Introduction
Chylous ascites is the build-up of lymphatic fluid
in the peritoneal cavity. Chylous ascites occurring following
surgery is uncommon, but may result from injury to lymphatic
vessels during retroperitoneal surgery. Treatment mainly includes
conservative measures aimed at decreasing chyle formation.
Treatment methods may involve introducing a low-fat diet with
medium-chain triglycerides, total parenteral nutrition,
administration of octreotide and paracentesis. For patients who are
refractory to these methods, lymphangiography is a proven
beneficial diagnostic tool, and may also be used as a therapeutic
intervention for lymphatic leakage to help avoid extensive surgery
(1–3).
We herein describe the case of a patient with
ovarian cancer who developed severe chylous ascites following
pelvic and para-aortic lymph node dissection and was effectively
managed by ultrasound-guided intranodal lymphangiography.
Case report
A 33-year-old pregnant woman (gravida 2, para 2)
presented with a small left ovarian tumor that started to increase
in size after delivery. The patient's medical and surgical history
was unremarkable and the findings on general physical examination
were normal. The hemoglobin level was 11.6 g/dl, the carbohydrate
antigen 19–9 level was markedly elevated at 107.4 U/ml (normal,
<37.0 U/ml), the cancer antigen 125 level was mildly elevated at
39.8 U/ml (normal, <35.0 U/ml), and the carcinoembryonic antigen
level was within the normal range.
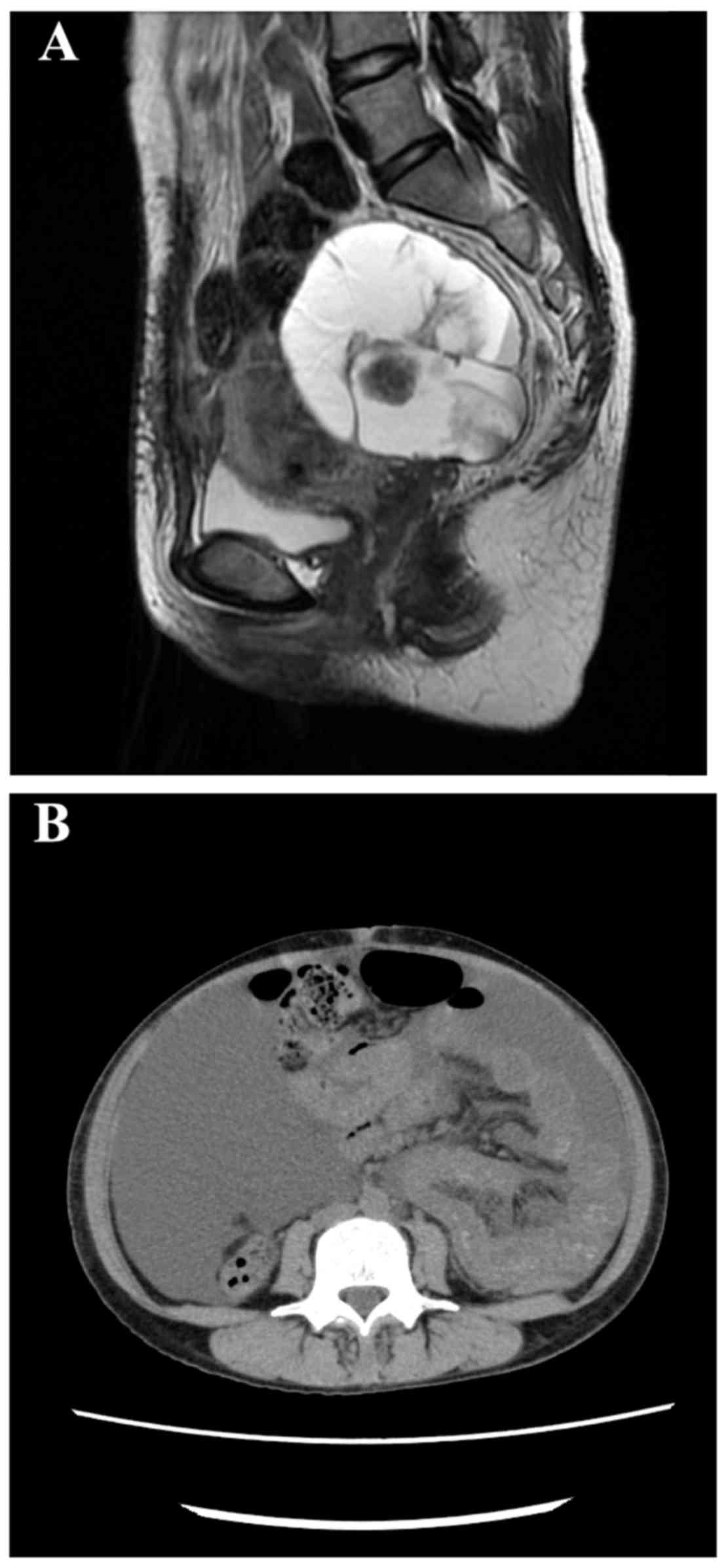
Pelvic magnetic resonance imaging (MRI) revealed a
mass sized 57×84×84 mm3 with multilocular cysts,
including small solid parts in the left adnexal region (Fig. 1A). MRI revealed no evidence of
peritonitis carcinomatosa, ascites, dissemination nodules, or
metastasis to the omentum. Contrast-enhanced computed tomography
(CT) revealed no lymphadenopathy or distant metastasis.
As the intraoperative rapid diagnosis based on a
right ovarian specimen was ovarian carcinoma, total abdominal
hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and
pelvic and para-aortic lymphadenectomy were performed. Upon
completion of the primary debulking surgery, no residual tumor was
identified. The resected specimens were examined by a pathologist
and the final diagnosis was seromucinous ovarian cancer (pT1c1N0M0,
stage IC1).
On postoperative day (POD)3, milky appearance of the
drainage fluid (800–1,000 ml/day) was continuously observed from
the abdominal drainage tube. Although a fat-restricted diet was
initiated, the volume of the discharge did not decrease. Following
removal of the drainage tube, the patient developed increasing
abdominal distention due to the large volume of chylous ascites.
Thus, a daily subcutaneous injection of 100 µg octreotide was
administered, in addition to total parental nutrition starting on
POD21. However, the amount of chylous ascites gradually increased
(Fig. 1B), and ascites drainage was
required several times during treatment. As no improvement was
observed on POD60, lipiodol lymphangiography was conducted on POD62
following a consultation with an in-house radiologist.
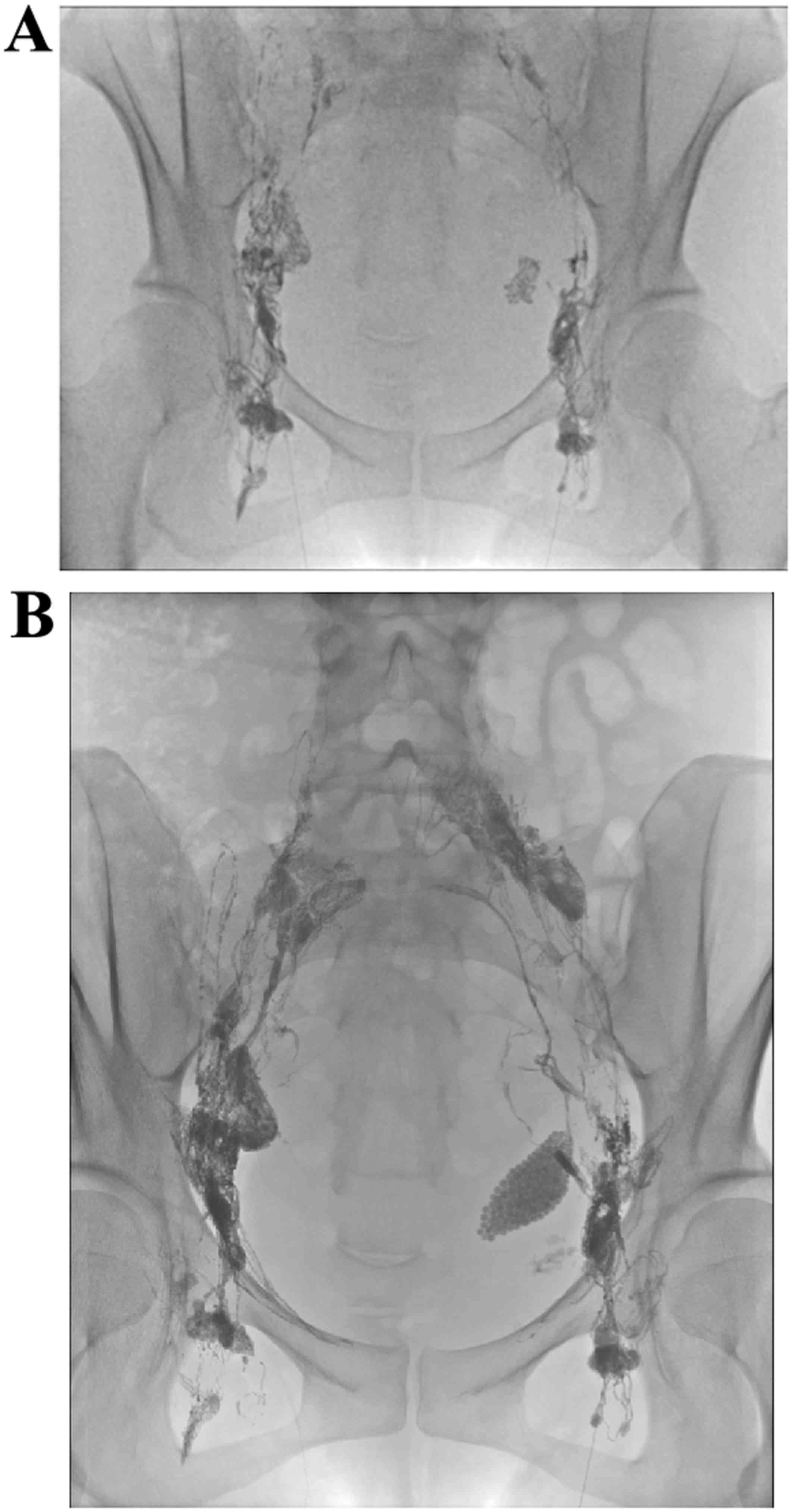
Ultrasound-guided access to the inguinal lymph nodes bilaterally
was achieved using a 27G needle, and lipiodol was slowly
administered to the lymph nodes at ~6 ml/h under fluoroscopic
guidance to ensure proper access (Fig.
2A). Following injection of ~12 ml of lipiodol, fluoroscopic
imaging demonstrated accumulation of lipiodol at the left pelvis
(Fig. 2B).
Following lymphangiography, the volume of discharge
slowly decreased, and the abdominal distention gradually improved.
On POD90 (28 days after lymphangiography) the patient resumed
normal oral nutrition and on POD95 (33 days after lymphangiography)
she was discharged from the hospital without recurrence of the
ascites. The patient has remained asymptomatic and progression-free
for 1 year.
Discussion
Postoperative chylous leakage is an uncommon but
established complication that occurs following general surgery.
Chylous ascites may occur due to conditions such as filariasis,
tuberculosis, trauma, hepatic cirrhosis and complications
associated with surgery. The reported incidence ranges from 1.3 to
3.4% following procedures involving the thorax and neck (4,5).
Prophylactic ligation of the lymphatic duct is commonly conducted
at the time of lymphadenectomy for gynecological cancer to prevent
chylous leakage. Chylous leakage is potentially fatal due to the
large amount of fluid, plasma protein, fat and immunoregulatory
lymphocyte loss. Furthermore, affected patients exhibit clinical
symptoms of severe malnutrition, hyponatremia, acidosis,
hypocalcemia and susceptibility to infection. Therefore,
uncontrolled chylous leakage is associated with a high mortality
rate (3,6).
Initial treatment for chylous leakage generally
comprises conservative methods, such as fasting, introducing a
modified diet (low-fat with medium-chain triglycerides, parenteral
nutrition and supplementation), drainage of the effusion, and
administration of octreotide (7,8) and
etilefrine (9). If conservative
therapy is unsuccessful, surgical interventions, such as thoracic
duct ligation, may be considered. However, it may not be easy to
locate the thoracic duct or the site of chylous leakage at
reoperation. Furthermore, surgical intervention is considered to
increase the risk of complications, necessitating extreme care
during the procedure.
Lipiodol lymphangiography has traditionally been
used as a method for diagnosing chylous leakage and enables the
identification of the leakage point for surgical intervention
(3,10). However, lipiodol lymphangiography may
also be applied as a treatment method. A previous study
demonstrated that lipiodol lymphangiography was efficacious in 35%
of patients with chylothorax, even when the volume of pleural
drainage fluid was >500 ml/day. The procedure is also reportedly
successful in 51% of patients who are refractory to non-surgical
treatments (2). A study by Matsumoto
et al (3) demonstrated that
no surgical reintervention was required in 89% of patients with
postoperative chylous leakage, and that chylous leakage may resolve
following lipiodol lymphangiography in the remaining patients.
Chylous leakage cessation following lipiodol lymphangiography is
hypothesized to occur due to accumulation of lipiodol at the
leakage point, which activates an inflammatory reaction and acts as
an embolic agent (2).
The lymphangiography procedure traditionally
includes subcutaneous injection of an oily contrast medium, such as
lipiodol, into each foot. However, this procedure is associated
with various problems. First, it is invasive, due to the need for
dorsal incisions, thereby posing an infection risk. Second, it is
technically difficult due to the need to isolate and cannulate the
fine lymphatic vessels on the feet. Even following successful
placement of the lymphangiogram needles, small movements by the
patient may cause needle displacement. By contrast, intranodal
lymphangiography using ultrasound has reduced the invasiveness of
the procedure compared with the traditional method, as it does not
require an incision. Instead, ultrasound-guided access to the
inguinal nodes is achieved using a 23-26G spinal needle under local
anesthesia, making intranodal lymphangiography a simpler and easier
procedure.
Lipiodol lymphangiography was performed by gaining
direct access to the bilateral inguinal lymph nodes using
ultrasound guidance. This procedure was effective for treating this
patient, who developed severe chylous leakage following surgery for
ovarian cancer. This shows that lipiodol lymphangiography is an
efficacious conservative treatment method for chylous leakage.
This single case report has obvious limitations.
While lipiodol lymphangiography using bilateral inguinal lymph node
puncture suggests the usefulness of thoracic duct embolization,
additional prospective studies are required to determine the safety
and efficacy of such methods.
Severe chylous leakage is uncommon, and the optimal
management methods remain controversial. However, the outcome of
the present case suggests that lipiodol lymphangiography is
clinically effective for resolving chylous leakage following
surgery for ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN has been involved in drafting the manuscript and
participated in the experiment design and patient treatment. MI,
TM, TI, KO, HY, RO, HS, SR and SKa carried out the treatment. KeN
participated in the design of the study. SKy conceived of the
study, and participated in its design and coordination and helped
to draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shimane University School of Medicine (Izumo, Japan;
approval no. 960). The research was conducted in accordance with
the Declaration of Helsinki and Title 45, US Code of Federal
Regulations, Part 46, Protection of Human Subjects, effective
December 13, 2001.
Consent for publication
The patient provided written informed consent for
the publication of the case details and associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
POD
|
postoperative day
|
References
|
1
|
Kos S, Haueisen H, Lachmund U and Roeren
T: Lymphangiography: forgotten tool or rising star in the diagnosis
and therapy of postoperative lymphatic vessel leakage. Cardiovasc
Intervent Radiol. 30:968–973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alejandre-Lafont E, Krompiec C, Rau WS and
Krombach GA: Effectiveness of therapeutic lymphography on lymphatic
leakage. Acta Radiol. 52:305–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsumoto T, Yamagami T, Kato T, Hirota T,
Yoshimatsu R, Masunami T and Nishimura T: The effectiveness of
lymphangiography as a treatment method for various chyle leakages.
Br J Radiol. 82:286–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaburagi T, Takeuchi H, Oyama T, Nakamura
R, Takahashi T, Wada N, Saikawa Y, Kamiya S, Tanaka M, et al:
Intraoperative fluorescence lymphography using indocyanine green in
a patient with chylothorax after esophagectomy: report of a case.
Surg Today. 43:206–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul S, Altorki NK, Port JL, Stiles BM and
Lee PC: Surgical management of chylothorax. Thorac Cardiovasc Surg.
57:226–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parvinian A, Mohan GC, Gaba RC, Saldanha
DF, Knuttinen MG, Bui JT and Minocha J: Ultrasound-guided
intranodal lymphangiography followed by thoracic duct embolization
for treatment of postoperative bilateral chylothorax. Head Neck.
36:E21–E24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Epaud R, Dubern B, Larroquet M, Tamalet A,
Guillemot N, Maurage C, Clement A and Fauroux B: Therapeutic
strategies for idiopathic chylothorax. J Pediatr Surg. 43:461–465.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Snow AL, Uller W, Kim HB and Alomari AI:
Percutaneous embolization of a chylous leak from thoracic duct
injury in a child. Cardiovasc Intervent Radiol. 37:1111–1113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohkura Y, Ueno M, Iizuka T, Haruta S,
Tanaka T and Udagawa H: New combined medical treatment with
etilefrine and octreotide for chylothorax after esophagectomy: a
case report and review of the literature. Medicine. 94:e22142015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ngan H, Fok M and Wong J: The role of
lymphography in chylothorax following thoracic surgery. Br J
Radiol. 61:1032–1036. 1988. View Article : Google Scholar : PubMed/NCBI
|