakta H, Pais S, Kanel G and
Verma S: Gastrointestinal stromal tumors: Clinical profile,
pathogenesis, treatment strategies and prognosis. J Gastroenterol
Hepatol. 20:818–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida T, Goto O, Raut CP and Yahagi N:
Diagnostic and treatment strategy for small gastrointestinal
stromal tumors. Cancer. 122:3110–3118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti
H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A
consensus approach. Hum Pathol. 33:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park J, Rubinas TC, Fordham LA and
Phillips JD: Multifocal gastrointestinal stromal tumor (GIST) of
the stomach in an 11-year-old girl. Pediatr Radiol. 36:1212–1214.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osada T, Nagahara A, Kodani T, Namihisa A,
Kawabe M, Yoshizawa T, Ohkusa T and Watanabe S: Gastrointestinal
stromal tumor of the stomach with a giant abscess penetrating the
gastric lumen. World J Gastroenterol. 13:2385–2387. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cruz RJ Jr, Vincenzi R, Ketzer BM, Cecilio
AL and Cepeda LA: Spontaneous intratumoral bleeding and rupture of
giant gastric stromal tumor (> 30 cm) in a young patient. World
J Surg Oncol. 6:762008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu CC, Wu CC, Hwang JI, Wang J and Chang
CS: Thick calcification from a GIST of the stomach penetrating into
pericolic soft tissue-report of a case. World J Surg Oncol.
9:452011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Notani H, Kawamura T, Sato T, Hoshino A,
Sato Y and Nakajima A: A case of a giant gastrointestinal stromal
tumor of the stomach with extramural growth. Gan To Kagaku Ryoho.
40:2179–2181. 2013.(In Japanese). PubMed/NCBI
|
|
13
|
Zhu CC, Liu Y and Zhao G: Exophytic
gastrointestinal stromal tumor with cystic changes: A case report.
Oncol Lett. 7:1427–1429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okano H, Tochio T, Suga D, Kumazawa H,
Isono Y, Tanaka H, Matsusaki S, Sase T, Saito T, Mukai K, et al: A
case of a stomach gastrointestinal stromal tumor with extremely
predominant cystic formation. Clin J Gastroenterol. 8:197–201.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamza AM, Ayyash EH, Alzafiri R, Francis I
and Asfar S: Gastrointestinal stromal tumour masquerading as a cyst
in the lesser sac. BMJ Case Rep. 2016:bcr20162154792016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun KK, Xu S, Chen J, Liu G, Shen X and Wu
X: Atypical presentation of a gastric stromal tumor masquerading as
a giant intraabdominal cyst: A case report. Oncol Lett.
12:3018–3020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levy AD, Remotti HE, Thompson WM, Sobin LH
and Miettinen M: Gastrointestinal stromal tumors: Radiologic
features with pathologic correlation. Radiographics. 23:283–304,
456, quiz 532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Beer RA and Shinya H: Colonic lipomas.
An endoscopic analysis. Gastrointest Endosc. 22:90–91. 1975.
View Article : Google Scholar
|
|
19
|
Miettinen M, Furlong M, Sarlomo-Rikala M,
Burke A, Sobin LH and Lasota J: Gastrointestinal stromal tumors,
intramural leiomyomas, and leiomyosarcomas in the rectum and anus:
A clinicopathologic, immunohistochemical, and molecular genetic
study of 144 cases. Am J Surg Pathol. 25:1121–1133. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andea AA and Klimstra DS: Adenocarcinoma
arising in a tailgut cyst with prominent meningothelial
proliferation and thyroid tissue: Case report and review of the
literature. Virchows Arch. 446:316–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishida T, Hirota S, Yanagisawa A, Sugino
Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F and Kubota
T; GIST Guideline Subcommittee: Clinical practice guidelines for
gastrointestinal stromal tumor (GIST) in Japan: English version.
Int J Clin Oncol. 13:416–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuda T, Nunobe S, Kosuga T, Kawahira H,
Inaki N, Kitashiro S, Abe N, Miyashiro I, Nagao S, Nishizaki M, et
al: Society for the Study of Laparoscopy and Endoscopy Cooperative
Surgery: Laparoscopic and luminal endoscopic cooperative surgery
can be a standard treatment for submucosal tumors of the stomach: A
retrospective multicenter study. Endoscopy. 49:476–483. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joensuu H, Eriksson M, Sundby Hall K,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joensuu H, Eriksson M, Sundby Hall K,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frolov A, Chahwan S, Ochs M, Arnoletti JP,
Pan ZZ, Favorova O, Fletcher J, von Mehren M, Eisenberg B and
Godwin AK: Response markers and the molecular mechanisms of action
of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther.
2:699–709. 2003.PubMed/NCBI
|