Introduction
Lung cancer is the leading cause of cancer-related
mortality and NSCLC is responsible for ~85% of lung cancer cases
worldwide (1). Advanced NSCLC is
associated with a higher risk of leptomeningeal metastasis (LM)
compared with other tumors. Due to the limitations of the
blood-brain barrier, traditional cytotoxic drugs are not effective,
and patients with brain metastases form NSCLC have a poor
prognosis, as their condition deteriorates rapidly due to the lack
of standard and effective treatments. Radiotherapy of LM requires a
large irradiation field, is associated with severe adverse
reactions, and generally has a poor prognosis. Previous studies
have demonstrated that epidermal growth factor receptor-tyrosine
kinase inhibitors (EGFR-TKIs) can improve the prognosis of these
patients (2,3). However, EGFR mutation-positive patients
eventually develop resistance to EGFR-TKIs, the most frequent
reason being a secondary EGFR T790M mutation encoded in exon 20
(4).
Osimertinib, a third-generation EGFR-TKI, was
recently approved for use in NSCLC patients who have been
previously treated with EGFR-TKIs and/or chemotherapy and who
harbor the EGFR T790M mutation (5,6). Early
results from a phase I study (BLOOM) indicate that osimertinib is
effective for LM treatment, regardless of whether there is a T790M
mutation (7). We herein report the
case of a patient with advanced NSCLC with LM who was treated with
osimertinib.
Case report
A 59-year-old woman was admitted to The First
Affiliated Hospital of Jinzhou Medical University (Jinzhou, China)
in December 2015, following detection of a mass in her left lung on
routine physical examination. The patient had never been a smoker
and had no other significant medical history. A computed tomography
(CT) scan revealed a mass in the left lung and small nodules in the
inferior lobe of the right lung. The patient's carcinoembryonic
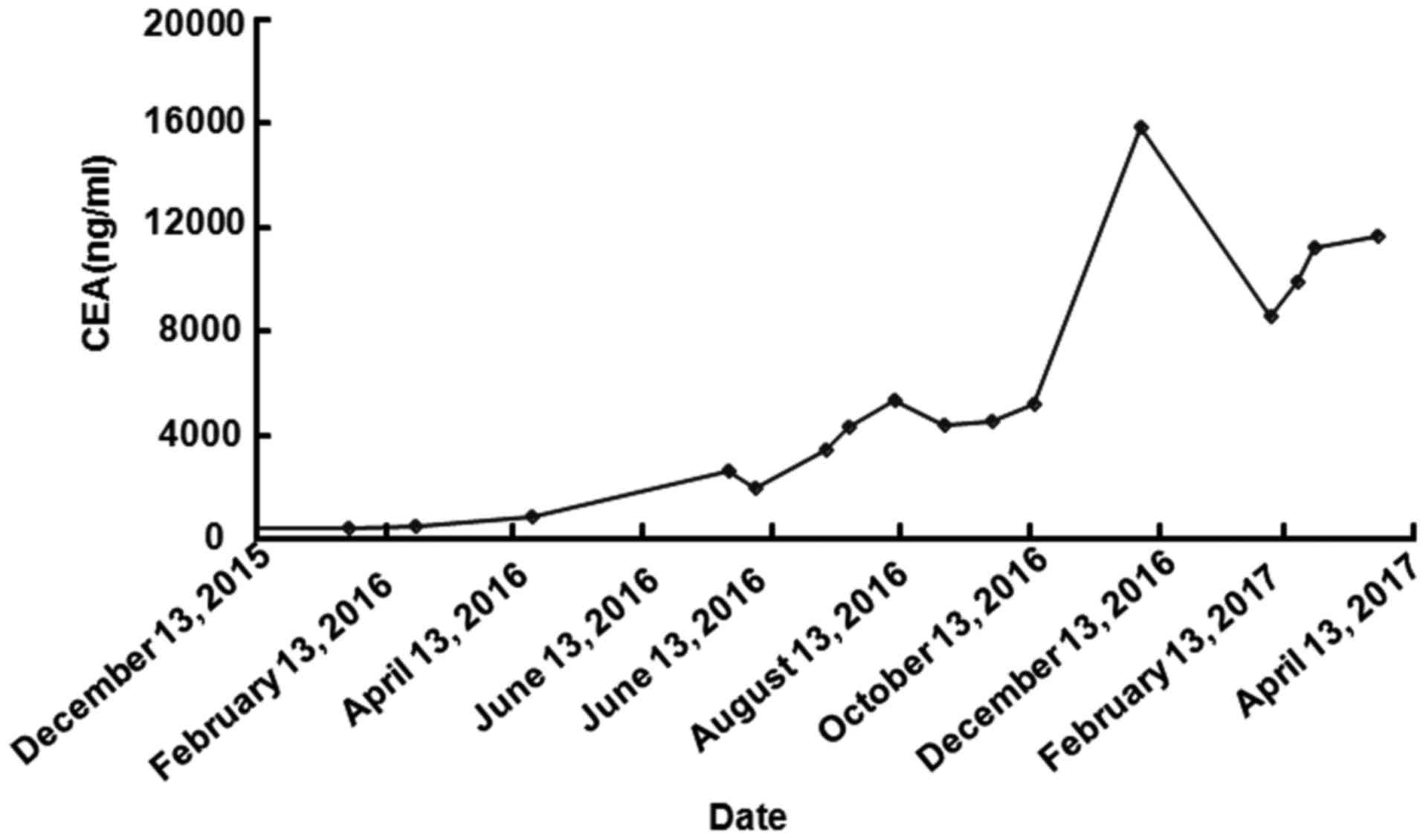
antigen (CEA) level was 422.0 ng/ml (normal range: 0.0–3.4 ng/ml)
higher than normal, and it significantly increased to 15854.2 ng/ml
when the patient's condition worsened (Fig. 1). The patient was diagnosed with lung
adenocarcinoma by fiberoptic bronchoscopy and biopsy. Mutation
analysis of EGFR in adenocarcinoma cells revealed an L858R mutation
in the EGFR gene. A bone emission computerized tomography scan
revealed metastasis to the thoracic and lumbar vertebrae. The
patient was initiated on 250 mg of gefitinib orally once a day in
January 2016. After 1 month of treatment with gefitinib, the lung
tumor exhibited partial response (PR). In April 2016, the mass in
the left lung had increased in size as detected during CT
examination, and gefitinib was replaced with erlotinib (150
mg/day). However, the patient developed progressive disease on
erlotinib.
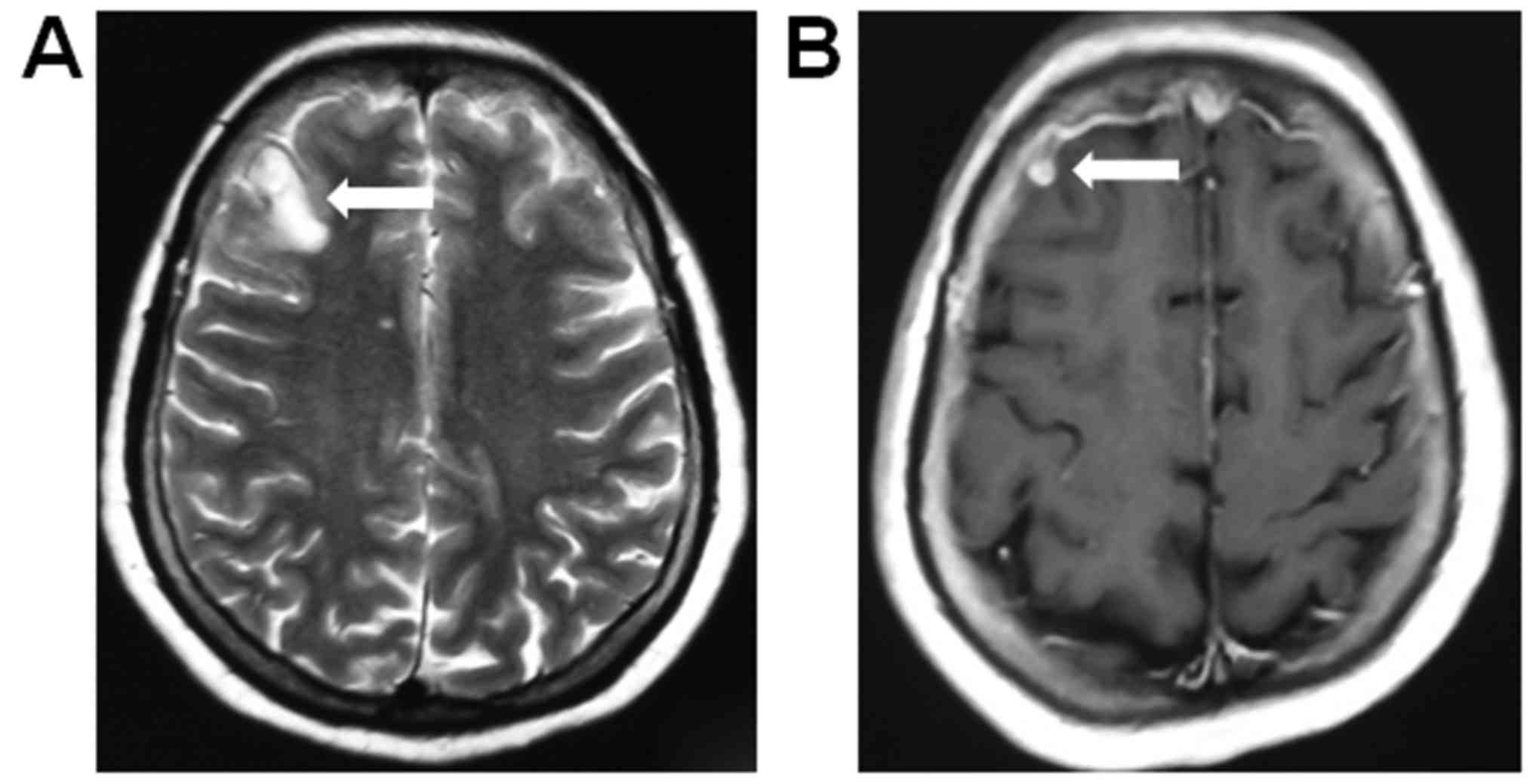
In September 2016, a new metastasis in the brain was
detected during brain magnetic resonance imaging (MRI) examination
(Fig. 2A). The patient was
administered four cycles of pemetrexed/cisplatin, and the cranial
tumor exhibited a PR that lasted for 5 months (Fig. 2B). However, in February 2017, the
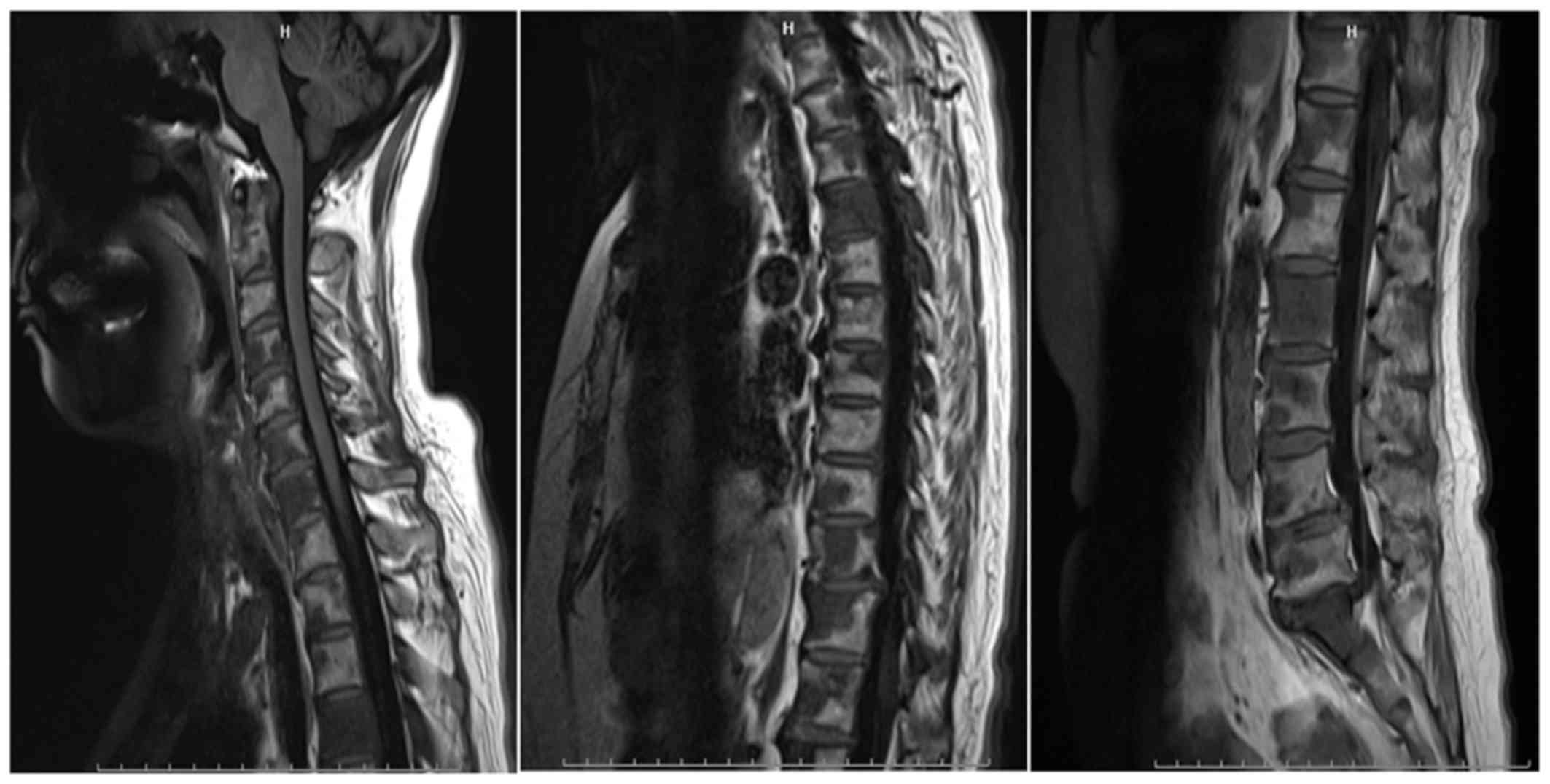
patient developed headache, dizziness and vomiting. An MRI of the
patients' spine revealed multiple metastases to the spine (Fig. 3). Based on this finding and taking
into consideration the risks of lumbar puncture, the options were
discussed with the patient's family, and the family declined both
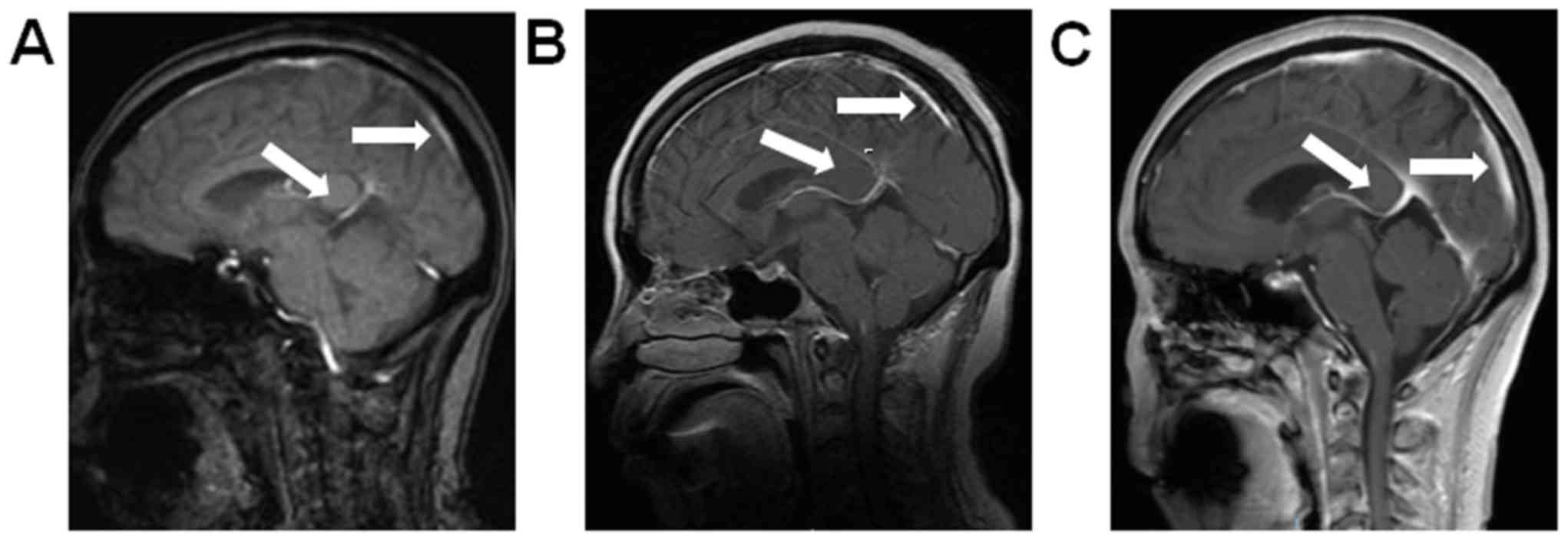
the lumbar puncture and cerebrospinal fluid examination. A brain
MRI revealed linear high-signal intensities in parts of the
meninges, and linear hardening was observed with enhanced scanning.
Thus, LM was diagnosed (Fig. 4A).
Despite receiving a series of symptomatic supportive treatments,
the patient's symptoms of intracranial metastases were not
relieved. It was recommended to the patient's family that the
pulmonary lesions were biopsied for pathological detection of gene
mutations. The family members considered the patient's current
symptoms and refused biopsy, but agreed to genetic testing of a
blood sample. In February 2017, The Beijing Genomics Institute was
commissioned to perform genetic testing on a blood sample. The EGFR
T790M mutation encoded in EGFR exon 20 was not detected in this
patient, but human epidermal growth factor receptor 2 (HER2)
amplification was detected instead. Treatment with oral osimertinib
was initiated at a dose of 80 mg per day in February 2017. A brain
MRI revealed that LM had progressed, and the patient's symptoms
worsened after 1 month of treatment with osimertinib (Fig. 4B). The dose of the drug was increased
to 160 mg per day. However, after 1 month of treatment, LM
progressed again (Fig. 4C), and the
CEA level also increased from 8613.7 to 11239.9 ng/ml (normal
range: 0.0–3.4 ng/ml) during treatment with osimertinib (Fig. 1). The patient's symptoms worsened,
and the LM continued to progress. Due to the deterioration of the
performance status (PS 4), the patient received supportive care and
succumbed to the disease 3 months following the worsening of the
meningeal metastasis symptoms.
This case report was approved by the Ethics
Committees of Jinzhou Medical University and written informed
consent was obtained from the patient regarding the publication of
the case details and associated images.
Discussion
Patients with advanced NSCLC and LM have a poor
prognosis. Our patient was a woman with advanced lung
adenocarcinoma who had no smoking history. The primary lesions
harbored exon-EGFR19 mutations. Based on genetic testing, the
patient was sensitive to EGFR-TKI treatment and the extracranial
conditions were briefly controlled. Erlotinib was used to replace
gefitinib as EGFR-TKI treatment following development of gefitinib
resistance and disease progression. However, brain metastases
occurred during gefitinib treatment with progression of peripheral
lesions. In addition to the standard whole-brain radiotherapy, some
studies have reported that EGFR-TKIs may improve the prognosis of
NSCLC patients. EGFR mutation is an oncogenic driver mutation, and
it has been demonstrated that individual NSCLC patients with
oncogenic drivers who receive a matched targeted agent exhibit
improved prognosis, and treatment with an EGFR-TKI is recommended
as first-line therapy for EGFR mutation-positive NSCLC patients
(8). However, resistance develops
rapidly, the main reason for which is a secondary T790M mutation
within the ATP site of the receptor (9,10), with
a mutation probability of ~60%. A third-generation EGFR-TKI,
osimertinib, was recently found to be of clinical value for NSCLC
patients who develop a secondary EGFR T790M mutation, which is the
most frequent reason for resistance to first-line treatment with
EGFR-TKIs (4,5). Recently, the BLOOM trial found
osimertinib to be effective in patients with advanced NSCLC who
developed LM, regardless of the presence of a T790M mutation
(7).
EGFR T790M has been identified as the most common
mechanism underlying acquired resistance, while MET amplification,
HER2 amplification and small-cell histological transformation occur
less frequently. Although EGFR T790M mutation encoded in EGFR exon
20 was not detected in our patient, HER2 and MYC amplification,
lack of CDKN2A, TP53 exon 4 and 6, ATP1B1 exon 21, and APC exon 5
mutations were detected. In fact, HER2 amplification may be a more
common finding at the time of resistance development (11). The HER2 copy number appeared to be
associated with gefitinib sensitivity in EGFR-positive patients
treated with gefitinib in North America and Europe (12). The prognostic role of EGFR in lung
cancer is not well defined, but previous studies have demonstrated
that patients overexpressing EGFR and HER2 have a poor prognosis
(13–15). Conversely, current findings suggest
that high copy numbers of the HER2 gene increase sensitivity to
gefitinib therapy (15). Preclinical
data have revealed that tumors overexpressing HER2 are the most
sensitive to gefitinib, possibly because this drug induces
sequestration of HER2 and HER3 receptors in an inactive heterodimer
configuration with the EGFR (16).
Conflicting results have also been reported regarding the
interaction between EGFR mutation and EGFR and HER2 copy numbers
(10,17–19).
The efficacy of EGFR-TKIs, such as gefitinib,
erlotinib and afatinib, in the treatment of NSCLC has been proven,
particularly in EGFR mutation-positive patients; however,
resistance develops rapidly. Osimertinib has exhibited good
penetration through the blood-brain barrier, delaying the
development of leptomeningeal carcinomatosis in EGFR
mutation-positive cases.
Unfortunately, our patient, who had lung
adenocarcinoma resistant to gefitinib and erlotinib treatment and
was negative for the EGFR T790M mutation, was not sensitive to
osimertinib. HER2 amplification may be the mechanism underlying
acquired resistance. Previous studies have reported that patients
overexpressing EGFR and HER2 have a poor prognosis. Conversely,
current findings suggest that high copy numbers of the HER2 gene
increase sensitivity to gefitinib therapy. Further studies are
required to elucidate this association and the role of HER2 protein
status among patients treated with reversible EGFR-TKIs.
Acknowledgements
Not applicable.
Funding
This study was supported by the Liaoning Province
Science and Technology Project (grant no. 2010010280-401).
Availability of data and materials
Not applicable.
Authors' contributions
JL collected the data and drafted the manuscript; XL
and CY analyzed and interpreted the patient data regarding
osimertinib resistance. JL, XL and CY were involved in revising the
manuscript, All the authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This case report was approved by the Ethics
Committees of Jinzhou Medical University.
Consent for publication
Written informed consent was obtained from the
patient regarding the publication of the case details and
associated images.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jorge SE, Kobayashi SS and Costa DB:
Epidermal growth factor receptor (EGFR) mutations in lung cancer:
Preclinical and clinical data. Braz J Med Biol Res. 47:929–939.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi HG, Kim HJ, Kim YJ, Han SW, Oh DY, Lee
SH, Kim DW, Im SA, Kim TY, Kim CS, et al: Epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for
leptomeningeal metastasis from non-small cell lung cancer patients
with sensitive EGFR mutation or other predictive factors of good
response for EGFR TKI. Lung Cancer. 65:80–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uemura T, Oguri T, Okayama M, Furuta H,
Kanemitsu Y, Takakuwa O, Ohkubo H, Takemura M, Maeno K, Ito Y and
Niimi A: Dramatic intracranial response to osimertinib in a poor
performance status patient with lung adenocarcinoma harboring the
epidermal growth factor receptor T790M mutation: A case report. Mol
Clin Oncol. 6:525–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Kim DS and Kim SW: Osimertinib
activity in patients (pts) with leptomeningeal (LM) disease from
non-small cell lung cancer (NSCLC): Updated results from BLOOM, a
phase I study. ASCO Meeting Abs. 34:2016.
|
|
8
|
Grigoriu B, Berghmans T and Meert AP:
Management of EGFR mutated nonsmall cell lung carcinoma patients.
Eur Respir J. 45:1132–1141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gainor JF and Shaw AT: Emerging paradigms
in the development of resistance to tyrosine kinase inhibitors in
lung cancer. J Clin Oncol. 31:3987–3996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chong CR and Jänne PA: The quest to
overcome resistance to EGFR-targeted therapies in cancer. Nat Med.
19:1389–1400. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cappuzzo F, Varella-Garcia M, Shigematsu
H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V,
Gregorc V, Toschi L, et al: Increased HER2 gene copy number is
associated with response to gefitinib therapy in epidermal growth
factor receptor-positive non-small-cell lung cancer patients. J
Clin Oncol. 23:5007–5018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim EK, Kim KA, Lee CY and Shim HS: The
frequency and clinical impact of HER2 alterations in lung
adenocarcinoma. PLoS One. 12:e01712802017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan D, Deeb G, Wang J, Slocum HK, Winston
J, Wiseman S, Beck A, Sait S, Anderson T, Nwogu C, et al: HER-2/neu
protein expression and gene alteration in stage I–IIIA
non-small-cell lung cancer: A study of 140 cases using a
combination of high throughput tissue microarray,
immunohistochemistry, and fluorescent in situ hybridization. Diagn
Mol Pathol. 12:201–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cappuzzo F, Varella-Garcia M, Shigematsu
H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V,
Gregorc V, Toschi L, et al: Increased HER2 gene copy number is
associated with response to gefitinib therapy in epidermal growth
factor receptor-positive non-small-cell lung cancer patients. J
Clin Oncol. 23:5007–5018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cappuzzo F, Hirsch FR, Rossi E, Bartolini
S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini
I, et al: Epidermal growth factor receptor gene and protein and
gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer
Inst. 97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takano T, Ohe Y, Sakamoto H, Tsuta K,
Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine
I, et al: Epidermal growth factor receptor gene mutations and
increased copy numbers predict gefitinib sensitivity in patients
with recurrent non-small-cell lung cancer. J Clin Oncol.
23:6829–6837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bell DW, Lynch TJ, Haserlat SM, Harris PL,
Okimoto RA, Brannigan BW, Sgroi DC, Muir B, Riemenschneider MJ,
Iacona RB, et al: Epidermal growth factor receptor mutations and
gene amplification in non-small-cell lung cancer: Molecular
analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol.
23:8081–8092. 2005. View Article : Google Scholar : PubMed/NCBI
|