Introduction
Breast cancer (BC) is the most common malignancy and
the leading cause of cancer death in women worldwide. Approximately
75% of BCs are hormone receptor positive (HR+), HER2-negative
(HER2-); systemic therapy with endocrine agents represents the
mainstay of treatment both in the early and advanced stages of
disease (1,2).
Considering the efficacy and the favorable safety
profile of endocrine-directed agents, sequential lines of endocrine
therapy (ET) should be the preferred treatment strategy in the
advanced setting, except in the case of immediate life-threatening
disease or rapid visceral recurrence during adjuvant ET (3,4).
Despite the effectiveness of ET in HR+ advanced BC,
disease progression occurs in the majority of patients due to
primary or acquired ET resistance.
In the last two decades, loss of estrogen receptor
(ER) expression, ER mutations, alterations in co-regulatory
proteins, and the upregulation of different signal transduction
pathways have been identified as mechanisms leading to ET failure
(5).
Among these mechanisms of resistance, cross-talk
between the phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(AKT)/mTOR axis and ER signaling plays a key role in BC
proliferation and progression and confers endocrine insensitivity
to ET.
Preclinical and clinical studies have shown that
co-targeting downstream elements of this pathway using mammalian
target of rapamycin (mTOR) inhibitors may synergistically increase
the antitumoral activity of ET and overcome anti-hormone therapy
resistance in BCs (6–8).
Everolimus (Eve) is a mTOR inhibitor which induces
apoptosis by blocking S6K1 and 4E-BP1 activation and inhibits cell
growth, proliferation, and G1-S transition. Eve in combination with
Exemestane (Exe), a steroidal aromatase inhibitor (SAI), was
approved in 2012 based on the results of the pivotal BOLERO-2
trial. In this study, dual-blockade significantly improved
progression-free survival (PFS), as compared with Exe alone, in
non-steroidal AI (NSAI)-pretreated post-menopausal patients
affected by HR+/HER2-MBC, while maintaining health-related quality
of life (HRQoL) (9–11).
Over the past two years, a new class of drugs,
namely, CDK4/6 inhibitors, in combination with letrozole or
fulvestrant as first or second-line therapy, respectively, has
increasingly been used in the treatment of luminal MBC disease.
Despite the impressive results from recently published studies
reporting a significant PFS benefit with CDK4-6 inhibitors
(palbociclib, ribociclib, abemaciclib) in combination with ET
compared with ET alone, overall survival (OS) data are still
awaited and the optimal sequence of ET, as monotherapy or in
combination with targeted agents, is still not well established
(12–15). However, combination of CDK4-6
inhibitors and letrozole is reasonably expected to become the new
gold standard as first-line therapy in post-menopausal patients
with HR+/HER2-endocrine sensitive MBC.
Nevertheless, it is crucial to acquire data about
long-term outcomes and toxicity profile of therapeutic options
currently used in everyday clinical practice in order to define the
best therapeutic strategy in HR+/HER2-advanced breast cancer (ABC).
Of note, the ongoing phase III randomized trial GIM-16 FEVEX
(EudraCT n. 2014-004035-38), where MBCs have been randomized to
receive Exe-Eve, followed, in case of progression disease, by
fulvestrant or vice versa, should define the best therapeutic
sequence in second and third line.
In our multicenter observational study, we
retrospectively analyzed data from 264 HR+/HER2-MBC patients who
received Exe-Eve combination, following NSAI failure, in different
lines of hormonal treatment to evaluate the efficacy and
tolerability of this combination in the ‘real world’ setting.
Patients and methods
The main aim of our retrospective study was the
analysis of activity, efficacy, and safety of Exe-Eve treatment
according to the line of therapy. From January 2012 to January
2017, 264 patients with HR+/HER-MBC who received Exe-Eve as first
or further line of ET, were eligible for the final analysis. For
all patients, inclusion criteria were histologically confirmed
diagnosis of HR+ and HER-locally advanced or MBC, age ≥18 years,
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
≤2, basal screening for hepatitis B and C, administration of at
least one cycle of Exe-Eve until disease progression, unacceptable
toxicity or patient refusal, availability of clinical-pathological,
radiologic, and laboratory parameters before Exe-Eve treatment,
response evaluation, and survival data.
Furthermore, prior therapy with AI was permitted if
recurrence had occurred during or within 12 months of completion of
adjuvant therapy, or in case of progression during treatment for
advanced stage disease; likewise, previous chemotherapy for
metastatic disease and palliative radiotherapy on bone and/or brain
were permitted. Eve starting dose was 10 mg once a day orally; a
dose reduction (5 mg) was chosen by the caring physician in some
instances. Supportive measures were allowed and implemented
according to individual daily practice. Toxicity data were
collected monthly at each patient visit and were classified
according to the National Cancer Institute Common Criteria for
Adverse Events (NCI-CTCAE), version 4 (16).
Accordingly, in case of toxicity, dose adjustments
or temporary interruptions of therapy as well as timing and
modality of evaluation were independently implemented by each
investigator (as a general rule, Eve dose was reduced for toxicity
of grade 2 or higher, while temporary interruption of treatment was
proposed for mild toxicities; tumor assessment was carried out
every 3 months). Data were retrieved after an anonymization
procedure from a centralized database at the Breast Unit of
Cardarelli Hospital. Four patients were lost to follow-up, and the
study was completed by January 31, 2017. All patients provided
written informed consent about the use of their data for future
medical research. The Institutional Review Board at ‘F. Magrassi’
Department of Clinical and Experimental Medicine of ‘Luigi
Vanvitelli’ University of Campania, Naples (Italy) approved the
study.
Statistical analysis
All continuous data were expressed as mean ± SD,
range and median value; frequencies and percentages were reported
for categorical variables. Fisher's exact test was used to analyze
associations with categorical variables. Survival distribution was
estimated by the Kaplan-Meier method with 95% confidence interval
(CI) (17). Progression Free
Survival (PFS) was defined as the time elapsed between the first
Exe-Eve dose to the detection of disease progression or death for
any cause. Patients who died of causes other than breast
cancer-without experiencing tumor progression-were regarded as
censored events at the date of death when computing the PFS rate.
Differences in PFS according to clinical parameters or line of
treatment were evaluated by the log-rank test and described by the
Kaplan-Meier method. For final analysis, the PFS status of all
patients was updated within 1 month of January 2017 deadline.
Overall survival (OS) was defined as the time from the first cycle
of therapy with Exe-Eve to the date of death or last contact. Cox
proportional-hazards model was applied to multivariate survival
analysis, and P-values and hazard ratios (HRs) with 95% CI were
obtained. All significant variables in the univariate model were
used to build the multivariate model of survival. Statistical
Package for the Social Sciences (SPSS) 20.0 software, (Chicago, IL,
USA) was used for statistical analysis and integrated with Medcalc
software V.9.4.2.0 (Mariakerke, Belgium). In all analyses, the
significance level was specified as P<0.05.
Results
Efficacy
Exe-Eve combination was used as first, second, or
third line of ET in 45 (17%), 115 (43.6%), and 104 (39.4%) patients
with MBC (total: 264; median age 56 years, range 49-64),
respectively. Of these, 192 (72.7%) and 229 (86.7%) received
adjuvant chemotherapy and ET, respectively. Furthermore, 128
patients (48.5%) treated with chemotherapy as first line treatment
for metastatic disease subsequently received Exe-Eve combination as
first (3 patients), second (56 patients), or third (69 patients)
hormonal line. The main characteristics of the series are reported
in Table I. At the end of the study,
104 patients (39.4%) had died and 156 patients (59.1%) were still
alive, while 4 (1.5%) patients were lost to follow-up; thus, 260
patients (43, 114, and 103 in first, second, or third line of
treatment, respectively) were eventually considered for survival
analysis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Exe-Eve as I line of
ET (%) | Exe-Eve as II line of
ET (%) | Exe-Eve as III line
of ET (%) | Total (%) | P-value |
|---|
| Total | 45 | 115 | 104 | 264 |
|
| Age (years) |
|
|
|
| 0.091 |
| ≤48 | 6 (13.3) | 31 (27.0) | 28 (26.9) | 65 (24.6) |
|
|
49-64 | 25 (53.3) | 65 (56.5) | 58 (55.8) | 147 (55.7) |
|
| ≥65 | 15 (33.3) | 19 (16.5) | 18 (17.3) | 52 (19.7) |
|
| ECOG PS |
|
|
|
| 0.724 |
| 0 | 37 (82.2) | 100 (87.0) | 90 (86.5) | 227 (86) |
|
| 1-2 | 8 (17.8) | 15 (13.0) | 14 (13.5) | 37 (14.0) |
|
| Surgery (primary
tumor) |
|
|
|
| 0.082 |
|
Quadrantectomy | 28 (62.2) | 56 (48.7) | 44 (42.3) | 128 (48.5) |
|
|
Mastectomy/biopsy | 17 (37.8) | 59 (51.3) | 60 (57.7) | 136 (51.5) |
|
| pT |
|
|
|
| 0.082 |
| 1.2 | 17 (37.8) | 91 (79.1) | 72 (69.2) | 201 (76.1) |
|
|
3.4.x | 7 (15.6) | 24 (20.9) | 32 (30.8) | 63 (23.9) |
|
| pN |
|
|
|
| 0.211 |
| 0 | 18 (40.0) | 24 (20.8) | 18 (17.3) | 60 (22.7) |
|
| 1 | 10 (22.2) | 26 (22.6) | 26 (25.0) | 62 (23.5) |
|
| 2 | 8 (17.8) | 34 (29.5) | 29 (27.8) | 71 (26.9) |
|
| 3 | 5 (11.1) | 21 (18.2) | 20 (19.2) | 46 (17.4) |
|
| X | 4 (8.8) | 10 (8.6) | 11 (6.7) | 25 (9.5) |
|
| Grading |
|
|
|
| 0.510 |
|
G1-G2 | 17 (37.8) | 52 (45.2) | 50 (48.1) | 119 (45.1) |
|
| G3 | 28 (62.2) | 63 (54.8) | 54 (51.9) | 145 (54.9) |
|
| ER, median
(IQR) | 80 (70-90) | 80 (70-90) | 80 (60-90) | 80 (70-90) | 0.252 |
| PgR, median
(IQR) | 60 (30-80) | 60 (30-80) | 60 (20-80) | 60 (30-80) | 0.804 |
| KI67 |
|
|
|
| 0.852 |
|
≤20% | 22 (48.9) | 56 (48.7) | 47 (45.2) | 125 (47.4) |
|
|
>20% | 23 (51.1) | 59 (51.3) | 57 (54.8) | 139 (52.7) |
|
| Adjuvant
chemotherapy |
|
|
|
| 0.641 |
|
Yes | 35 (77.8) | 81 (70.4) | 76 (73.1) | 192 (72.7) |
|
| No | 10 (22.2) | 34 (29.6) | 28 (26.9) | 72 (27.3) |
|
| Adjuvant
radiotherapy |
|
|
|
| 0.002 |
|
Yes | 38 (84.4) | 64 (60) | 56 (53.9) | 163 (61.7) |
|
| No | 7 (15.6) | 46 (40) | 48 (46.2) | 101 (38.3) |
|
| Adjuvant ET |
|
|
|
| 0.015 |
|
Yes | 45 (100) | 96 (83.5) | 88 (84.6) | 229 (86.7) |
|
| No | 0 (0) | 19 (16.5) | 16 (15.4) | 35 (13.3) |
|
| Median duration of
adjuvant ET, months (IQR) | 42 (21-60) | 38 (12-60) | 35.5 (16-60) | 36 (15-60) | 0.711 |
| Disease free
interval (from surgery) | 42 (25-93) | 44 (18-84) | 48.5 (22-84) | 45 (21-85) | 0.710 |
| 1st site of
metastases |
|
|
|
| 0.005 |
|
Liver/lung | 11 (24.4) | 13 (11.3) | 10 (9.6) | 34 (12.9) |
|
|
Skin/lymph
nodes/peritoneo/pleura | 4 (8.9) | 21 (18.3) | 32 (30.8) | 57 (21.6) |
|
|
Bone | 30 (66.7) | 81 (70.4) | 62 (59.6) | 173 (65.5) |
|
| 2 site of
metastases |
|
|
|
| 0.406 |
| No | 20 (44.4) | 61 (53.0) | 56 (53.9) | 137 (51.9) |
|
| Liver,
lung | 13 (28.9) | 20 (17.4) | 16 (15.4) | 49 (18.6) |
|
|
Other | 12 (26.7) | 34 (29.6) | 32 (30.8) | 78 (29.6) |
|
| 3 site of
metastases |
|
|
|
| 0.999 |
| No | 41 (91.1) | 105 (91.3) | 95 (91.4) | 241 (91.3) |
|
|
Yes | 4 (8.9) | 10 (8.7) | 9 (8.7) | 23 (8.7) |
|
| 1st line CT for
metastatic disease |
|
|
|
| <0.001 |
|
Yes | 3 (6.7) | 56 (48.7) | 69 (66.4) | 128 (48.5) |
|
| No | 42 (93.3) | 59 (51.3) | 35 (33.7) | 136 (51.5) |
|
Exe-Eve combination was shown to be active in all
lines of therapy. Particularly, although no complete response was
observed, 105 (39.8%) partial responses, 88 (33.3%) disease
stabilizations, and 71 (26.9%) disease progressions were recorded.
The overall Disease Control Rate (DCR) was 73.1%, with no
statistically significant difference between the different settings
(73.3, 79.2 and 66.3% in first, second and third-line therapy,
respectively; P=0.105).
At the time of data censoring, 4.1% of patients were
receiving Exe-Eve without evidence of disease progression; median
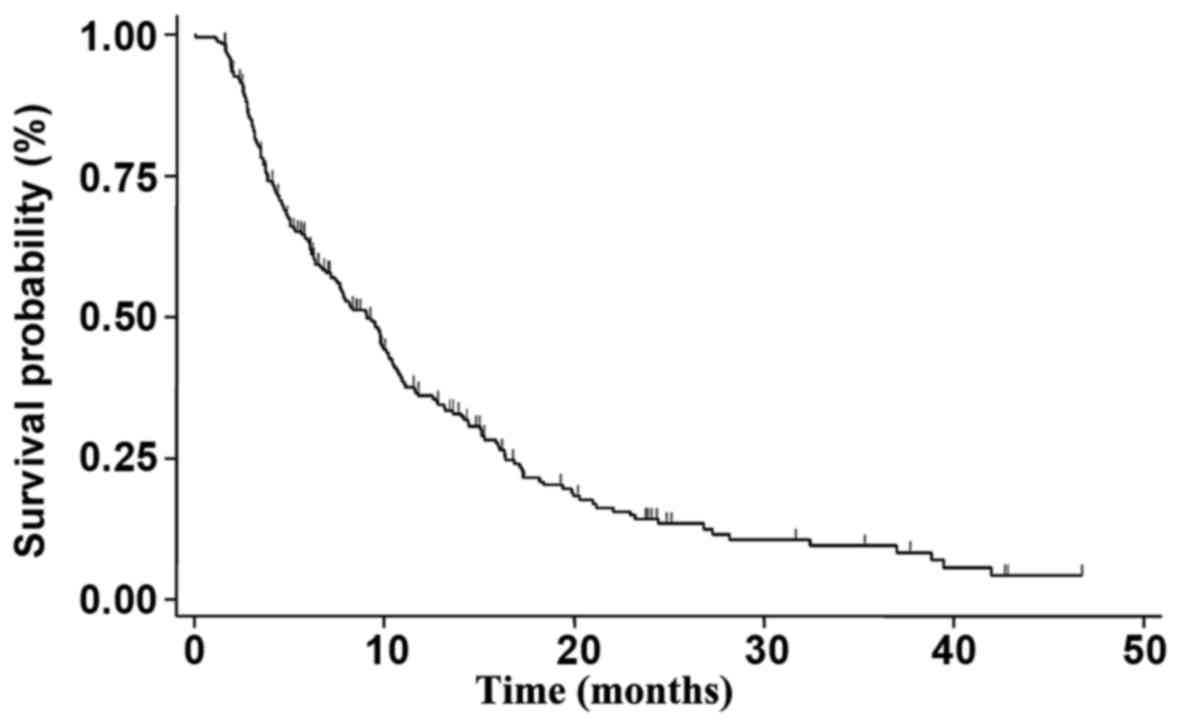
PFS was 9.1 months (95% CI 7.4-10.2) (Fig. 1). At a median follow-up of 42 months,
median PFS was 11.6 (95% CI 5.8-17.3), 9.7 (95% CI7.7-10.8), and
7.5 (95% CI 6.0-9.8) months for patients treated with Exe-Eve as
first, second, or third line of treatment, respectively, with a
statistically significant correlation on univariate analysis with
younger age (P=0.024), no previous adjuvant chemotherapy (P=0.028),
no previous adjuvant ET (P=0.030), adjuvant ET duration ≥36 months
(P=0.013), involvement of liver and/or lung (P=0.054), no previous
chemotherapy for metastatic disease (P=0.0050), PS=0 at the start
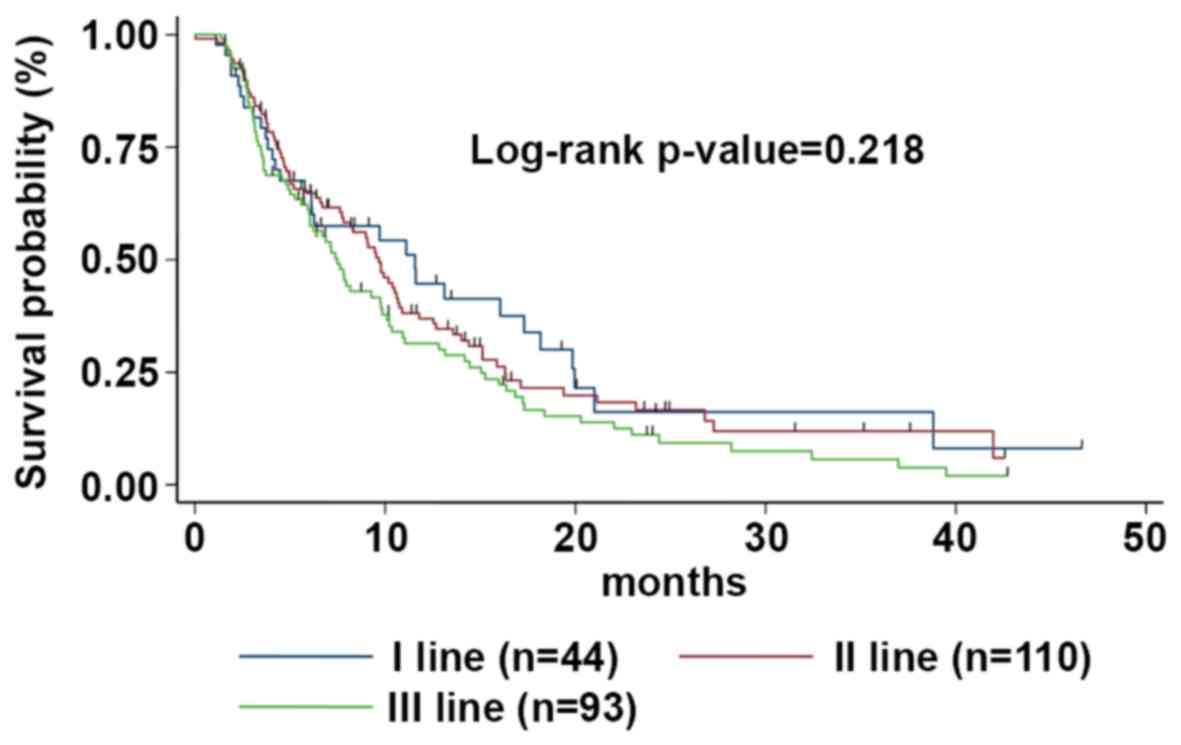
of treatment (P<0.0001). The risk of disease progression between
the different subgroups was not statistically significant (log-rank
P-value=0.218) (Fig. 2).
On multivariate analysis, previous adjuvant ET
(P=0.001; HR=2.78; 95% CI: 1.68-4.60), previous chemotherapy for
advanced disease (P=0.0027; HR=1.39; 95% CI: 1.03-1.89), and poor
PS (P=0.002; HR=2.70; 95% CI: 1.67-4.37) were shown to be
independent prognostic factors related to poor recurrence rate.
Conversely, the risk of progression was significantly lower in
patients treated with hormonal drugs for 36 months or longer
(HR=0.48; 95% CI: 0.34-0.68) in the adjuvant setting.
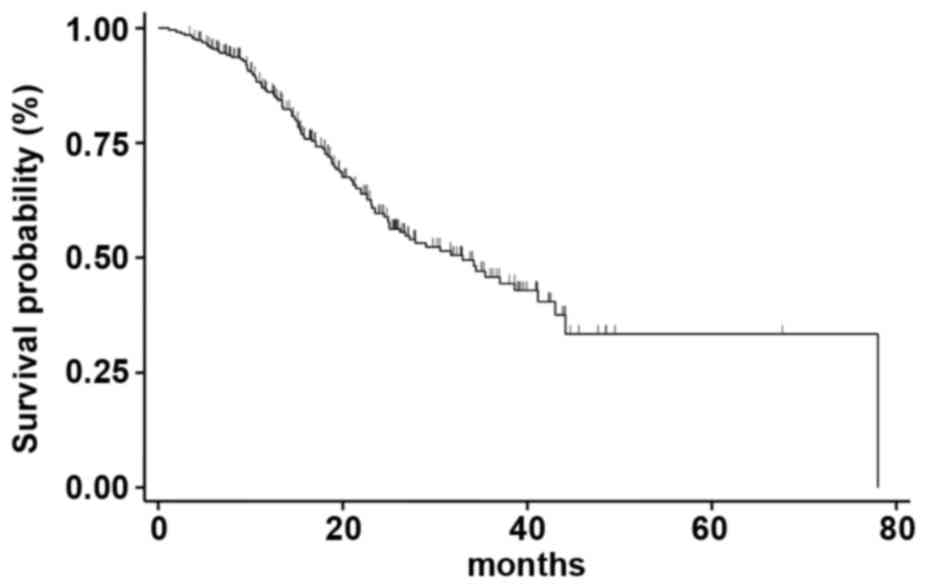
Median OS was 33.0 months (95% CI 25.2-41.2)
(Fig. 3); at a median follow-up of
67 months, median OS was 43.1 months (95% CI 23.1-53.2), 31.7
months (95% CI 23.5-44.1) and 27.9 months (95% CI 22.7-38.9) for
patients treated with Exe-Eve as first, second, or third line of
treatment, respectively (the difference between the different OS
medians was not statistically significant, P=0.538) (Fig. 4). On univariate analysis, the
variables related to better survival were younger age (≤48 years)
(P=0.041), Ki67 ≤20% (P=0.032), duration of adjuvant ET ≥36 months
(P=0.028), disease-free interval ≥45 months (P=0.010), no previous
chemotherapy for metastatic disease (P=0.001), ECOG PS=0 (P=0.032),
duration of treatment with Exe-Eve longer than 6.3 months
(P=0.012), partial response (P=0.009), presence of stomatitis
(P=0.049), and absence of diabetes during the treatment (P=0.001).
Finally, on multivariate analysis, the variables related to a worse
outcome were diabetes [P=0.001; HR=2.35; 95% CI 1.52-3.58)] and
previous chemotherapy for metastatic disease (P=0.032; HR=1.58; 95%
CI 0.92-2.26); conversely, the presence of mucositis correlated
with long-term survival (P=0.042; HR=0.64; 95% CI 0.30-0.82).
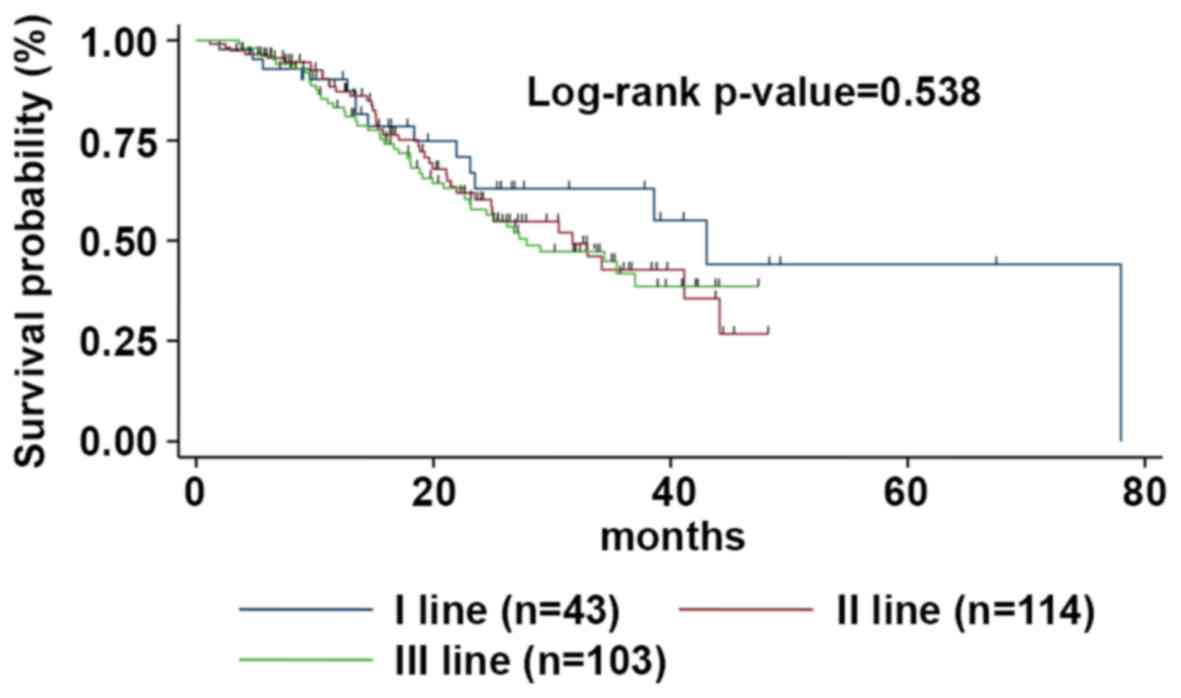 | Figure 4.mOS according to the line of
treatment. The mOS was 43.1 (95% CI, 23.1-53.2), 31.7 (95% CI,
23.5-44.1), and 27.9 months (95% CI, 22.7-38.9) for patients
treated with Exe-Eve at first, second or third line of treatment,
respectively. mOS, median overall survival; Eve-Exe,
Everolimus-Exemestane; CI, confidence interval. |
Toxicity
Overall, Exe-Eve was fairly well tolerated, most
toxicities being G1 or 2, while treatment discontinuation was
required in 15 patients (5.7%) due to unacceptable toxicity.
Particularly, one patient who received Exe-Eve as first line
treatment suffered from G4 diarrhea and refused the treatment, 3
patients developed interstitial lung disease requiring
hospitalization, and 1 case of persistent G4 neutropenia was
registered among second line patients. Finally, in patients
subjected to a third line of therapy, 3 cases of G3 pneumonitis, 3
cases of G4 diarrhea, 1 case of persistently increased hepatic
transaminases, 1 case of hyperglycemia requiring insulin therapy
and a brief hospitalization, and 2 cases of G4 stomatitis were
recorded.
Two hundred and fifty patients were started on full
dose Eve, while 16 patients (6%) began treatment with half dose per
physician choice; due to toxicities, Eve dosage was reduced to 5 mg
in 25 patients (9.45%).
Overall, hematologic toxicity was mild and no G4
events were recorded, with the exception of the neutropenic patient
described above. Of note, a significant difference among the three
groups of patients was recorded in terms of incidence of G1-3
thrombocytopenia and neutropenia, which occurred more frequently in
second and third line, respectively (Table II).
 | Table II.Haematological and non-haematological
toxicities. |
Table II.
Haematological and non-haematological
toxicities.
| Haematological
toxicities | All patients
(%) | First line (%) | Second line
(%) | Third line (%) |
P-valuea |
|---|
| Neutropenia |
|
|
|
| 0.002 |
|
G1-2 | 36 (13.6) | 2 (4.4) | 12 (10.4) | 22 (21.1) |
|
|
G3-4 | 6 (2.2) | – | 2 (1.7) | 4 (3.8) |
|
| Anemia |
|
|
|
| 0.788 |
|
G1-2 | 65 (24.6) | 13 (28.9) | 28 (24.3) | 24 (23.0) |
|
|
G3-G4 | 13 (4.9) | 2 (4.4) | 4 (3.5) | 7 (6.7) |
|
|
Thrombocytopenia |
|
|
|
| 0.010 |
|
G1-2 | 32 (12.1) | – | 18 (15.6) | 14 (13.5) |
|
|
G3-4 | 6 (2.28) | – | 3 (2.6) | 3 (2.9) |
|
| Non-haematological
toxicities |
|
|
|
|
|
| Stomatitis |
|
|
|
| 0.828 |
|
G1-2 | 120 (45.4) | 15 (33.3) | 55 (47.8) | 50 (48.0) |
|
|
G3-4 | 25 (9.5) | 4 (8.9) | 10 (8.7) | 11 (10.6) |
|
| Fatigue |
|
|
|
| 0.279 |
|
G1-2 | 95 (36.0) | 15 (33.3) | 39 (34.0) | 41 (39.4) |
|
|
G3-4 | 9 (3.4) | – | 3 (2.6) | 6 (5.8) |
|
| Rash |
|
|
|
| 0.778 |
|
G1-2 | 84 (31.8) | 16 (35) | 36 (31.3) | 32 (30.8) |
|
|
G3-G4 | 4 (1.5) | 1 (2.2) | 1 (<0.1) | 2 (1.9) |
|
| Diarrhea |
|
|
|
| 0.876 |
|
G1-2 | 76 (28.8) | 15 (33.3) | 29 (25.2) | 32 (30.7) |
|
|
G3-4 | 12 (4.5) | 2 (4.0) | 4 (3.5) | 6 (5.7) |
|
| Nausea |
|
|
|
| 0.627 |
|
G1-2 | 76 (28.7) | 12 (26.7) | 32 (27.8) | 32 (30.8) |
|
|
G3-G4 | 3 (1.1) | – | 1 (<0.1) | 2 (1.9) |
|
| Decreased
appetite |
|
|
|
| 0.727 |
|
G1-2 | 73 (27.6) | 10 (22.2) | 28 (24.3) | 35 (33.6) |
|
|
G3-G4 | 3 (1.1) | – | 1 (<0.1) | 2 (1.9) |
|
| AST increase |
|
|
|
| 0.781 |
|
G1-2 | 28 (10.6) | 5 (11.1) | 11 (9.6) | 12 (11.5) |
|
|
G3-4 | 9 (3.4) | 1 (2.2) | 3 (2.6) | 5 (4.8) |
|
| Cough |
|
|
|
| 0.192 |
|
G1-2 | 31 (11.7) | 5 (11.1) | 12 (10.4) | 14 (13.5) |
|
|
G3-G4 | 3 (1.1) | – | – | 3 (2.9) |
|
| ALT increase |
|
|
|
| 0.375 |
|
G1-2 | 24 (9.1) | 5 (11.1) | 10 (8.7) | 9 (8.6) |
|
|
G3-4 | 7 (2.6) | – | 3 (2.6) | 4 (3.8) |
|
| Hyperglicemia |
|
|
|
| 0.472 |
|
G1-2 | 17 (6.4) | 3 (6.7) | 8 (6.9) | 6 (5.8) |
|
|
G3-4 | 5 (1.8) | – | 2 (1.7) | 3 (2.9) |
|
|
Pneumonitis/interstitial lung disease |
|
|
|
| 0.592 |
|
G1-2 | 13 (4.9) | 2 (4.4) | 4 (3.5) | 7 (6.7) |
|
|
G3-G4 | 6 (2.3) | – | 2 (1.7) | 4 (3.8) |
|
The most commonly reported extra-hematologic
toxicities (all grades) were stomatitis (54.8%), fatigue (39.3%),
rash (33.3%), and diarrhea (33.2%), which occurred in more than one
third of patients (Table II).
Nausea and decreased appetite were recorded in 28.7 and 27.6% of
patients, respectively, even if G3-4 adverse events (AEs) were rare
(about 1-2%). Elevated serum transaminases, cough, hyperglycemia,
pneumonitis/interstitial lung disease were also observed and the
majority of G3-4 toxicities occurred in patients treated with
Exe-Eve as third line therapy.
Discussion
The outcome of MBC patients is continuously
improving because of the availability of new active therapeutic
options. The challenge for clinicians is to balance
treatment-related toxicity with the likelihood of benefit and
cancer-related symptom relief deriving from therapy. For this
reason, current clinical guidelines advocate the use of ET as the
preferred treatment for HR+, HER2-MBC, unless visceral crisis or
concern/proof of endocrine resistance is observed.
Recently, the introduction of CDK4/6 inhibitors in
combination with endocrine agents resulted in a significant PFS
benefit, however, overall survival data are still missing and there
is no consensus on the optimal sequence of ET, that is, as
monotherapy or in combination with targeted agents (12–15).
Currently, the combination of Eve and Exe is a
widely used regimen for treatment of endocrine sensitive
postmenopausal MBC patients progressing after NSAIs therapy.
However, current international guidelines suggest taking into
account the relevant class-effect AEs of this regimen with regard
to the decision to treat, which nonetheless should be made on a
case by case basis.
For this purpose, more experience has to be
collected on toxicity profile and efficacy in different subsets of
patients (i.e., differing in metastatic sites, performance status,
number of prior therapies etc.) in order to help physicians to
optimize the therapeutic strategy for each patient.
The main aim of our observational study was to
explore the efficacy and tolerability of Eve-Exe combination,
according to the line of therapy, in an unselected population from
nine Cancer Centers in Campania region (South Italy). The
hypothesis of the association between the onset of side effects and
efficacy was also addressed in our analysis.
The pivotal phase III BOLERO-2 trial showed that
dual-blockade based on the association of Eve plus Exe doubled the
median PFS compared to Exe alone (7.8 vs. 3.2 by investigator
review and 11 vs. 4.1 months by central review, respectively) in
patients with HR+, HER2-MBC progressing after prior NSAIs. The
clinical benefit rate (CBR), defined as CR+PR+SD ≥24 months, was
also better in the Eve-Exe arm with respect to Exe alone arm (51.3%
vs. 26.4%, respectively).
OS was a secondary endpoint but did not meet
statistical significance despite it was shown to increase (4.4
months) following addition of Eve to Exe (31.0 months in the
experimental arm vs. 26.6 months in patients receiving Exe alone)
(9–10,18).
The lack of a statistically significant survival
gain could be due to the fact that BOLERO-2 trial was powered to
detect only an eight-month OS improvement. Furthermore, the small
imbalance in post-study salvage chemotherapy use (63 vs. 53%,
control vs. experimental arm, respectively) has probably influenced
OS results leading to a reduction in the survival gap between the
two groups.
In our retrospective analysis, Exe-Eve combination
was shown to be active in all lines of therapy. Particularly,
although no complete response was observed, DCR was 73.1%, with no
statistically significant difference between the different
settings. At a median follow-up of 42 months, median PFS was 11.6,
9.7, and 7.5 months for patients treated with Exe-Eve as first,
second, or third line of treatment, respectively. Median OS was
33.0 months; at a median follow-up of 67 months, median OS was
43.1, 31.7, and 27.9 months for patients treated with Exe-Eve as
first, second or third line of treatment, respectively. No
statistically significant difference in terms of PFS and OS between
the different subgroups was detected. Our results confirmed the
considerable activity and efficacy of Eve-Exe observed in BOLERO-2,
although the safety profile of this combination remains debated. In
the BOLERO-2 trial, the most commonly reported AEs, affecting at
least one-third of patients in the Eve-Exe arm, were stomatitis,
rash, fatigue, diarrhea, nausea, decreased appetite, and
pneumonitis. While most AEs were low grade (G1-2), one-half of
patients in the Eve-Exe arm experienced grade 3-4 toxicities vs.
27% of patients in the placebo plus Exe arm. The discontinuation
rate due to AEs was higher with Eve-Exe (9%) compared with the
control arm (3%); the most common toxiticies leading to treatment
discontinuation in the experimental group included pneumonitis,
stomatitis, dyspnea, and fatigue (19). Consistent with BOLERO-2 results, in
our analysis, the most frequently described toxicities (all grades)
were stomatitis (54.8%), fatigue (39.3%), rash (33.3%), and
diarrhea (33.2%), which occurred in more than one third of
patients. Treatment discontinuation was registered only in 5.7% of
patients and the most common AEs leading to treatment
discontinuation were pneumonitis, stomatitis, and diarrhea. In the
Italian expanded-access, multicenter BALLET trial, permanent
treatment discontinuation due to side effects was reported to be
more frequent (17.1% of patients) with respect to our series and
was mainly caused by non infectious pneumonitis (NIP), stomatitis,
asthenia, and dyspnea. The majority of these toxicities occurred
within the first 12 weeks of start of therapy, consistently across
all clinical trials (20). Since
2012 approval, several retrospective trials have been carried out
to assess the role of Eve-Exe combination in daily clinical
practice, providing clinicians with valuable additional data to
guide treatment decisions. To our knowledge, five large
observational studies have strengthened the role of this
combination in patients with HR+, HER2-MBC. With the exception of
the Italian trial by Moscetti et al (21), the remaining studies were only
presented as abstracts at scientific international conferences on
breast cancer.
The Austrian non-interventional phase 4 STEPAUT
study aimed at evaluating efficacy and safety of Eve-Exe according
to clinical routine. The second interim analysis on 225 out of 300
enrolled patients was recently presented at San Antonio Breast
Cancer Symposium in 2016. Overall, median PFS was 9.5 months, in
line with our PFS data (9.1 months). A subgroup analysis was also
performed according to Eve dosing and, interestingly, PFS was lower
for the 5 mg-group compared to the 10-mg group (6.5 vs. 9.1 months,
respectively). However, it should be noted that patients receiving
the 5 mg starting dose had unfavorable prognostic factors as well
as worse ECOG PS and more prior therapies. The safety profile was
consistent with data previously reported in BOLERO-2 trial and,
noteworthy, in accordance with our findings, occurrence of
stomatitis did not negatively affect PFS. The EVEREXES phase IIIb
trial investigated safety and tolerability profiles as primary
endpoints and efficacy as a secondary objective. In this trial, 232
post-menopausal women affected by HR+, HER2-MBC were recruited in
Eastern countries. The planned interim analysis presented at San
Antonio Breast Cancer Symposium by Im et al in December 2015
showed a median PFS of 9.45 months, which is also consistent with
our results. The majority of AEs were grade 1-2 and the most common
G3-4 toxicities, as expected, included stomatitis, fatigue,
hyperglycemia, and NIP. Therefore, this trial confirmed the role of
Eve-Exe combination even for the treatment of patients from Asia,
Africa and middle Est, a population poorly represented in BOLERO-2
(<10% of all series). Furthermore, a large, multicenter, non
interventional study from Germany (BRAWO trial) including 3,000
patients provided data on the routine clinical use of Eve-Exe
therapy. The second interim analysis of BRAWO, presented at the
European Society of Medical Oncology (ESMO) in September 2014 by
Fasching et al was carried out on 500 patients: A median PFS
of 8 months was recorded, with a higher median PFS (10.1 months)
for patients treated with Exe-Eve in the first-line setting. These
data are still in accordance with our results as an increased PFS
of 11.6 months was observed in patients receiving Eve-Exe as first
line treatment, even though no statistically significant
correlation in terms of risk of disease progression between the
different lines of treatment was found. Of note, the third interim
analysis of BRAWO study, presented at ESMO in 2015, showed a
significantly longer duration of treatment in patients with younger
age, better ECOG performance status, and no comorbidities. 4EVER is
another German phase IIIb study evaluating the efficacy and safety
of Eve-Exe in a broader patient population than that of BOLERO-2.
The final efficacy analysis, calculated on 281 patients, was
presented at the San Antonio Breast Cancer Symposium in 2015.
Efficacy data were poorer compared with those from BOLERO-2, being
the median PFS equal to 5.6 months. This may be due to the
different patient population enrolled in this trial. In fact,
unlike the BOLERO-2 trial, there were less limitations in
enrollment criteria, including the number of previous CT lines, the
prior use of Exe, or the time to progression after NSAI therapy.
Finally, Moscetti et al (21)
recently published an Italian retrospective trial assessing the
safety of Eve-Exe combination and the possible association between
toxicities and previous treatments in 181 unselected patients. On
multivariate analysis, no association between the number of prior
therapies and toxic events was found, which is in line with the
real-life data of the Italian BALLETT-related cohort (20). In this study, a switch from 10 mg Eve
starting dose to 5 mg, due to AEs, was reported in 27% of patients,
which is a figure quite similar to that reported in BOLERO-2 and
EAP. Conversely, in our series, Eve dosage was reduced to 5 mg in
about 10% of patients only, perhaps due to the wide use of
prophylactic measures and therapeutic interventions for stomatitis
management.
Another important aspect to focus on is the
correlation between adverse events and patient characteristics and
response to treatment. In this regard, our multivariate analysis
showed diabetes and previous chemotherapy for metastatic disease as
the variables related to a worse outcome; conversely, the presence
of mucositis correlated with long-term survival. Recently, a
meta-analysis of data from seven randomized phase III trials of Eve
assessing the clinical impact of stomatitis on efficacy in
different solid tumors was published (22). Specifically, in BOLERO-2 study, the
median PFS was 8.5 vs. 6.9 months for patients receiving Eve with
or without stomatitis within 8 weeks, respectively. These results
and those of the above mentioned STEPAUT trial are in accordance
with our findings and suggest a careful and close monitoring of
patients in the first weeks of treatment in order to better manage
and prevent stomatitis. In this scenario, the SWISH trial
demonstrated that a prophylactic use of a commercially available,
non-expensive oral dexamethasone mouthwash resulted in a marked
reduction in the incidence and severity of stomatitis in patients
receiving Eve-Exe combination for treatment of HR+, HER2-MBC, when
compared with data from BOLERO-2. On the basis of these findings,
this measure can be considered as a new standard of oral care for
BC patients receiving Eve and Exe therapy (23).
In conclusion, with the limitations due to the
observational nature of our findings, addition of Eve to Exe is an
effective therapeutic option for HR+, HER2-MBC patients after
failure of NSAI therapy in the real word scenario. Patient
characteristics, costs, and side effects have to be integrated in
the treatment decision making process.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FR and MO conceived and designed the study. GiuC,
AD, CM, GiaC, RL, AF, FN, RA, OM and PI collected the data from the
source. Analysis and interpretation of data was performed by FR. AD
wrote the first draft for the manuscript. FC and SD performed data
collection and critical revision of the article for important
intellectual content. All authors approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Review Board at ‘F. Magrassi’
Department of Clinical and Experimental Medicine of ‘Luigi
Vanvitelli’ University of Campania, Naples (Italy) approved the
study. All patients provided written informed consent for the use
of their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson WF, Chatterjee N, Ershler WB and
Brawley OW: Estrogen receptor breast cancer phenotypes in the
Surveillance, Epidemiology, and End Results database. Breast Cancer
Res Treat. 76:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardoso F, Costa A, Senkus E, Aapro M,
André F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso
MJ, et al: 3rd ESO-ESMO International Consensus Guidelines for
Advanced Breast Cancer (ABC 3). Ann Oncol. 28:16–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Comprehensive Cancer Network, :
NCCN Clinical Practice Guidelines in Oncology Breast Cancer Version
1.2018. https://www.nccn.org/professional/physician_gls/pdf/breast.pdfApril
6–2018
|
|
5
|
Osborne CK and Schiff R: Mechanisms of
endocrine resistance in breast cancer. Annu Rev Med. 62:233–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselga J, Semiglazov V, van Dam P,
Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R,
Bianchi G, et al: Phase II randomized study of neoadjuvant
everolimus plus letrozole compared with placebo plus letrozole in
patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2630–2637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boulay A, Rudloff J, Ye J, Zumstein-Mecker
S, O'Reilly T, Evans DB, Chen S and Lane HA: Dual inhibition of
mTOR and estrogen receptor signaling in vitro induces cell death in
models of breast cancer. Clin Cancer Res. 11:5319–5328. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yardley DA, Noguchi S, Pritchard KI,
Burris HA III, Baselga J, Gnant M, Hortobagyi GN, Campone M,
Pistilli B, Piccart M, et al: Everolimus plus exemestane in
postmenopausal patients with HR(+) breast cancer: BOLERO-2 final
progression-free survival analysis. Adv Ther. 30:870–884. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burris HA III, Lebrun F, Rugo HS, Beck JT,
Piccart M, Neven P, Baselga J, Petrakova K, Hortobagyi GN,
Komorowski A, et al: Health-related quality of life of patients
with advanced breast cancer treated with everolimus plus exemestane
versus placebo plus exemestane in the phase 3, randomized,
controlled, BOLERO-2 trial. Cancer. 119:1908–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al:
Palbociclib in hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cristofanilli M, Turner NC, Bondarenko I,
Ro J, Im SA, Masuda N, Colleoni M, De Michele A, Loi S, Verma S, et
al: Fulvestrant plus palbociclib versus fulvestrant plus placebo
for treatment of hormone-receptor-positive, HER2-negative
metastatic breast cancer that progressed on previous endocrine
therapy (PALOMA-3): Final analysis of the multicentre,
double-blind, phase 3 randomised controlled trial. Lancet Oncol.
17:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,
Winer EP, et al: Ribociclib as first-line therapy for HR-positive,
advanced breast cancer. N Engl J Med. 375:1738–1748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE), Version 4.03. June 14–2010, US Department of Health
and Human Services. National Institutes of Health National Cancer
Institute; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_Quick
Reference_5×7.pdfOctober 8–2017
|
|
17
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
18
|
Piccart M, Hortobagyi GN, Campone M,
Pritchard KI, Lebrun F, Ito Y, Noguchi S, Perez A, Rugo HS, Deleu
I, et al: Everolimus plus exemestane for hormone-receptor-positive,
human epidermal growth factor receptor-2-negative advanced breast
cancer: Overall survival results from BOLERO-2. Ann Oncol.
25:2357–2362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rugo HS, Pritchard KI, Gnant M, Noguchi S,
Piccart M, Hortobagyi G, Baselga J, Perez A, Geberth M, Csoszi T,
et al: Incidence and time course of everolimus-related adverse
events in postmenopausal women with hormone receptor-positive
advanced breast cancer: Insights from BOLERO-2. Ann Oncol.
25:808–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jerusalem G, Mariani G, Ciruelos EM,
Martin M, Tjan-Heijnen VC, Neven P, Gavila JG, Michelotti A,
Montemurro F, Generali D, et al: Safety of everolimus plus
exemestane in patients with hormone-receptor-positive,
HER2-negative locally advanced or metastatic breast cancer
progressing on prior non-steroidal aromatase inhibitors: Primary
results of a phase IIIb, open-label, single-arm, expanded-access
multicenter trial (BALLET). Ann Oncol. 27:1719–1725. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moscetti L, Vici P, Gamucci T, Natoli C,
Cortesi E, Marchetti P, Santini D, Giuliani R, Sperduti I, Mauri M,
et al: Safety analysis, association with response and previous
treatments of everolimus and exemestane in 181 metastatic breast
cancer patients: A multicenter Italian experience. Breast.
29:96–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rugo HS, Hortobagyi GN, Yao J, Pavel M,
Ravaud A, Franz D, Ringeisen F, Gallo J, Rouyrre N, Anak O and
Motzer R: Meta-analysis of stomatitis in clinical studies of
everolimus: Incidence and relationship with efficacy. Ann Oncol.
27:519–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rugo HS, Seneviratne L, Beck JT, Glaspy
JA, Peguero JA, Pluard TJ, Dhillon N, Hwang LC, Nangia C, Mayer IA,
et al: Prevention of everolimus-related stomatitis in women with
hormone receptor-positive, HER2-negative metastatic breast cancer
using dexamethasone mouthwash (SWISH): A single-arm, phase 2 trial.
Lancet Oncol. 18:654–662. 2017. View Article : Google Scholar : PubMed/NCBI
|