Introduction
Symptoms of depression are present in ~55% of cancer
patients, and Selective serotonin reuptake inhibitor (SSRI)
anti-depressant medications are prescribed in up to 20% of these
individuals (1). SSRIs work by
blocking reuptake of the biogenic monoamine serotonin
[5-hydroxytryptamine (5-HT)], thus systemically elevating serotonin
levels and leading to feelings of well-being and happiness. While
serotonin is most noted as a neurotransmitter in the central
nervous system, a local mediator in the gut, and a vasoactive agent
in the blood, a connection between 5-HT receptor signaling and
proliferation of diverse non-diseased cell types has been reported
over the past two decades (2–5). Several
studies have linked serotonin signaling to the promotion of tumor
growth and metastasis in hepatocellular carcinoma, melanoma, as
well as pancreatic, prostate, bladder, and breast cancer (6–19).
Moreover, high levels of serotonin are capable of transforming
non-tumorigenic cell lines such as NIH3T3 fibroblasts (20,21), and
serum levels of this neurotransmitter have been used as a
prognostic marker for urothelial, prostate, and renal cell
carcinoma (22).
Serotonin can exert multiple and sometimes opposing
actions on its target cells. These effects are determined by the
characteristics of the 5-HT receptor/s with which it interacts and
the intracellular signaling pathways coupled to each receptor. In
humans, there are seven 5-HT receptor families (six families of
G-protein coupled receptors and one ion channel family) that are
expressed in a tissue-specific manner across a variety of normal
and tumor cells, and the levels of a handful of these receptors
have been correlated with increased tumorigenicity. For instance,
strong expression of 5-HT1A and B have been correlated with high
Gleason grades, as well as lymph node and bone metastasis in
prostate cancer (23). 5-HT2B
expression has been linked to induction of tumor formation in nude
mice (24), while 5-HT4 is
overexpressed in high grade prostate tumors and has been shown to
facilitate cell growth in an androgen depleted environment
(25,26).
Though some 5-HT receptors have been associated with
oncogenic processes, a comprehensive analysis of 5-HT receptors
across both carcinomas and sarcomas has not yet been performed. In
this study, we examined the expression of 5-HT receptors across a
large panel of cancers, and employed pharmacological inhibitors of
each of the seven classes of 5-HT receptors to evaluate their
effects on tumor cell viability and oncogenic signaling. We then
performed a retrospective clinical analysis of a large cohort of
breast cancer patients looking specifically at the correlation
between SSRI use and tumor proliferation rates.
Materials and methods
Meta-analysis of genomic expression
data
Meta-analysis was performed to examine the HTR mRNA
expression levels on 1036 cancer cell lines housed in the Cancer
Cell Line Encyclopedia (CCLE) (www.broadinstitute.org/ccle/home) (27). Normalized heatmap data was generated
in Cluster 3.0 software (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
(using unsupervised hierarchical clustering analysis (uncentered
correlation similarity metric, centroid linkage). Heatmaps were
visualized using Java Treeview software (http://jtreeview.sourceforge.net/). Meta-analysis of
5-HT protein expression was performed using existing annotated
databases from the Human Protein Atlas (HPA; www.protein atlas.org). Antigen staining was evaluated
as positive or negative, and represented as a percentage of the
total number of cancer tissues tested for each cancer type.
Meta-analysis of HTR and TPH genes in normal mouse breast tissue
(N=5), non-metastatic breast tumors from 67NR xenografts (N=5), and
metastatic breast tumors from 4T1 ×enografts (N=4) was performed on
Affymetrix mouse Genome 430 2.0 Array data housed in Gene
Expression Omnibus (GEO; cat. no. GSE62817).
Cell culture
Sarcoma and breast cancer cell lines were cultured
in incubators maintained at 37°C in the presence of 5%
CO2. AU565 (cat. no. CRL-2351), HCC70 (cat. no.
CRL-2115), and BT-549 (cat. no. HTB-122) human breast cancer lines,
and SW872 human liposarcoma cells (cat. no. HTB-92) were grown in
RPMI-1640 medium supplemented with 10% fetal bovine serum and
penicillin/streptomycin antibiotics (all from ATCC, Manassas, VA,
USA). The SK-BR-3 human breast cancer line (cat. no. HTB-30; ATCC)
was grown in McCoy's medium supplemented with 10% fetal bovine
serum and penicillin/streptomycin antibiotics. The A673 human
Ewing's sarcoma cell line (cat. no. CRL-1598) and the HOS human
osteosarcoma cell line (cat. no. CRL-1543) (both from ATCC) were
grown in Dulbecco's modified eagle medium supplemented with 10%
fetal bovine serum and penicillin/streptomycin. The COSB canine
hemangiosarcoma cell line (28) was
grown in EGM-2 basal medium with the EGM-2 Bullet kit (cat. no.
CC-3162; Lonza, Basel, Switzerland). The epidemiology and cancer
biology of the canine cell line has been shown to extrapolate
directly to humans (28).
Immunoblotting
Cell lysates were collected as indicated for each
experiment, subjected to SDS-PAGE, and transferred to
polyvinylidene fluoride membranes using the Trans-Blot Turbo
Transfer System (Bio-Rad, Hercules, CA, USA). Membranes were
blocked in tris buffered saline plus 3% bovine serum albumin and
0.05% Tween-20, and incubated with the following antibodies as
indicated for each experiment: Ki-67 (cat. no. ab16667; 1:1,000
dilution, 1 h incubation at 25°C; Abcam, Cambridge, UK), Kinome
View Profiling kit (cat. no. 9812; 1:2,000 dilution for all
antibodies, 1 h incubation at 25°C; Cell Signaling, Danvers, MA,
USA) or anti-actin (cat. no. sc8432; 1:1,000 dilution, 1 h
incubation at 25°C; Santa Cruz Biotech, Dallas, TX, USA). Each
primary antibody was detected with 1:1,000 HRP-conjugated secondary
antibody [Santa Cruz Biotechnology cat. no. sc-2357 (anti-rabbit)
or cat. no. sc-2005 (anti-mouse); 1:1,000 dilution, 1 h incubation
at 25°C], subjected to Supersignal West Dura Extended Duration
Substrate (Thermo Scientific, Waltham, MA, USA), and digitally
captured using a GE Image Quant Las4000 imaging system.
Antibody arrays
The Phospho-Mitogen-activated protein kinase (MAPK)
Antibody Array (cat. no. ARY002B; R&D Systems, Minneapolis, MN,
USA) was performed on HOS cells as indicated according to the
manufacturer's instructions. Normalized heatmap data was generated
in Cluster 3.0 software (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
(using unsupervised hierarchical clustering analysis (uncentered
correlation similarity metric, centroid linkage). Heatmaps were
visualized using Java Treeview software (http://jtreeview.sourceforge.net/).
Proliferation/viability assays
To measure the effects of serotonin or 5-HT
pharmacological inhibitors on tumor cell viability, sarcoma and
breast cancer cells were plated in 96-well plates at approximately
~75% confluence, treated as indicated, and cell viability was
measured after 24 h via fluorescent excitation at 530 nm with the
Alamar Blue cell viability assay (ThermoFisher Scientific).
Tumor spheroid model
HOS cells were grown in hanging drops (3,000
cells/drop) for 48 h as previously described for other cell lines
(29), and transferred to
non-adherent well plates. Spheroids were treated as indicated for
48 h and photos were taking using bright field microscopy.
In ovo tumor assay
All chicken embyo experiments were performed prior
to hatching, thus these experiments were considered exempt from
ethics approval based on PHS policy. Chorioallantoic membrane (CAM)
tumor assays were performed as previously described using rainbow
hen eggs (Gallus gallus domesticus) (30). A false air-sac was generated directly
over the CAM of fertilized chicken eggs (7 days
post-fertilization). 20,000 dissociated CosB tumor cells were
soaked onto a 5 mm2 gelatin sponge and then placed onto
the CAM. A sham solution of isotonic saline solution (N=3) or 100
nM SB-269970 (N=3) was added daily directly onto the CAM tumor.
After 72 h of treatment (13 days post-fertilization), the tumors
were collected, weighed, and photographed on a lightbox.
Retrospective clinical analysis
Retrospective analysis of clinical data was carried
out with the approval of the Texas Tech University Health Sciences
Center Institutional Review Board. Analysis of 419 female patients
diagnosed with invasive ductal carcinoma at the Texas Tech Breast
Care Center between the years of 2010 to 2014 was performed. The
demographic characteristics of the study population are described
in Table I. IHC for estrogen
receptor (ER), progesterone receptor (PR), Her-2/neu, and Ki-67
tumor proliferative index were available for all patients. Breast
cancer clinico-pathological features (age at diagnosis, tumor size,
tumor grade, lymph node status, hormonal receptor status) were
extracted from each case report. Patients were considered positive
for SSRI usage if they had been prescribed SSRIs at any point in
the year prior to or at the time of diagnosis. Of a total of 419
patients included in this study, we identified 28 patients taking
SSRIs and 391 patients who were not taking SSRIs during the defined
time frame.
 | Table I.Demographic characteristics of
retrospective study population. |
Table I.
Demographic characteristics of
retrospective study population.
| Demographic | Number | Percentage |
|---|
| Caucasian,
hispanic | 378 | 90 |
| Caucasian,
white | 4 | 1 |
| Native
American | 5 | 1 |
| Other | 32 | 8 |
Statistical analysis
All in vitro experiments were performed at least
three independent times, with at least four technical replicates
per assay. Unpaired t-tests or one-way analysis of variance (ANOVA)
followed by Dunnett's multiple comparison post hoc test were used
to determine the statistical significance for all in vitro
experiments. Differences were considered statistically significant
if the P-value was less than 0.05. The retrospective relationship
between SSRI usage and the Ki-67-based proliferative index of the
breast tumors was determined with the Mann-Whitney rank sum test.
Comparisons of SSRI usage to tumor hormonal receptor status and
tumor staging were calculated with the Fisher's exact test.
Statistical analyses were carried out using Graphpad Prism.
Differences were considered statistically significant if the
P-value was less than 0.05.
Results
5-HT receptor expression across
diverse cancers
To evaluate HTR gene (encoding 5-HT receptors)
expression in human cancers, we performed meta-analysis on the
global gene expression profiles obtained from microarray analysis
housed in the Cancer Cell Line Encyclopedia (CCLE; http://portals.broadinstitute.org/ccle).
This database contains quantitative data on over 1,000 different
cell lines representing a diverse array of cancer types including
carcinomas, sarcomas, and hematopoietic cancers. Unique expression
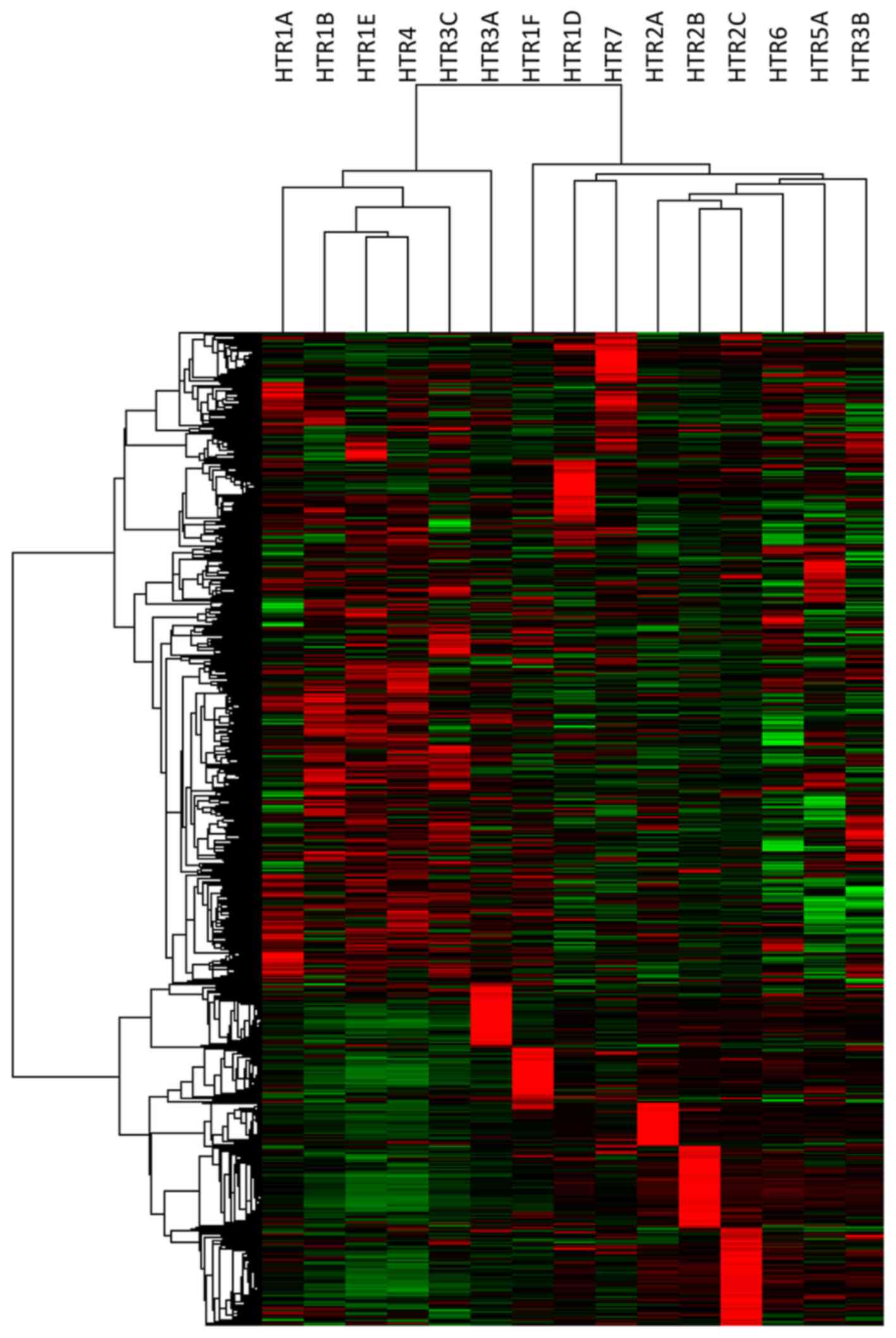
patterns emerged for many of the HTR mRNAs (Fig. 1). Analysis of the CCLE data revealed
clear clustering of gene over-expression dependent on tumor origin
was observed for several HTR mRNAs including HTR3A (largely lung
and hematopoietic/lymphoid tumor cell lines), HTR1F (largely
hematopoietic/lymphoid, bone sarcoma, and soft tissue sarcoma cell
lines), HTR1D (largely mixed digestive track cancer cell lines),
HTR2A (largely central nervous system, breast, and bone sarcoma
cell lines), and HTR2B (largely skin cancer cell lines). Though
clustering of expression patterns were observed other HTR mRNAs,
the cell lines composing these clusters were varied among cancer
origins. As mRNA expression is not always reflective of protein
levels, we performed meta-analysis of tissue pathology data housed
in the Human Protein Atlas to analyze 5-HT protein expression
across cancers. This repository contains stained tissue samples
representing the most common forms of cancer, totaling 216
different cancer samples on which immunohistochemistry data is
reported for many proteins. Our meta-analysis of the
pathology-based annotation of 5-HT1A, 1D, 1E, 1F, 2A, 2B, 3B, 4,
5A, and 7 protein expression levels is illustrated in Table II. A handful of the 5-HT receptors
were not reported due to their absence from the Human Protein
Atlas, therefore IHC analysis for these proteins is not included in
our results. Our meta-analysis revealed, with the exception of
5-HT1A and 2A, that the majority of 5-HT receptors analyzed were
expressed across many cancers.
 | Table II.Percentage of cancer tissues
expressing 5-hydroxytryptamine (5-HT) receptors based on IHC
staining. |
Table II.
Percentage of cancer tissues
expressing 5-hydroxytryptamine (5-HT) receptors based on IHC
staining.
|
| 5-HT1A | 5-HT1D | 5-HT1E | 5-HT1F | 5-HT2A | 5-HT2B | 5-HT3B | 5-HT4 | 5-HT5A | 5-HT7 |
|---|
| Breast | 0 | 100 | 100 | 67 | 9 | 92 | 55 | 91 | 100 | 100 |
| Cervical | 17 | 73 | 100 | 82 | 8 | 73 | 50 | 50 | 83 | 92 |
| Colorectal | 9 | 100 | 91 | 91 | 0 | 73 | 92 | 100 | 100 | 82 |
| Glioma | 13 | 100 | 100 | 18 | 0 | 0 | 55 | 36 | 9 | 0 |
| Head and neck | 0 | 100 | 75 | 75 | 50 | 50 | 75 | 25 | 75 | 0 |
| Liver | 17 | 100 | 73 | 55 | 10 | 20 | 67 | 75 | 45 | 73 |
| Lung | 0 | 100 | 100 | 45 | 8 | 67 | 82 | 54 | 67 | 100 |
| Lymphoma | 0 | 67 | 70 | 8 | 0 | 0 | 50 | 58 | 27 | 17 |
| Melanoma | 8 | 100 | 100 | 33 | 17 | 0 | 91 | 45 | 50 | 42 |
| Pancreatic | 0 | 100 | 100 | 67 | 10 | 80 | 83 | 100 | 80 | 58 |
| Prostate | 0 | 100 | 100 | 67 | 0 | 36 | 82 | 92 | 55 | 92 |
| Non-Mel skin | 0 | 100 | 100 | 90 | 18 | 8 | 67 | 36 | 58 | 36 |
Serotonin modulates intracellular
signaling pathways
To determine if serotonin is capable of enhancing
the proliferation of rate of cancer cells, we serum starved a panel
of diverse tumor cell lines representing 4 breast cancers (AU565,
BT549, HCC70, and SK-BR-3), 2 soft tissue sarcomas (CosB
hemangiosarcoma cells and SW872 liposarcomas cells), and 2 bone
cancer (A673 Ewing's sarcoma cells and HOS osteosarcoma cells) for
24 h. We then treated each cell line with increasing concentrations
of serotonin (1 to 1×105 nM), and cell viability was
accessed after 48 h. No major changes in the proliferation rate
were observed for any of the cell lines following serotonin
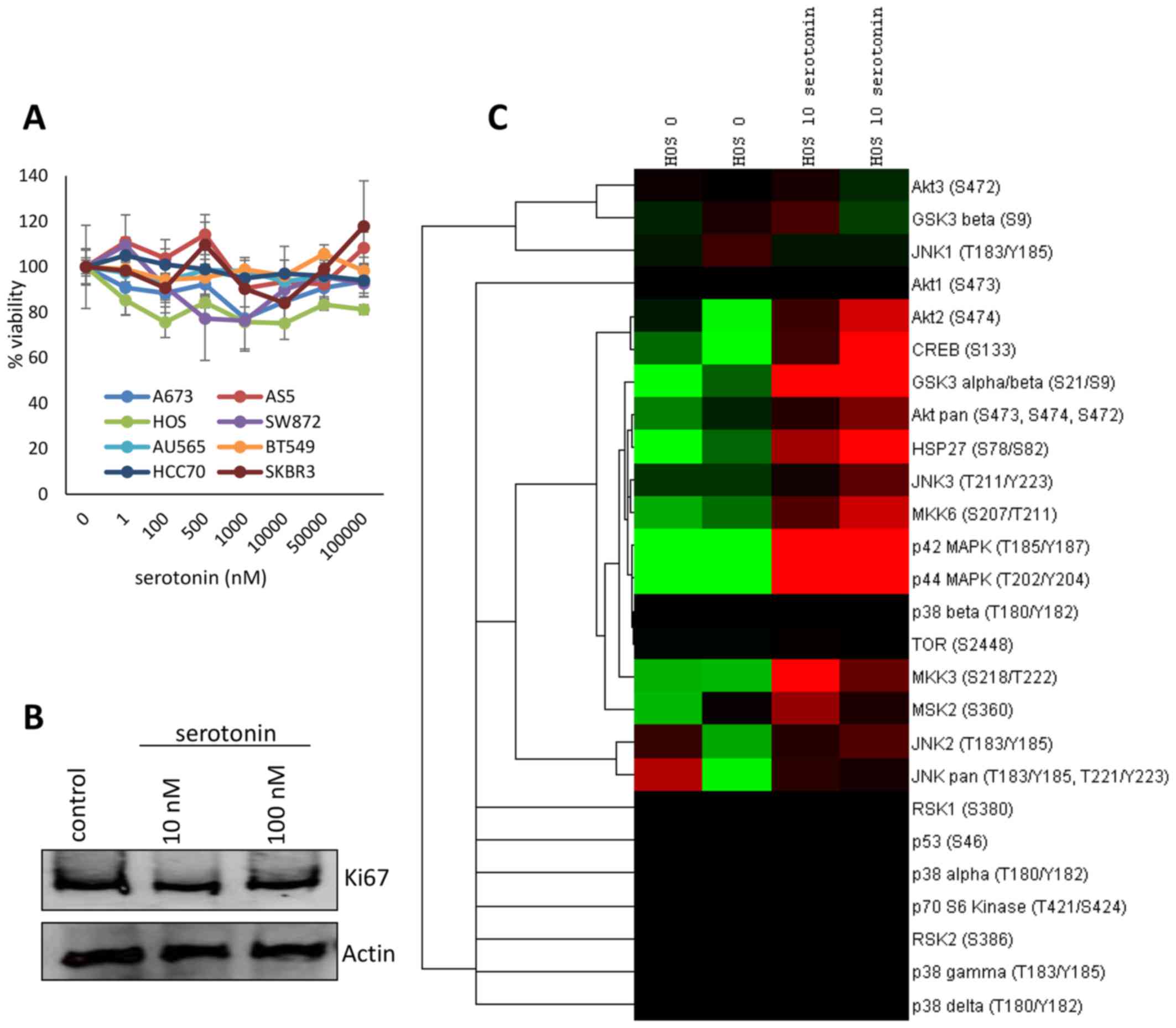
treatment relative to the untreated control (Fig. 2A). These data were confirmed in HOS
osteosarcoma cells via immunoblotting by using the proliferative
marker Ki-67 (Fig. 2B).
We next sought to determine if the presence of
serotonin alters intracellular signaling of the cancer cell lines.
To accomplish this, HOS osteosarcoma cells were serum starved
overnight and stimulated with 10 nM serotonin or a sham control for
10 min. Lysates were collected and the phosphorylation status of 24
kinases implicated in mitogenic and survival processes were
examined using antibody arrays. Serotonin stimulation resulted in a
marked increase in the activation of Akt2, CREB, GSK3, HSP27, and
multiple MAPK signaling mediators (Fig.
2C).
Selective 5-HT receptor blockade
reduces cancer cell viability and tumor growth
Because serotonin modulated the levels of kinases
involved in cancer cell signaling pathways, we determined whether
inhibition of 5-HT receptor activity was capable of affecting
cancer cell viability. We subjected the cell line panel consisting
of 4 breast cancers (AU565, BT549, HCC70, and SK-BR-3), 2 soft
tissue sarcomas (CosB hemangiosarcoma cells and SW872 liposarcomas
cells), and 2 bone cancer (A673 Ewing's sarcoma cells and HOS
osteosarcoma cells) to highly selective 5-HT receptor antagonists
(Table III), and cell viability
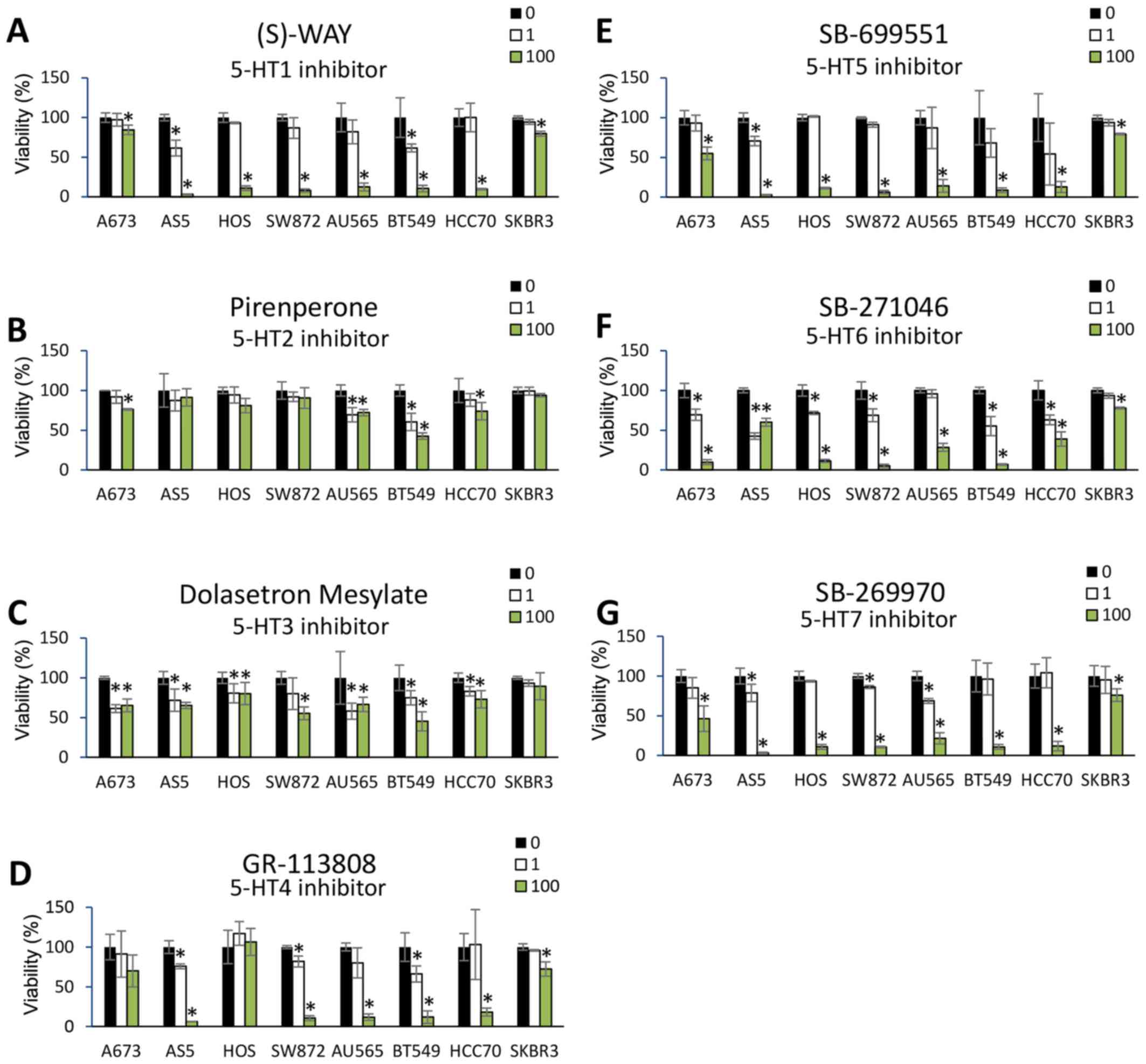
was assayed 24 h post-treatment. Dose-dependent reductions in cell
viability were observed for most of the 5-HT receptor antagonists
across the panel of cancer cell lines (Fig. 3A-G). Notable exceptions were
pirenperone (5-HT2 antagonist) and dolasetron mesylate (5-HT3
antagonist), which did not reduce cell viability across any of the
cancer cell lines. Moreover, A673, HOS, and SK-BR-3 tumor cells
were relatively more resistant to 5-HT antagonism compared to the
other cells lines, despite expression of selected HRT mRNAs in each
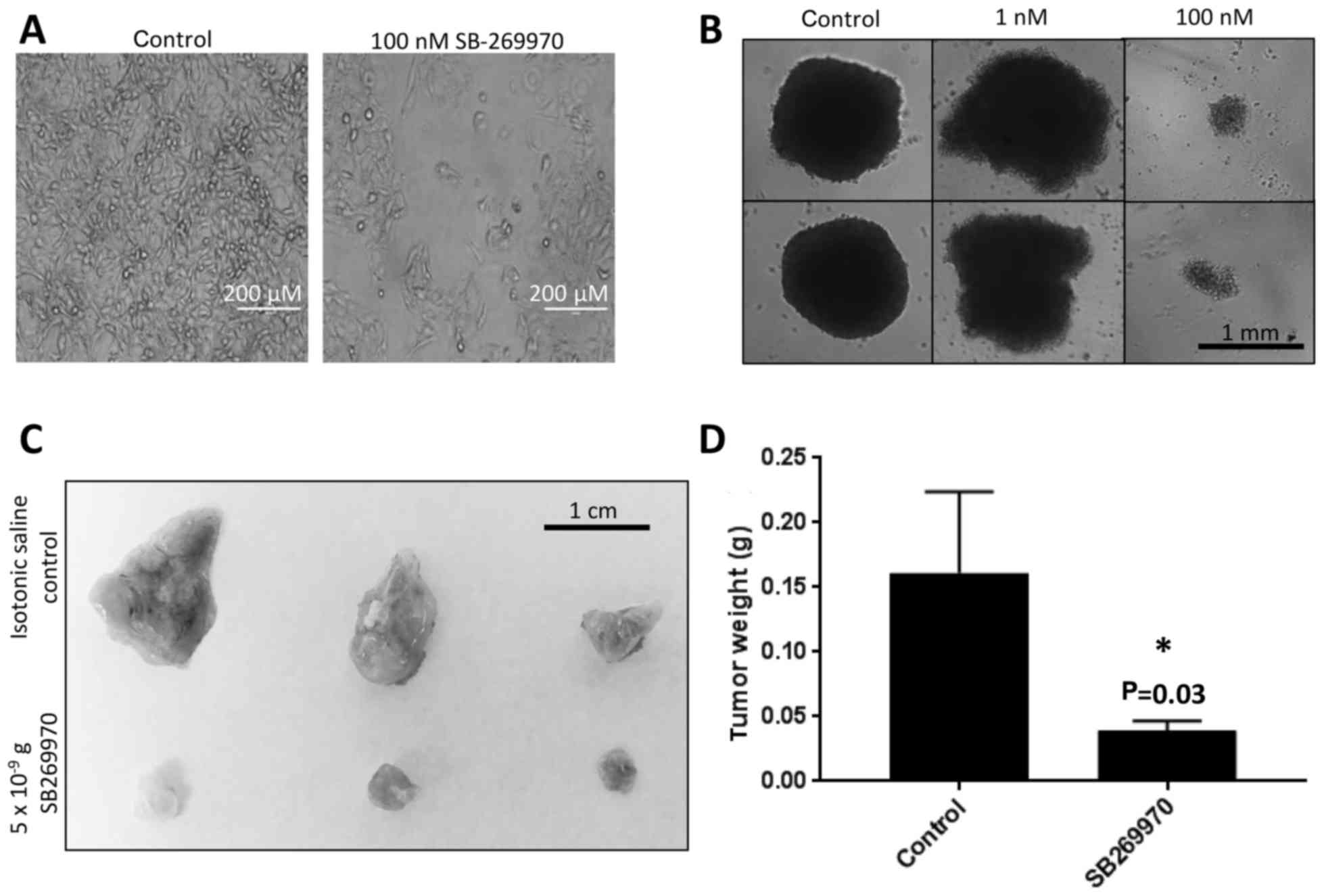
of these cell lines (based on CCLE data). A representative image of
the SW872 liposarcoma cell line treated for 24 h with SB-269970
(5-HT7 antagonist) is shown in Fig.
4A. We confirmed the efficacy of SB-269970 to abrogate cell
viability in the HOS osteosarcoma cell line using a
three-dimensional tumor spheroid model, whereby the 5-HT7 receptor
antagonist reduced tumor cell viability in a dose dependent manner
after 48 h (Fig. 4B). To expand our
in vitro results into an in vivo xenograft tumor model, we
subjected CosB hemangiosarcoma tumors grown on CAMs to daily
treatments of the 5-HT7 antagonist (5×10−9 grams/day) or
a control sham. Tumors treated with the 5-HT7 antagonist exhibited
significantly smaller tumor sizes compared to the sham control
(Fig. 4C and D).
 | Table III.5-Hydroxytryptamine (5-HT) receptor
antagonists used in this study. |
Table III.
5-Hydroxytryptamine (5-HT) receptor
antagonists used in this study.
| Antagonist | Target
receptor |
|---|
| (S)-WAY 100135 | 5-HT1 |
| Pirenperone | 5-HT2 |
| Dolasetron
mesylate | 5-HT3 |
| GR-113808 | 5-HT4 |
| SB-699551 | 5-HT5 |
| SB-271046 | 5-HT6 |
| SB-269970 | 5-HT7 |
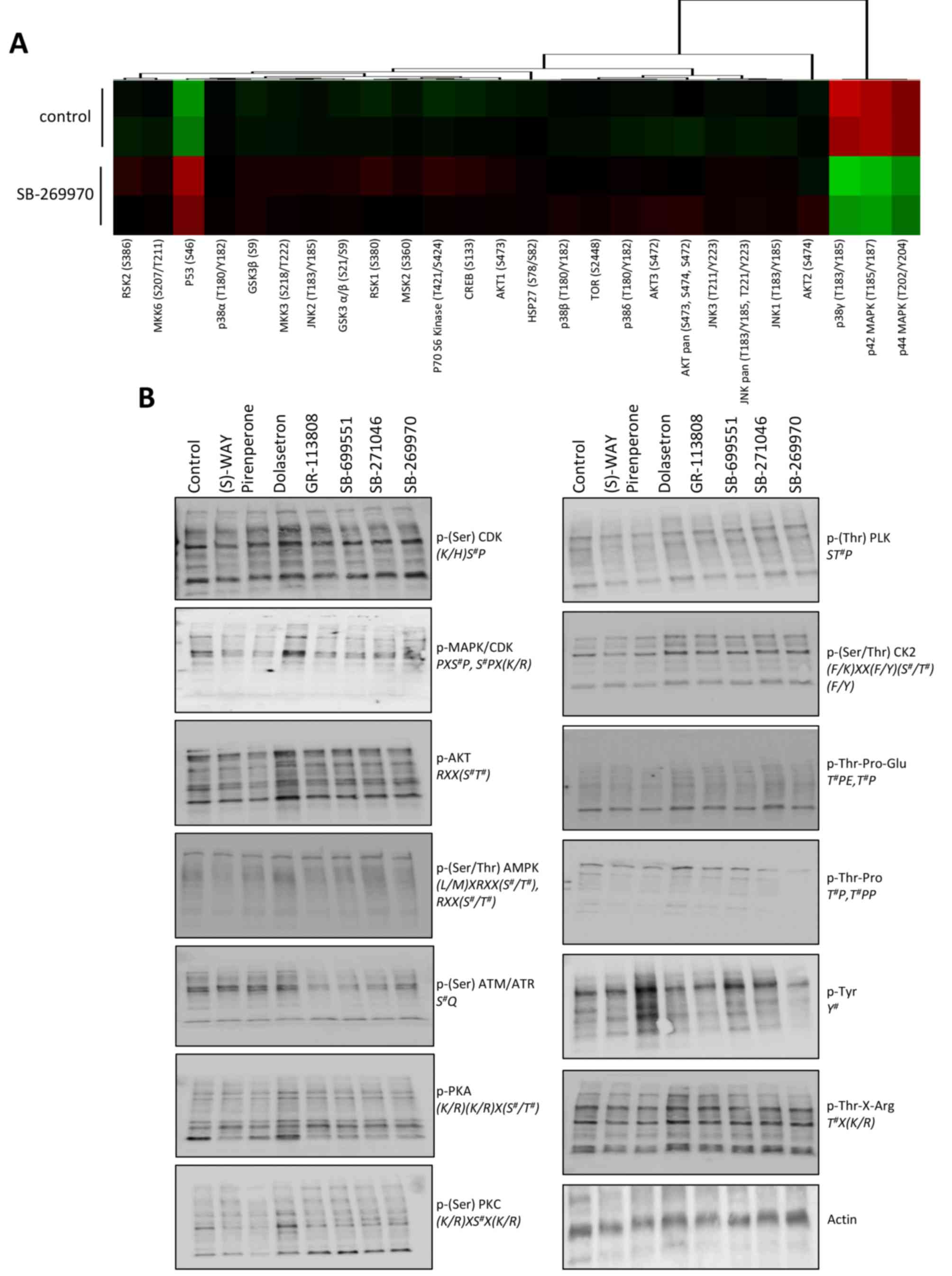
Selective 5-HT7 receptor blockade
modulates intracellular signaling in cancer cells
To detect the phosphorylation status of
proliferation and survival regulators following 5-HT antagonism, we
treated HOS osteosarcoma cells with the 5-HT7 receptor antagonist
for three h, collected cell lysates, and performed antibody arrays
to quantify changes in protein phosphorylation. Antagonist
treatment resulted in a marked increase in p53 phosphorylation and
reductions in the phosphorylation status of both p38 and p42 MAPK
(Fig. 5A). To more broadly evaluate
the panel of selective 5-HT receptor antagonists, we collected
protein lysates from HOS cells treated for three h with each
selective 5-HT antagonist (100 nM), and performed immunoblots using
a set of phospho-motif antibodies that cover a large portion of the
kinome regulated by diverse kinase families. Our data revealed,
with the exception of 5-HT3 antagonists, treatment with many of the
antagonists resulted in reductions in substrate phosphorylation for
signaling pathways including CDK, MAPK, and AKT (Fig. 5B).
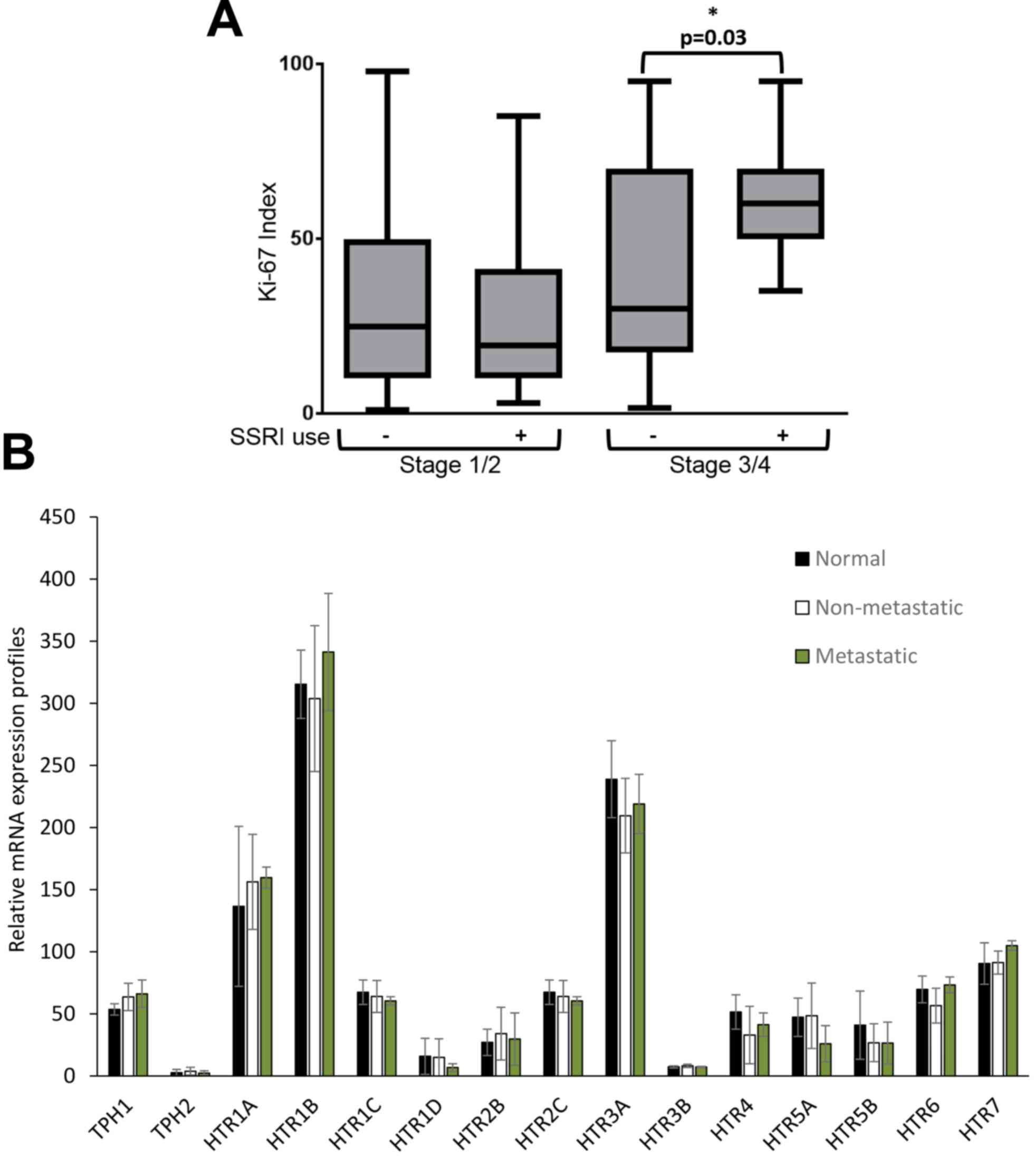
SSRI use is associated with increased
tumor cell proliferation in late stage breast cancer patients
We carried out a retrospective clinical analysis of
419 patients diagnosed with breast cancer to assess the association
between the use of SSRIs and breast tumor proliferation rates.
Patients were stratified based on SSRI use. A description of the
various SSRIs taken by these patients is exhibited in Table IV. The Ki-67-based proliferative
index was determined from pathology samples taken at each patient's
diagnostic biopsy. No difference was found in tumor staging or
hormone receptor status between users of SSRIs and non-users;
however, in patients with late stage breast cancer, use of SSRIs
was significantly associated with increased tumor proliferative
index (2.3-fold increase) compared to patients who were non-users
of SSRIs (P=0.03) (Table V, Fig. 6A). To determine if expression changes
in 5HT receptors or biosynthetic enzymes contribute to
SSRI-dependent alterations in proliferation rates between early and
late stage breast cancer, meta-analysis of HTR and TPH genes was
performed for normal mouse breast tissue, non-metastatic breast
tumors from 67NR xenografts, and metastatic breast tumors from 4T1
×enografts based on data from the Gene Expression Omnibus (GEO cat.
no. GSE62817). No statistical difference in expression patterns was
observed for HTR or TPH mRNAs between normal, non-metastatic, and
metastatic breast tissue (Fig.
6B).
 | Table IV.Selective serotonin reuptake
inhibitor (SSRI) usage identified in the retrospective study. |
Table IV.
Selective serotonin reuptake
inhibitor (SSRI) usage identified in the retrospective study.
| SSRI | No. of
patients | Dose range |
|---|
| Escitalopram | 4 | 10-20 mg daily |
| Fluoxetine | 1 | 20 mg daily |
| Paroxetine | 1 | 20 mg daily |
| Sertraline | 21 | 25-100 mg
daily |
| Unknown | 1 | Unknown |
 | Table V.Association between SSRI usage and
clinicopathological characteristics in breast cancer patients. |
Table V.
Association between SSRI usage and
clinicopathological characteristics in breast cancer patients.
|
Characteristics | No SSRIs n=391
(93.3%) | SSRIs n=28
(6.7%) | P-value | Significant |
|---|
| Tumor stage |
| Stage
I/II | 281 | 20 | 0.98 | No |
| Stage
III/IV | 110 | 8 |
|
|
| Hormonal
status |
| ER (no.
of patients) |
| – | 101 | 11 | 0.58 | No |
| + | 274 | 17 |
|
|
| PR (no.
of patients) |
| – | 144 | 11 | 0.93 | No |
| + | 231 | 16 |
|
|
| HER2 (no. of
patients) |
| – | 100 | 8 | 0.80 | No |
| + | 257 | 19 |
|
|
| Ki-67 index |
| Stage
I/II mean (SEM) | 33.32 (1.61) | 27.10 (5.46) | 0.29 | No |
| Stage
III/IV mean (SEM) | 42.37 (2.66) | 62.14 (6.97) | 0.03 | Yes |
Discussion
In this study, we demonstrated that several 5-HT
receptors are expressed across a diverse array of cancers, and that
serotonin signaling through the 5-HT receptors plays a role in the
control of cancer cell viability through modulating key mitogenic
signaling pathways. Furthermore, we revealed that use of SSRIs at
the time of breast cancer diagnosis is correlated with increased
tumor proliferative indices in late stages of the disease.
Expression of 5-HT receptors has been reported in
hepatocellular carcinoma, leiomyosarcoma, and ovarian and breast
cancer (6–8,17,31–34).
Moreover, 5-HT1D, 5-HT2B and 5-HT7 receptors are overexpressed in
hepatocellular carcinoma (7) and
5-HT1A and B are correlated with high Gleason grades and metastasis
in prostate cancer (23,25,26).
Increases in serum serotonin itself have been shown to serve as a
marker for early stage hepatocellular carcinoma development
(35). In the current study, we took
advantage of existing gene expression and pathology databases and
analyzed the expression of HTR mRNAs and their 5-HT receptor
protein products across a large panel of cell lines and tissues
representing the most common cancers in humans. We observed clear
gene expression clustering of multiple HTR mRNAs based on
cancer cell line origin and that many of the 5-HT receptors were
present in the majority of the cancer types examined. While the
Human Protein Atlas database did not house enough matching normal
controls for statistical analysis of over/underexpression of the
5-HT receptors in cancer, future studies should take advantages of
tumor tissue array technologies to comprehensively evaluate this
possibility in order to identify specific tumor types that may show
benefit from blocking serotonin signaling.
A handful of molecular studies have attempted to
identify downstream signaling mediators of the 5-HT receptors that
contribute to serotonin-induced tumor growth. One study identified
gut-derived serotonin stimulation of RUNX2, a transcription factor
involved in bone and cartilage development and maintenance, as a
facilitator for breast cancer metastasis to the bone (19). Moreover, serotonin has been shown to
promote the activation of β catenin (7,17), a
protein known to induce tumor cell growth, migration, and
pluripotency (36). A meta-analysis
of the Metabric dataset, which characterized the genomic landscape
of 2000 breast cancer patients, identified active serotonin
metabolism as a major metabolic feature of the poor prognosis
cluster of patients (37), and
serotonin has been shown to contribute to pancreatic tumor growth
promotion via its regulation of the Warburg effect in cells under
metabolic stress (9). Serotonin may
exert its effect not only on the tumor cells, but also on the tumor
stroma as this neurotransmitter enhances tumor growth via
modulation of the angiogenic properties of tumor endothelial cells
(12,38,39). In
the current study, we did not observe serotonin-mediated increases
in tumor cell proliferation for a panel of breast cancer, soft
tissue sarcoma, and bone sarcoma cells, however the addition of
this neurotransmitter did indeed enhance the activating
phosphorylation of key mitogenic regulators in cancer cells.
Through the use of pharmacological inhibitors that
selectively and specifically block individual 5-HT receptors, roles
for several of the 5-HT receptors have been implicated in cancer
cell proliferation. For instance, 5-HT1 receptors are essential for
proliferation of bladder cancer, colorectal cancer, leiomyosarcoma,
and small cell lung carcinoma (15,32,40);
5-HT2 receptors for breast and prostate cancer proliferation
(25,34); 5-HT3 receptors for breast and
colorectal cancer proliferation (34,41);
5-HT4 receptors for prostate cancer proliferation (25); and 5-HT7 receptors for hepatocellular
carcinoma proliferation (7). Despite
the number of studies that examined only one or two of the 5-HT
receptors, no report, to our knowledge, has described the efficacy
of comprehensively blocking each 5-HT receptor across a panel of
cancer cell lines. The current study individually blocked the
activation of each known 5-HT receptor using highly selective
pharmacological antagonists, revealing dose dependent decreases in
tumor cell viability across most cell lines when treating with
inhibitors of 5-HT1, 4, 5, 6 and 7. The viability of some cell
lines, such as the SK-BR-3 breast cancer cell line, were not
greatly affected by the antagonists, suggesting cell-type
dependence on some 5-HT receptors, but not on others. Selective
inhibition of the 5-HT7 receptor resulted in increased levels of
activated p53 and decreased levels of active MAPKs, as well as
reduced tumor size following treatment of a CAM angiosarcoma tumor
model. Based on kinome-level profiling, the majority of 5-HT
receptor antagonists reduced the activation of downstream signaling
proteins involved in proliferation and survival. In contrast,
inhibition of 5-HT3 (which had no effect on tumor cell viability in
our assays) exhibited opposite results, resulting in increased
activation of signaling proteins involved in proliferation and
survival. 5-HT3 is markedly distinct both structurally and
functionally from the other 5-HT receptors. For instance, while all
other 5-HT receptors are G-protein coupled receptors, 5-HT3 is a
ligand gated ion channel that is permeable to sodium, potassium,
and calcium ions (42).
Given that several selective 5-HT receptor
antagonists (Ketanserin, Clozapine, Agomelatine, Buspirone, etc.)
are clinically used for conditions including hypertension, anxiety,
depression, and psychosis, and this class of drugs is one of the
most widely prescribed therapeutics, it seems logical that
retrospective and/or prospective analysis could easily determine if
a correlation exists between use of these SSRIs and cancer
risk/prognosis; however retrospective clinical analysis of patient
data attempting to correlate cancer survival or time to progression
with SSRI usage have led to mixed results. For instance, 33%
(20/61) of studies have found a positive correlation between
antidepressant use and breast or ovarian cancer (43), however many of these studies were
supported financially by pharmaceutical sponsors and freedom from
bias could not be confirmed. On closer examination, industry-backed
studies were significantly less likely than those funded by
non-industry financial streams to report a correlation between
antidepressants and cancer risk (0% [0/15] for industry-back
studies; 43.5% [20/46] for non-industry backed studies) (43). Similarly conflicting results have
been reported outside of breast or ovarian cancer, with no
SSRI-mediated decreases in patient survival or time to disease
progression observed for oral cancer (44), gastric cancer (45), cervical cancer (46), colorectal cancer (47), glioblastoma (48), or hepatocellular carcinoma (49). In contrast, SSRI use has been
correlated with an enhanced risk of lung, colorectal, and prostate
cancer (50–53), and increased mortality rates and
tumor metastasis in melanoma (10,54). Our
findings from this report support the notion that SSRI use may
contribute to enhanced tumor growth, given that we observed SSRIs
were associated with a significantly increased breast tumor
proliferative index in late stage cancer patients. Future studies
should attempt to prospectively correlate SSRI use with
accompanying changes in tumor proliferation and intracellular
signaling pathways.
Interestingly, our data revealed that SSRI-use did
not influence tumor proliferation rates in early stage breast
tumors. Collectively, these many conflicting studies indicate that
while serotonin signaling and use of SSRIs may contribute to some
degree toward cancer progression, future studies are necessary to
elucidate these conundrums. We demonstrated that the mRNA
expression of neither HTR nor TPH genes were significantly
different between normal breast tissue and non-metastatic or
metastatic xenograft breast tumor models. Unaccounted and
uncontrolled factors that may contribute to the use of SSRIs could
confound the outcome of these studies. Such factors could include
lifestyle (e.g., diet), use of prescription and/or non-prescription
drugs, and comorbidities (e.g., diabetes or heart disease)-all of
which potentially could lead to increased SSRI use, and all of
which could be attributed to a later stage of cancer at diagnosis
and worse patient prognosis completely independent of SSRI use.
Along these lines, a growing amount of literature has shown that
stress in general, and specifically sympathetic nervous system
responses through epinephrine and/or norepinephrine regulation of
the β adrenergic receptor pathways have been implicated in cancer
progression (29,55–57).
Indeed, targeting the β adrenergic stress response pathways has
shown clinical efficacy against benign vascular tumors (58,59) as
well as rare, lethal sarcomas (60–63). It
is very possible that greater psychological stress in late stage
patients, which impacts a number of physiological pathways and
would be factor leading to higher SSRI use, is a confounding
contributor to increased cancer risk and poor clinical outcomes
associated with antidepressant use.
Our findings suggest that serotonin influences tumor
cell viability and behavior at the cellular level. Whether this
translates to clinical outcomes needs to be confirmed in future
randomized trials given the mixed results reported from a large
number of retrospective studies and the likely confounders
associated with lifestyle, drug use, comorbidities, or overall
stress levels. The data presented here and those from other
laboratories suggest an underlying psychophysiological regulation
of tumor cells, yet how these characteristics manifest at the
clinical level is yet to be definitively determined. Pending
further studies, the likely association between SSRIs and worsening
cancer outcome should be a reason to pause, especially in view of
availability of other lines of medications for depression and
anxiety that do not depend on similar serotonin-dependent
pathways.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants to BAB
from the Sarcoma Foundation of America, Angiosarcoma Awareness
Foundation to EBD from the Sarcoma Foundation of America, and to ZN
from the Cancer Prevention and Research Institute of Texas (CPRIT
RP120528).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Cancer Cell Line Encyclopedia
(CCLE; www.broadinstitute.org/ccle/home), Human Protein Atlas
repository (www.proteinatlas.org) and Gene Expression Omnibus
(GEO) repository (https://www.ncbi.nlm.nih.gov/geo/; GEO no. GSE62817).
All other data are included in this published article.
Authors' contributions
YB, AR, AB, LP, CAN, SL and TK acquired and
interpreted the data. ERD, ZN conceived and designed the study and
critically revised the manuscript. BAB conceived and designed the
study and drafted the manuscript.
Ethics approval and consent to
participate
Retrospective analysis of clinical data was
performed with the approval of the Texas Tech University Health
Sciences Center Institutional Review Board. All animal experiments
used embryonated eggs prior to day 16, therefore these experiments
were considered exempt from approval by the Texas Tech University
Health Sciences Center Institutional Animal Care and Use Committee
regulations for the care and use of animals in experimental
procedures.
Patient consent for publication
Not applicable.
Competing of interests
The authors declare no conflict of interest.
References
|
1
|
Sanjida S, Janda M, Kissane D, Shaw J,
Pearson SA, DiSipio T and Couper J: A systematic review and
meta-analysis of prescribing practices of antidepressants in cancer
patients. Psychooncology. 25:1002–1016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Collet C, Schiltz C, Geoffroy V, Maroteaux
L, Launay JM and de Vernejoul MC: The serotonin 5-HT2B receptor
controls bone mass via osteoblast recruitment and proliferation.
FASEB J. 22:418–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fanburg BL and Lee SL: A new role for an
old molecule: Serotonin as a mitogen. Am J Physiol. 272:L795–L806.
1997.PubMed/NCBI
|
|
4
|
Lesurtel M, Graf R, Aleil B, Walther DJ,
Tian Y, Jochum W, Gachet C, Bader M and Clavien PA:
Platelet-derived serotonin mediates liver regeneration. Science.
312:104–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raymond JR, Mukhin YV, Gelasco A, Turner
J, Collinsworth G, Gettys TW, Grewal JS and Garnovskaya MN:
Multiplicity of mechanisms of serotonin receptor signal
transduction. Pharmacol Ther. 92:179–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christensen DK, Armaiz-Pena GN, Ramirez E,
Matsuo K, Zimmerman B, Zand B, Shinn E, Goodheart MJ, Bender D,
Thaker PH, et al: SSRI use and clinical outcomes in epithelial
ovarian cancer. Oncotarget. 7:33179–33191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fatima S, Shi X, Lin Z, Chen GQ, Pan XH,
Wu JC, Ho JW, Lee NP, Gao H, Zhang G, et al: 5-Hydroxytryptamine
promotes hepatocellular carcinoma proliferation by influencing
β-catenin. Mol Oncol. 10:195–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gautam J, Bae YK and Kim JA: Up-regulation
of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent
serotonin signaling correlates with triple negativity of human
breast cancer. Breast Cancer Res. 161:29–40. 2017. View Article : Google Scholar
|
|
9
|
Jiang SH, Li J, Dong FY, Yang JY, Liu DJ,
Yang XM, Wang YH, Yang MW, Fu XL, Zhang XX, et al: Increased
serotonin signaling contributes to the warburg effect in pancreatic
tumor cells under metabolic stress and promotes growth of
pancreatic tumors in mice. Gastroenterology. 153:277–291. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubera M, Grygier B, Wrona D, Rogóż Z,
Roman A, Basta-Kaim A, Budziszewska B, Leskiewicz M, Jantas D,
Nowak W, et al: Stimulatory effect of antidepressant drug
pretreatment on progression of B16F10 melanoma in high-active male
and female C57BL/6J mice. J Neuroimmunol 240-241. 1–44. 2011.
|
|
11
|
Liang C, Chen W, Zhi X, Ma T, Xia X, Liu
H, Zhang Q, Hu Q, Zhang Y, Bai X and Liang T: Serotonin promotes
the proliferation of serum-deprived hepatocellular carcinoma cells
via upregulation of FOXO3a. Mol Cancer. 12:142013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peters MA, Walenkamp AM, Kema IP, Meijer
C, de Vries EG and Oosting SF: Dopamine and serotonin regulate
tumor behavior by affecting angiogenesis. Drug Resist Updat.
17:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarrouilhe D, Clarhaut J, Defamie N and
Mesnil M: Serotonin and cancer: What is the link? Curr Mol Med.
15:62–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siddiqui EJ, Shabbir M, Mikhailidis DP,
Thompson CS and Mumtaz FH: The role of serotonin
(5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell
proliferation. J Urol. 176:1648–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui EJ, Shabbir MA, Mikhailidis DP,
Mumtaz FH and Thompson CS: The effect of serotonin and serotonin
antagonists on bladder cancer cell proliferation. BJU Int.
97:634–639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siddiqui EJ, Thompson CS, Mikhailidis DP
and Mumtaz FH: The role of serotonin in tumour growth (review).
Oncol Rep. 14:1593–1597. 2005.PubMed/NCBI
|
|
17
|
Sui H, Xu H, Ji Q, Liu X, Zhou L, Song H,
Zhou X, Xu Y, Chen Z, Cai J, et al: 5-hydroxytryptamine receptor
(5-HT1DR) promotes colorectal cancer metastasis by regulating
Axin1/β-catenin/MMP-7 signaling pathway. Oncotarget. 6:25975–25987.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vicaut E, Laemmel E and Stucker O: Impact
of serotonin on tumour growth. Ann Med. 32:187–194. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zong JC, Wang X, Zhou X, Wang C, Chen L,
Yin LJ, He BC and Deng ZL: Gut-derived serotonin induced by
depression promotes breast cancer bone metastasis through the
RUNX2/PTHrP/RANKL pathway in mice. Oncol Rep. 35:739–748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Julius D, Huang KN, Livelli TJ, Axel R and
Jessell TM: The 5HT2 receptor defines a family of structurally
distinct but functionally conserved serotonin receptors. Proc Natl
Acad Sci USA. 87:928–932. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Julius D, Livelli TJ, Jessell TM and Axel
R: Ectopic expression of the serotonin 1c receptor and the
triggering of malignant transformation. Science. 244:1057–1062.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jungwirth N, Haeberle L, Schrott KM,
Wullich B and Krause FS: Serotonin used as prognostic marker of
urological tumors. World J Urol. 26:499–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dizeyi N, Bjartell A, Nilsson E, Hansson
J, Gadaleanu V, Cross N and Abrahamsson PA: Expression of serotonin
receptors and role of serotonin in human prostate cancer tissue and
cell lines. Prostate. 59:328–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Launay JM, Birraux G, Bondoux D, Callebert
J, Choi DS, Loric S and Maroteaux L: Ras involvement in signal
transduction by the serotonin 5-HT2B receptor. J Biol Chem.
271:3141–3147. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dizeyi N, Bjartell A, Hedlund P, Tasken
KA, Gadaleanu V and Abrahamsson PA: Expression of serotonin
receptors 2B and 4 in human prostate cancer tissue and effects of
their antagonists on prostate cancer cell lines. Eur Urol.
47:895–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dizeyi N, Hedlund P, Bjartell A, Tinzl M,
Austild-Tasken K and Abrahamsson PA: Serotonin activates MAP kinase
and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol
Oncol. 29:436–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The cancer cell line encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Modiano JF: Comparative pathogenesis of
cancers in animals and humans. Vet Sci. 3:2016.PubMed/NCBI
|
|
29
|
Stiles JM, Amaya C, Rains S, Diaz D, Pham
R, Battiste J, Modiano JF, Kokta V, Boucheron LE, Mitchell DC and
Bryan BA: Targeting of beta adrenergic receptors results in
therapeutic efficacy against models of hemangioendothelioma and
angiosarcoma. PLoS One. 8:e600212013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spencer C, Montalvo J, McLaughlin SR and
Bryan BA: Small molecule inhibition of cytoskeletal dynamics in
melanoma tumors results in altered transcriptional expression
patterns of key genes involved in tumor initiation and progression.
Cancer Genomics Proteomics. 8:77–85. 2011.PubMed/NCBI
|
|
31
|
Gautam J, Banskota S, Regmi SC, Ahn S,
Jeon YH, Jeong H, Kim SJ, Nam TG, Jeong BS and Kim JA: Tryptophan
hydroxylase 1 and 5-HT7 receptor preferentially expressed in
triple-negative breast cancer promote cancer progression through
autocrine serotonin signaling. Mol Cancer. 15:752016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gurbuz N, Asoglu MR, Ashour AA, Salama S,
Kilic GS and Ozpolat B: A selective serotonin 5-HT1B receptor
inhibition suppresses cells proliferation and induces apoptosis in
human uterine leiomyoma cells. Eur J Obstet Gynecol Reprod Biol.
206:114–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gwynne WD, Hallett RM, Girgis-Gabardo A,
Bojovic B, Dvorkin-Gheva A, Aarts C, Dias K, Bane A and Hassell JA:
Serotonergic system antagonists target breast tumor initiating
cells and synergize with chemotherapy to shrink human breast tumor
xenografts. Oncotarget. 8:32101–32116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hejazi SH, Ahangari G and Deezagi A:
Alternative Viewpoint Against Breast Cancer Based on Selective
Serotonin Receptors 5HTR3A and 5HTR2A Antagonists that can Mediate
Apoptosis in MCF-7 Cell Line. Curr Drug Discov Technol. 12:240–249.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdel-Hamid NM, Shehata DE, Abdel-Ghany
AA, Ragaa A and Wahid A: Serum serotonin as unexpected potential
marker for staging of experimental hepatocellular carcinoma. Biomed
Pharmacother. 83:407–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kourtidis A, Lu R, Pence LJ and
Anastasiadis PZ: A central role for cadherin signaling in cancer.
Exp Cell Res. 358:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leoncikas V, Wu H, Ward LT, Kierzek AM and
Plant NJ: Generation of 2,000 breast cancer metabolic landscapes
reveals a poor prognosis group with active serotonin production.
Sci Rep. 6:197712016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asada M, Ebihara S, Yamanda S, Niu K,
Okazaki T, Sora I and Arai H: Depletion of serotonin and selective
inhibition of 2B receptor suppressed tumor angiogenesis by
inhibiting endothelial nitric oxide synthase and extracellular
signal-regulated kinase 1/2 phosphorylation. Neoplasia. 11:408–417.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banskota S, Gautam J, Regmi SC, Gurung P,
Park MH, Kim SJ, Nam TG, Jeong BS and Kim JA: BJ-1108, a
6-amino-2,4,5-trimethylpyridin-3-ol analog, inhibits
serotonin-induced angiogenesis and tumor growth through PI3K/NOX
pathway. PLoS One. 11:e01481332016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cattaneo MG, Palazzi E, Bondiolotti G and
Vicentini LM: 5-HT1D receptor type is involved in stimulation of
cell proliferation by serotonin in human small cell lung carcinoma.
Eur J Pharmacol. 268:425–430. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ataee R, Ajdary S, Zarrindast M, Rezayat
M, Shokrgozar MA and Ataee A: Y25130 hydrochloride, a selective
5HT3 receptor antagonist has potent antimitogenic and apoptotic
effect on HT29 colorectal cancer cell line. Eur J Cancer Prev.
19:138–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Engel M, Smidt MP and van Hooft JA: The
serotonin 5-HT3 receptor: A novel neurodevelopmental target. Front
Cell Neurosci. 7:762013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cosgrove L, Shi L, Creasey DE,
Anaya-McKivergan M, Myers JA and Huybrechts KF: Antidepressants and
breast and ovarian cancer risk: A review of the literature and
researchers' financial associations with industry. PLoS One.
6:e182102011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung CM, Kuo TM, Chiang SL, Wang ZH, Hung
CC, Lane HY, Liu CS and Ko YC: Antidepressants in association with
reducing risk of oral cancer occurrence: A nationwide
population-based cohort and nested case-control studies.
Oncotarget. 7:11687–11695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsieh YH, Chiu WC, Lin CF, Chan HL, Liang
HY, Lee Y, McIntyre RS and Chen VC: Antidepressants and gastric
cancer: A nationwide population-based nested case-control study.
PLoS One. 10:e01436682015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan HL, Hsieh YH, Lin CF, Liang HY, Huang
KY, Chiu WC, Lee Y, McIntyre RS and Chen VC: Invasive cervical
cancer and antidepressants: A nationwide population-based study.
Medicine. 94:e18662015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee HC, Chiu WC, Wang TN, Liao YT, Chien
IC, Lee Y, McIntyre RS, Chen PC and Chen VC: Antidepressants and
colorectal cancer: A population-based nested case-control study. J
Affect Disord. 207:353–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Caudill JS, Brown PD, Cerhan JH and
Rummans TA: Selective serotonin reuptake inhibitors, glioblastoma
multiforme and impact on toxicities and overall survival: The mayo
clinic experience. Am J Clin Oncol. 34:385–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pocha C, Knott A, Rector TS and Dieperink
E: Are selective serotonin reuptake inhibitors associated with
hepatocellular carcinoma in patients with hepatitis C? J Clin
Psychiatry. 75:e1122–e1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boursi B, Lurie I, Haynes K, Mamtani R and
Yang YX: Chronic therapy with selective serotonin reuptake
inhibitors and survival in newly diagnosed cancer patients. Eur J
Cancer Care(Engl). 27:e126662018. View Article : Google Scholar
|
|
51
|
Boursi B, Lurie I, Mamtani R, Haynes K and
Yang YX: Anti-depressant therapy and cancer risk: A nested
case-control study. Eur Neuropsychopharmacol. 25:1147–1157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Haukka J, Sankila R, Klaukka T, Lonnqvist
J, Niskanen L, Tanskanen A, Wahlbeck K and Tiihonen J: Incidence of
cancer and antidepressant medication: Record linkage study. Int J
Cancer. 126:285–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu W, Tamim H, Shapiro S, Stang MR and
Collet JP: Use of antidepressants and risk of colorectal cancer: A
nested case-control study. Lancet Oncol. 7:301–308. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Brandes LJ, Arron RJ, Bogdanovic RP, Tong
J, Zaborniak CL, Hogg GR, Warrington RC, Fang W and LaBella FS:
Stimulation of malignant growth in rodents by antidepressant drugs
at clinically relevant doses. Cancer Res. 52:3796–3800.
1992.PubMed/NCBI
|
|
55
|
Montoya A, Amaya CN, Belmont A, Diab N,
Trevino R, Villanueva G, Rains S, Sanchez LA, Badri N, Otoukesh S,
et al: Use of non-selective β-blockers is associated with decreased
tumor proliferative indices in early stage breast cancer.
Oncotarget. 8:6446–6460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Watkins JL, Thaker PH, Nick AM, Ramondetta
LM, Kumar S, Urbauer DL, Matsuo K, Squires KC, Coleman RL,
Lutgendorf SK, et al: Clinical impact of selective and nonselective
beta-blockers on survival in patients with ovarian cancer. Cancer.
121:3444–3451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Leaute-Labreze C, Hoeger P,
Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ,
Caceres H, Lopez Gutierrez JC, Ballona R, et al: A randomized,
controlled trial of oral propranolol in infantile hemangioma. N
Engl J Med. 372:735–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Leaute-Labreze C and Taieb A: Efficacy of
beta-blockers in infantile capillary haemangiomas: The
physiopathological significance and therapeutic consequences. Ann
Dermatol Venereol. 135:860–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Banavali S, Pasquier E and Andre N:
Targeted therapy with propranolol and metronomic chemotherapy
combination: Sustained complete response of a relapsing metastatic
angiosarcoma. Ecancermedicalscience. 9:4992015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chow W, Amaya CN, Rains S, Chow M,
Dickerson EB and Bryan BA: Growth attenuation of cutaneous
angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol.
151:1226–1229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Daguze J, Saint-Jean M, Peuvrel L,
Cassagnau E, Quéreux G, Khammari A and Dréno B: Visceral metastatic
angiosarcoma treated effectively with oral cyclophosphamide
combined with propranolol. JAAD Case Rep. 2:497–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pasquier E, Andre N, Street J, Chougule A,
Rekhi B, Ghosh J, Philip DSJ, Meurer M, MacKenzie KL, Kavallaris M
and Banavali SD: Effective management of advanced angiosarcoma by
the synergistic combination of propranolol and vinblastine-based
metronomic chemotherapy: A bench to bedside study. EBioMedicine.
6:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|