Introduction
Myocardial ischemia/reperfusion (I/R) injury is a
phenomenon that is frequently evident in clinical practice
(1). Although ischemic
preconditioning (IPC) and remote ischemic preconditioning (RIPreC)
significantly reduce myocardial injury (2,3), the
applicability of IPC is limited by the unpredictable nature of
ischemic events in clinical practice. Ischemic postconditioning
(IPostC) is triggered during the clinically applicable time period
of reperfusion, with the major limitation being the invasive
protocol (4).
Remote ischemic postconditioning (RIPostC) is
another endogenous cardioprotective method (5). Brief application of ischemic stimulus
on an organ at a distance from the heart may render the heart more
tolerant to the subsequential prolonged period of ischemia. RIPostC
does not require invasive intervention, unlike other types of
intervention, and may have a promising future in clinical practice
(6). Therefore, the present study
concerning the mechanism by which RIPostC induces cardioprotection
against I/R injury is beneficial for further studies and may help
to identify a novel interventional method for use in clinical
practice (7,8).
Myocardial I/R injury leads to an overload of
oxidative stress, an imbalance of calcium homeostasis and cell
apoptosis, and thereby induces tissue damage. Mitochondria have a
fundamental role in the maintenance of the normal structure and
function of tissues. A decline in mitochondrial function has a
critical role in the promotion of cell apoptosis. Aldehyde
dehydrogenase 2 (ALDH2), a type of mitochondrial protein enzyme
involved in the metabolism of acetaldehyde and other toxic
aldehydes (9), is considered to be
responsible for the oxidation and detoxification of reactive
aldehydes in different organs and cell types (10,11).
ALDH2 is highly expressed in the heart, liver, kidney and muscles
(12). Chen et al (13) indicated an inverse correlation
between the levels of ALDH2 activity and infarct size in a
myocardial infarction model. ALDH2 is the crucial enzyme for the
removal of aldehydes in the heart, and activated ALDH2
significantly reduces cardiac I/R injury (14). A previous study has shown that the
cardiac ALDH2 expression levels were further reduced with the
development of diabetes. Thus, ALDH2 may be an endogenous cardiac
protective factor in myocardial injury. Furthermore, it was also
demonstrated in a previous study that RIPostC produced a protective
effect through inhibition of the opening of the mitochondrial
permeability transition pore (mPTP) and apoptosis (15). However, the role of ALDH2 in
RIPostC cardioprotection has not been elucidated. The purpose of
the present study was to investigate whether ALDH2 is involved in
the protective role of RIPostC.
The reperfusion injury salvage kinase (RISK)
signaling pathway refers to a group of prosurvival kinases,
including the phosphatidylinositol-3 kinase (PI3K)/Akt cascade,
which confer cardioprotection when specifically activated at the
onset of myocardial reperfusion following ischemia (16). Furthermore, a previous study has
established that activation of the PI3K/Akt signaling pathway
contributes to IPostC-mediated cardioprotection (17). However, it remains unknown whether
the PI3K/Akt signaling pathway is involved in the cardioprotection
of RIPostC.
In the present study, the objectives were: i) To
investigate the role of ALDH2 in RIPostC; ii) to determine whether
RIPostC mediates its protective effect through the activation of
the PI3K/Akt-dependent signaling pathway; and iii) to clarify the
association of ALDH2 and the PI3K/Akt signaling pathway in the
protective effect of RIPostC.
Materials and methods
Animals and materials
Male Sprague-Dawley rats (250–300 g) were obtained
from the Animal Center of Bengbu Medical College (Bengbu, China).
All rats were housed in individual cages in a
temperature-controlled room with a 12-h light/dark cycle. The rats
were fed a normal diet and had free access to distilled water. All
the animal procedures were in accordance with the United States
National Institutes of Health Guide and were approved by the Animal
Use and Care Committee of Bengbu Medical College.
Chemicals and reagents
Wortmannin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Lactate dehydrogenase (LDH) and creatine kinase
(CK) assay kits were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). All primers were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). Mouse
ALDH2 and β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), and rabbit polyclonal
Akt, phospho-Akt (p-Akt), caspase-3 and cleaved caspase-3
antibodies were purchased from Anbo Biotechnology Co., Ltd (San
Francisco, CA, USA). A chemiluminescence reaction (ECL) system was
purchased from Millipore (Billerica, MA, USA).
Animal experiment
Male Sprague Dawley rats (n=48) were randomly
divided into the following four groups (n=12 in each group): Sham
group, I/R group (I/R), RIPostC group (RIPostC), and RIPostC +
wortmannin group (RIPostC+Wort). After the rats were anesthetized
with 60 mg/kg pentobarbital sodium through intraperitoneal
injection, the left anterior descending coronary arteries (LAD) of
all rats were encircled with a suture to make a snare after their
chests had been opened. With the exception of the Sham group, the
LAD were ligated for 45 min (ischemia) followed by l80 min of the
LAD open (reperfusion) in vivo. In the RIPostC group, three
cycles of lower limb I/R (right femoral artery clamping for 5 min
and declamping for 5 min) were performed prior to the onset of
myocardial reperfusion (6). In the
RIPostC+Wort group, wortmannin (15 μg/kg; a PI3K inhibitor) was
administered intravenously 30 sec prior to myocardial reperfusion
in RIPostC-treated animals. Throughout the whole process of the
experiment, the mean arterial pressure (MAP) and heart rate (HR)
were continuously monitored.
Assessment of myocardial infarct
size
The infarct size was measured in all groups at the
end of the reperfusion. The heart was removed with the reoccluded
LAD, and was retrogradely perfused with 1.5 ml 1% Evans blue dye to
delineate the risk area. Following freezing at -20°C, the heart was
cut into 5–6 sections from the apex to the base and incubated in 1%
triphenyltetrazolium chloride for 15 min. Subsequently, the
sections were fixed in 10% formalin buffer, and the infarct size
was quantified by computerized planimetry using ImageJ software
(version 1.40, National Institutes of Health, Bethesda, MD, USA).
The myocardial infarct size was expressed as the percentage of the
area at risk.
Measurement of LDH content and CK
activity levels in the plasma
At the end of the reperfusion, an arterial blood
sample was placed in test tubes with heparin and centrifuged at
1509 × g for 30 min. The supernatant was collected and stored at
−20°C, thawed once and assayed. The LDH content and CK activity
levels were measured spectrophotometrically at wavelengths of 440
and 660 nm using colorimetric assay kits according to the
manufacturer’s instructions (18,19).
Reverse transcription polymerase chain
reaction (RT-PCR) assay for determining the B-cell lymphoma 2
(Bcl-2) and Bcl-2-associated X protein (Bax) mRNA levels
Total RNA was extracted from the left anterior
myocardium using TRIzol reagent (Life Technologies Corporation,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The total RNA (2 μg) was reversely transcribed to cDNA, and PCR was
performed by a routine method. The sequences of the primers for
Bax, Bcl-2 and β-actin are shown in Table I. The PCR products were analyzed on
1% agarose gel. The densitometry results for the Bcl-2 and Bax
genes were compared with the corresponding β-actin levels to
account for loading differences.
 | Table IQuantitative polymerase chain
reaction primers for Bax, Bcl-2 and β-actin. |
Table I
Quantitative polymerase chain
reaction primers for Bax, Bcl-2 and β-actin.
| Gene | Primer | Sequence | Product (bp) |
|---|
| Bax | Forward | 5′-GGA TCG AGC AGA
GAG GAT GG-3′ | 464 |
| Reverse | 5′-TGG TGA GTG AGG
CAG TGA GG-3′ | |
| Bcl-2 | Forward | 5′-CTG GTG GAC AAC
ATC GCT CTG-3′ | 227 |
| Reverse | 5′-GGT CTG CTG ACC
TCA CTT GTG-3′ | |
| β-actin | Forward | 5′-GAT GGT GGG TAT
GGG TCA GAA GGA C-3′ | 630 |
| Reverse | 5′-GCT CAT TGC CGA
TAG TGA TGA CT-3′ | |
Western blot analysis of ALDH2, Akt and
p-Akt, caspase-3 and cleaved caspase-3
Left anterior myocardium tissues from each group
were collected and homogenized in a lysis buffer, which contained
20 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l
EGTA, 1% Triton, 0.1% SDS, and 1% protease inhibitor cocktail. The
homogenates were sonicated and centrifuged at 12,000 × g for 30 min
at 4°C. The protein concentration was determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). The total protein (80 μg) was
separated by SDS-PAGE, and transferred electrophoretically to
polyvinylidene difluoride filter membranes (14).
The membranes were blocked with 5% nonfat milk in
Tris-buffered saline with Tween 20 for 2 h, and then they were
incubated at 4°C overnight with the corresponding primary antibody,
including mouse ALDH2 antibody (1:500), β-actin antibody (1:500),
rabbit Akt antibody (1:1,000), rabbit p-Akt antibody (1:1,000),
rabbit caspase-3 antibody (1:1,000) and rabbit cleaved caspase-3
antibody (1:1,500). All membranes were incubated for 1 h with the
corresponding horseradish peroxidase (HRP)-linked anti-mouse
immunoglobulin (IgG) or HRP-linked anti-rabbit IgG secondary
antibody. The membranes were analyzed by an ECL system. The
autoradiographs were scanned and the band densities were determined
with ImageJ software.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. One-way analysis of variance followed by
Student-Newman-Keuls was used for multiple comparisons. Differences
with P<0.05 were considered statistically significant. All data
were analyzed using GraphPad Prism version 4.0 software (GraphPad
Software, Inc., San Diego, CA, USA).
Results
Hemodynamics
With the exception of the Sham group, the MAP and HR
were reduced in each group following the coronary artery occlusion,
and recovered to varying extents after 180 min of reperfusion. No
statistical differences in the MAP and HR among these groups were
identified (Table II).
 | Table IIHemodynamic data in the rats. |
Table II
Hemodynamic data in the rats.
| | Ischemia (min) | Reperfusion
(min) |
|---|
| |
|
|
|---|
| Group | Baseline | 45 | 10 | 60 | 180 |
|---|
| MAP (mmHg) |
| Sham | 109.58±3.10 | 111.33±7.95 | 103.33±3.02 | 102.50±3.32 | 97.83±3.73 |
| I/R | 108.42±2.84 | 82.92±2.76a | 63.83±3.83a | 80.92±3.82a | 72.42±4.05a |
| RIPostC | 111.58±2.66 | 84.42±3.96a | 74.42±2.56a | 81.00±3.63a | 71.42±4.77a |
| RIPostC+Wort | 104.42±3.66 | 77.50±3.78a | 65.42±2.40a | 78.00±3.04a | 72.42±3.70a |
| HR (beats/min) |
| Sham | 413.58±9.33 | 399.25±4.42 | 395.50±3.87 | 384.00±7.35 | 386.92±3.19 |
| I/R | 412.33±7.67 |
354.75±10.65a | 322.00±8.49a | 340.83±7.85a | 318.50±7.51a |
| RIPostC | 413.33±8.47 | 369.00±9.09a | 341.17±5.88a | 371.92±11.58 | 364.08±8.27 |
| RIPostC+Wort | 403.08±7.91 |
353.58±11.05a | 323.67±8.53a | 344.67±8.07a | 309.75±8.73a |
Myocardial infarct size and lactate
dehydrogenase and creatine kinase levels in the plasma
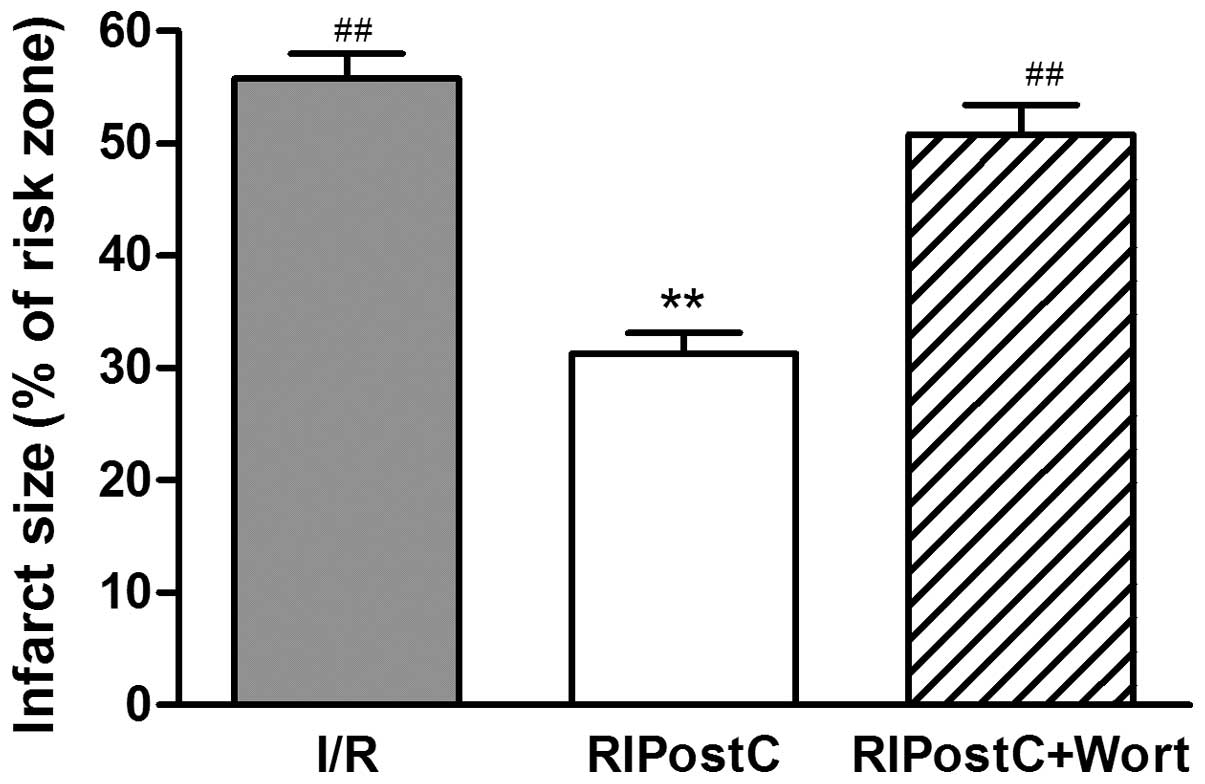
The myocardial infarct size (percentage infarct
size/area at risk) was reduced by ≥55% in the RIPostC group
(31.25±1.89%) compared with that in the I/R group (55.75±2.21%;
P<0.01). However, wortmannin (15 μg/kg), an inhibitor of the
PI3K/Akt signaling pathway, attenuated the effect of RIPostC
(RIPostC+Wort, 49.67±3.48 vs. RIPostC, 31.25±1.89%, P<0.01;
Fig. 1).
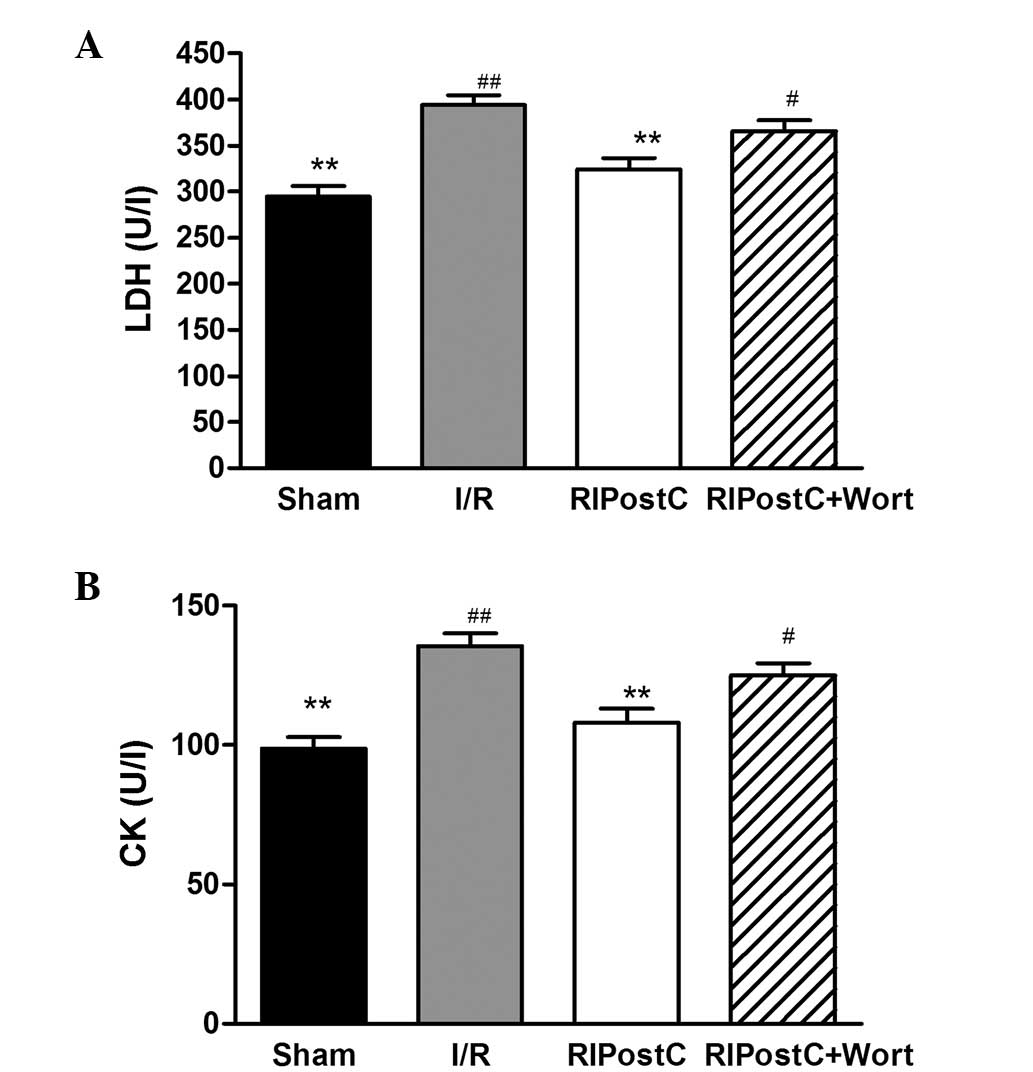
As displayed in Fig.
2, compared with those in the I/R group, the LDH content
(RIPostC, 324.25±12.46 vs. I/R, 394.08±10.83 U/l, P<0.01) and CK
activity levels (RIPostC, 108.08±4.96 vs. I/R, 135.58±4.61 U/l,
P<0.01) were significantly reduced in the RIPostC group.
However, in contrast to those of the RIPostC group, the LDH content
(RIPostC+Wort, 365.92±11.51 vs. RIPostC, 324.25±12.46 U/l, n=12,
P<0.05) and CK activity levels (RIPostC+Wort, 125.00±4.48 vs.
RIPostC, 108.08±4.96 U/l, n=12, P<0.05) were markedly increased
in the RIPostC+Wort group.
Changes in the expression levels of
myocardial Bcl-2 and Bax at the mRNA level and cleaved caspase-3
and caspase-3 at the protein level
The RT-PCR results revealed that, compared with
those in the I/R group, the Bcl-2/Bax ratio at the mRNA level was
elevated in the Sham and RIPostC groups (Sham, 1.43±0.05; RIPostC,
1.32±0.06 vs. I/R, 0.31±0.03, n=4, P<0.01; Fig. 3). However, compared with that in
the RIPostC group, the ratio was significantly reduced in the
RIPostC+Wort group (0.43±0.04).
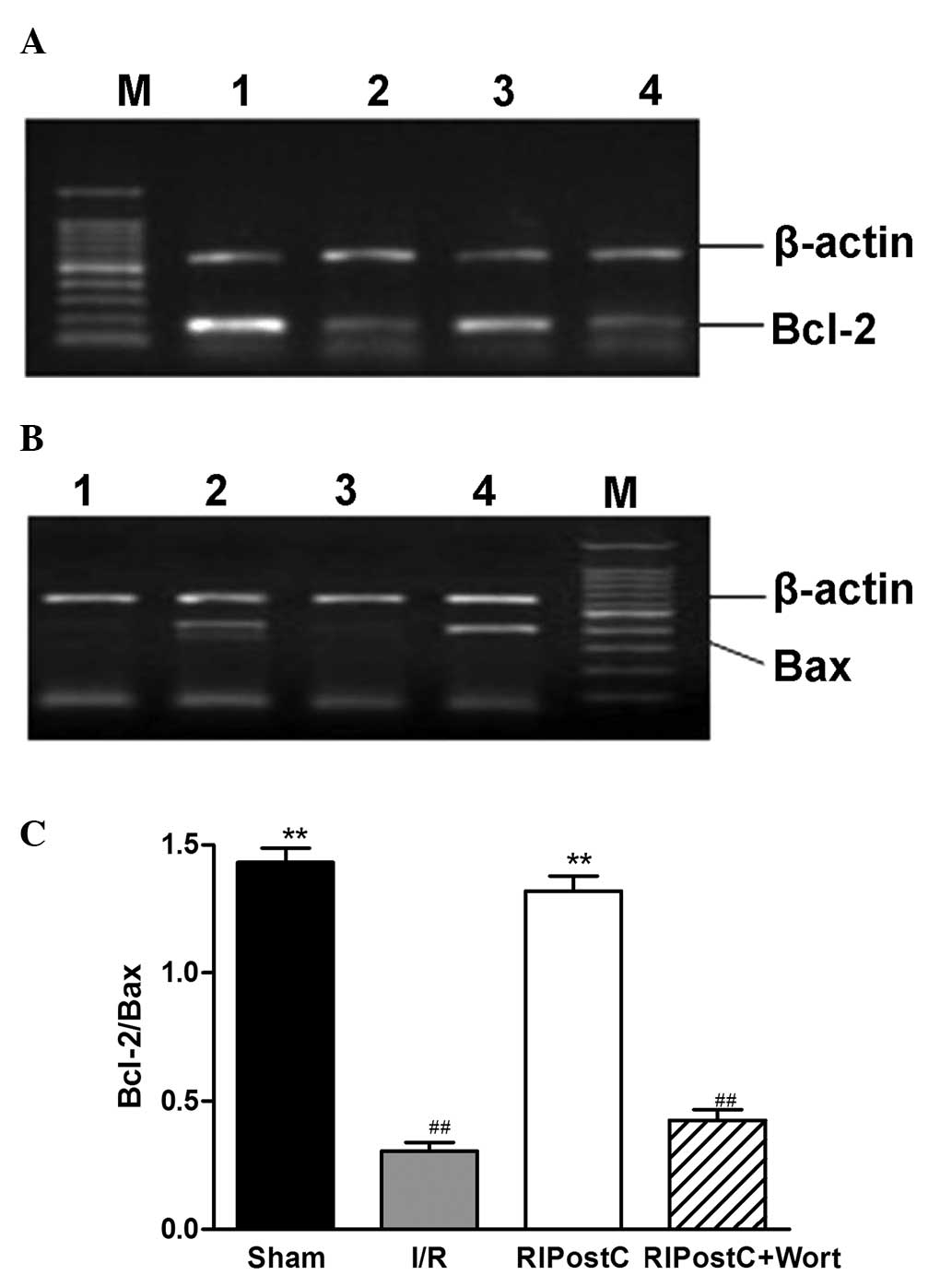 | Figure 3Expression levels of myocardial (A)
Bcl-2 and (B) Bax mRNA and (C) quantification of the Bcl-2/Bax
ratio for the different groups. Values are the mean ± standard
error (n=4). **P<0.01 vs. I/R; ##P<0.01
vs. RIPostC. M, marker; 1, Sham; 2, I/R; 3, RIPostC; 4,
RIPostC+Wort. I/R, ischemia/reperfusion; RIPostC, remote ischemic
postconditioning; Wort, wortmannin; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein. |
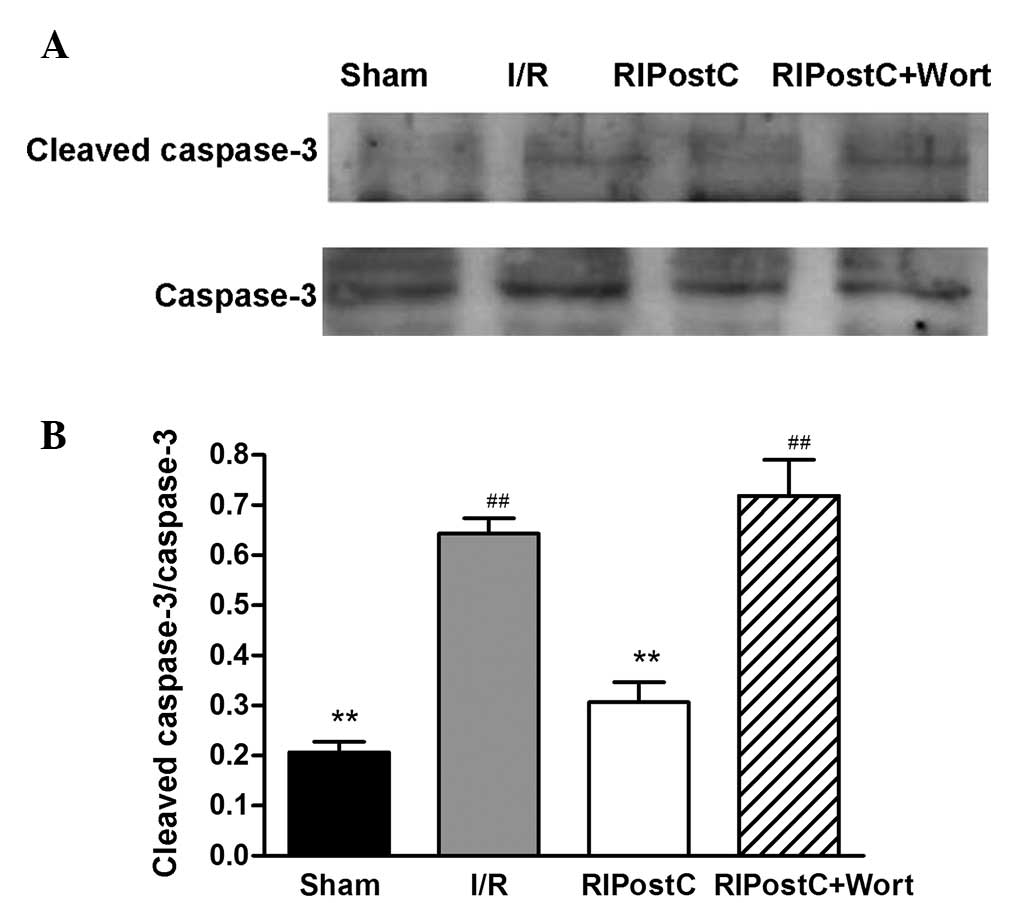
As shown in Fig. 4,
only small amounts of cleaved caspase-3 were detected in the left
anterior myocardium in the Sham group. In contrast to those of the
Sham group, the expression levels of cleaved caspase-3 and the
ratio of cleaved caspase-3/caspase-3 were increased in the I/R
group (I/R, 0.64±0.03 vs. Sham, 0.20±0.02, n=4, P<0.01).
Compared with those in the I/R group, the levels of cleaved
caspase-3 were reduced and the ratio of cleaved caspase-3/caspase-3
was suppressed in the RIPostC group (RIPostC, 0.31±0.04 vs. I/R,
0.64±0.03, P<0.01). However, treatment with wortmannin
significantly attenuated the effect of RIPostC (0.72±0.07).
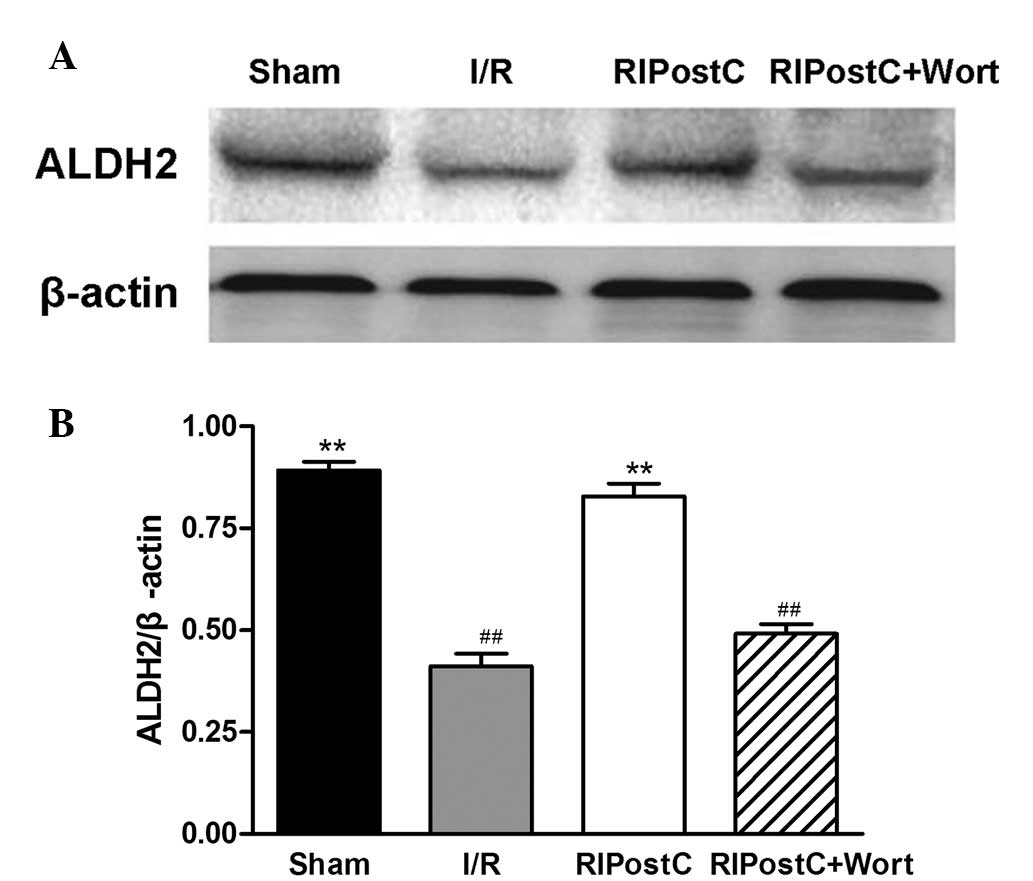
Changes in the myocardial ALDH2, Akt and
p-Akt protein levels
The western blot analysis revealed that, compared
with that of the I/R group, the ratio of ALDH2/β-actin at the
protein level in the RIPostC group was increased (RIPostC,
0.83±0.03 vs. I/R, 0.41±0.03, n=4, P<0.01). Compared with that
of the RIPostC group, the ratio in the RIPostC+Wort group was
reduced (0.49±0.03; Fig. 5).
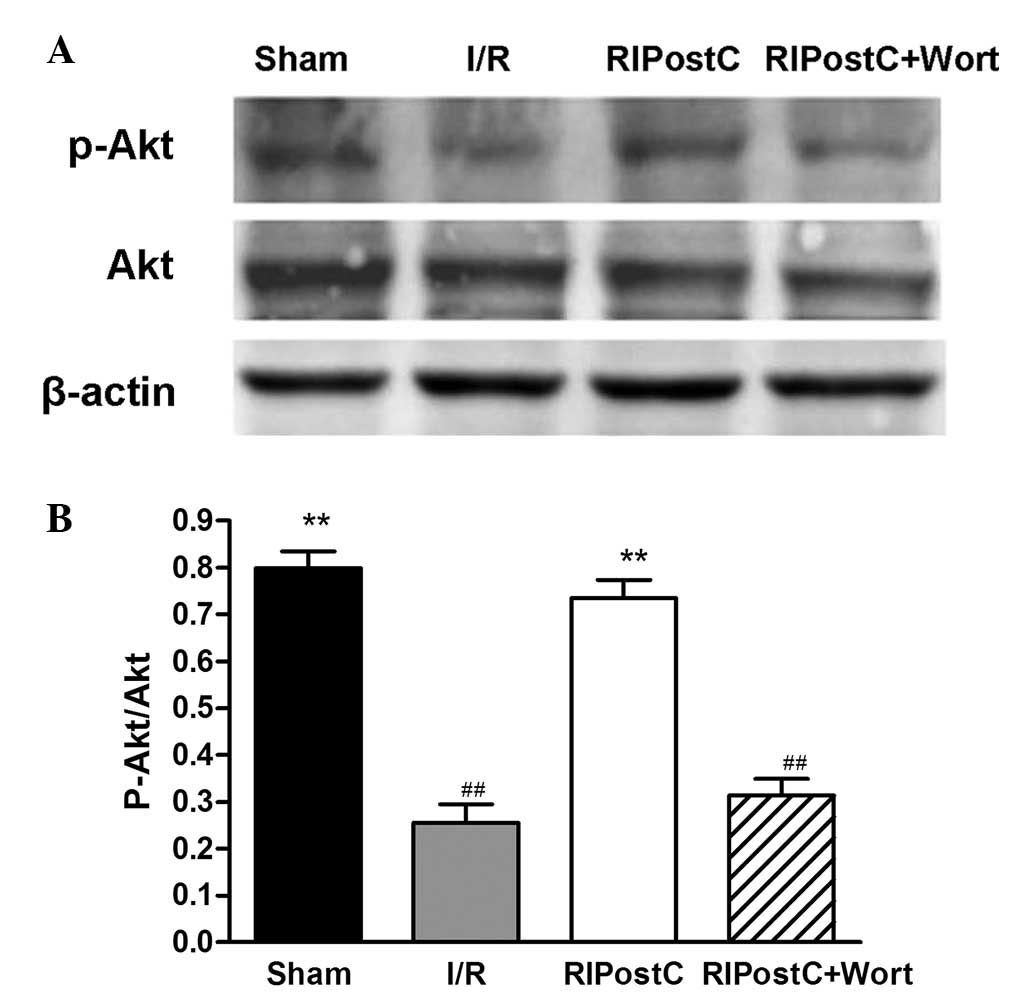
Also in the western blot analysis, compared with
that of the I/R group, the ratio of p-Akt/Akt in the RIPostC group
was increased (RIPostC: 0.74±0.04 vs. I/R: 0.26±0.04, n=4,
P<0.01), and compared with that of the RIPostC group, the ratio
in the RIPostC+Wort group was reduced (0.31±0.04; Fig. 6).
Discussion
Studies have demonstrated that ALDH2 is a
mitochondrial enzyme involved in protecting the heart against I/R
injury (20,21). In the present study, the effect of
RIPostC was evaluated and the role of ALDH2 in the RIPostC
cardioprotective effect on myocardial I/R injury was observed. As
the results showed, the expression levels of ALDH2 were reduced
following reperfusion in the I/R model compared with those in the
Sham group. The findings of the present study also demonstrated
that RIPostC reduced the myocardial infarct size, the levels of
plasma LDH and CK release, and the levels of cleaved caspase-3
protein, while the expression levels of ALDH2 protein, and the
p-Akt/Akt and Bcl-2/Bax ratio levels were increased in contrast to
those of the I/R group. All the results suggested that ALDH2 may
participate in the RIPostC cardioprotective effect and activation
of ALDH2 expression may reduce the levels of cardiomyocyte
apoptosis. The present study further identified that when the
RIPostC rats were treated with wortmannin, a PI3K inhibitor, the
protective effect was abrogated, the p-Akt/Akt ratio was markedly
reduced, and was associated with decreases in the ALDH2 and
Bcl-2/Bax levels, and the cleaved caspase-3 expression levels were
increased. All these findings indicated that RIPostC had a
cardioprotective and anti-apoptotic effect through activation of
the PI3K/Akt-dependent signaling pathway, and upregulation of the
ALDH2 expression levels; thus, ALDH2 may be an important mediator
of RIPostC.
RIPostC, which had been demonstrated to confer
cardioprotection, was first reported by Kerendi et al in
2005 (5). RIPostC was later
reported to have a cardioprotective effect in various animal
models, including rabbits, pigs and rats (22–24).
Furthermore, an increasing number of clinical studies investigated
the possible protection by remote ischemic-conditioning, mostly in
cardiac and major vascular surgery (25,26).
Remote ischemic-conditioning, which consists of transient limb
ischemia applied prior to or during a prolonged period of ischemia,
improved the outcome of patients suffering from acute ischemia or
who were at risk of developing ischemia during surgery (27). Although the protective role has
been widely recognized, the underlying mechanisms remain to be
fully elucidated. The objective of the present study was to confirm
whether cardiac function is protected through RIPostC by an
increase in the expression levels of ALDH2 and a reduction in the
levels of cardiomyocyte apoptosis.
ALDH2 is a mitochondrial enzyme which is abundantly
expressed in the heart. ALDH2 has a key role in the metabolism of
acetaldehyde and other toxic aldehydes to confer cardioprotection
(28). However, excessive levels
of aldehydes severely damage cardiomyocytes and cardiac function.
Therefore, the myocardial infarction and the levels of LDH and CK
are frequently used to quantify the amount of myocardial damage.
The findings of the present study showed that RIPostC reduced the
myocardial infarct size and inhibited the release of LDH and CK,
while increasing the levels of ALDH2 protein compared with those in
the I/R group. RIPostC may have protected the heart by upregulating
the ALDH2 expression levels against the I/R injury in the rat model
in vivo.
Numerous studies (13,29)
have indicated that ALDH2 has a key role in the cardioprotection
against I/R injury, as the overexpression of ALDH2 improves cardiac
function and reduces the levels of cardiomyocyte apoptosis. Prior
to the present study, it had already been reported that damage of
the myocardial ultrastructure accompanied the aggravation of
oxidative stress in diabetic rats. However, when ethanol was
administered to the diabetic rats in order to induce ALDH2
activity, the events of oxidative stress, the destruction of the
myocardial function and the occurrence of apoptosis were attenuated
(14,30). Those findings suggested that a
ALDH2 deficiency aggravated cardiac dysfunction, while ALDH2
activation protected the heart against myocardial injury, possibly
through detoxification of toxic aldehyde. Evidence from previous
studies also revealed that ethanol postconditioning had a
cardioprotective effect by upregulating the levels of ALDH2 mRNA
expression and inhibiting the opening of the mPTP (31), which is considered to be an
important factor in apoptosis. In regard to RIPostC
cardioprotection, the present study aimed to observe whether ALDH2
reduced the levels of apoptosis.
Cardiomyocyte apoptosis is one of the major
pathogenic mechanisms underlying myocardial I/R injury (32). Bcl-2 family proteins are potent
regulators of the mitochondrial changes during apoptosis. The
balance in the expression levels of the antiapoptotic Bcl-2 and the
proapoptotic Bax proteins has a major role in the regulation of
myocardial apoptotic cell death. The Bcl-2/Bax ratio represents the
extent of apoptosis (33,34). Caspases are regarded as the central
executors of the apoptotic pathway. Among the family of caspases,
caspase-3 ultimately executes the apoptotic signal. High levels of
cleaved caspase-3 confirm that cell death is due to apoptosis
(35). In the present study, it
was shown that in the RIPostC group, the upregulation of ALDH2 was
accompanied by an elevated myocardial Bcl-2/Bax ratio and cleaved
caspase-3 levels compared with those in the I/R group. The results
revealed that the upregulation of the ALDH2 expression levels may
contribute to reducing the occurrence of cardiomyocyte apoptosis.
Although ALDH2 is essential for cell survival, the mechanism of how
ALDH2 expression levels are upregulated in RIPostC remains to be
fully elucidated.
Activation of the RISK pathway contributes to
RIPreC-induced cardioprotection within the remote organ (36,37).
Breivik et al (36)
confirmed that RIPreC exhibited strong cardioprotective properties
via the PI3K/Akt-dependent signaling pathway. Akt, a
serine/threonie kinase, is an important mediator of the downstream
effects of RISK signaling. Once Akt is activated, phosphorylated
substrates are translocated from the cell membrane to various
subcellular compartments. Inhibition of RISK with wortmannin
reduced Akt phosphorylation and abolished ischemic pre- and
postconditioning-induced protection in previous studies (38,39).
Lang et al (20)
demonstrated that isoflurane preconditioning confers
cardioprotection by activation of ALDH2 from the RISK pathway
against I/R injury. The present study investigated whether the
activation of ALDH2 in RIPostC also proceeded via the PI3K/Akt
signaling pathway. The results demonstrated that when the rats were
treated with the PI3K inhibitor wortmannin during the RIPostC
intervention, the myocardial protective effect was reduced. This
result further supports that RIPostC had a strong cardioprotective
effect through the RISK pathway to upregulate the levels of ALDH2
expression. Furthermore, a previous study has already revealed that
activation of the PI3K/Akt signaling pathway attenuates
mitochondrial-mediated apoptosis. Upon activation, the endogenous
protein kinase Akt confers broad cardioprotection from suppression
of cell apoptosis to promotion of cellular survival in I/R injury
(33). Overexpression of Akt
preserves the Bcl-2 levels in mitochondria. In the present study,
it was observed that RIPostC upregulated the levels of ALDH2
expression as well as inhibited the occurrence of myocardial
apoptosis; however, this effect was blocked in the RIPostC+Wort
group. These observations suggested that RIPostC had a
cardioprotective and anti-apoptotic effect through activation of
the PI3K/Akt-dependent signaling pathway to upregulate the ALDH2
expression levels. Thus, ALDH2 may have a pivotal role in cell
survival and myocardial function via the PI3K/Akt-dependent
signaling pathway.
In conclusion, the present study demonstrated that
RIPostC protects the heart by upregulating the levels of ALDH2
against I/R-induced cardiomyocyte apoptosis, and the activation of
the PI3K/Akt-dependent signaling pathway is involved in the
cardioprotective effect of ALDH2.
RIPostC has a significant protective effect against
myocardial ischemia and reperfusion injury in rats through
activation of ALDH2. ALDH2 may be an important mediator in
cardioprotection through the PI3K/Akt-dependent cell survival
signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81000074) and by the Research Fund
for the Doctoral Program of Bengbu Medical College, China (no.
BYkf13A05).
References
|
1
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar
|
|
3
|
Kharbanda RK, Mortensen UM, White PA, et
al: Transient limb ischemia induces remote ischemic preconditioning
in vivo. Circulation. 106:2881–2883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003.PubMed/NCBI
|
|
5
|
Kerendi F, Kin H, Halkos ME, Jiang R,
Zatta AJ, Zhao ZQ, Guyton RA and Vinten-Johansen J: Remote
postconditioning. Brief renal ischemia and reperfusion applied
before coronary artery reperfusion reduces myocardial infarct size
via endogenous activation of adenosine receptors. Basic Res
Cardiol. 100:404–412. 2005.
|
|
6
|
Gao Q, Hu J, Hu J, Yu Y, Ye H, Li Z and
Guan S: Calcium activated potassium channel and protein kinase C
participate in the cardiac protection of remote post conditioning.
Pak J Pharm Sci. 26:285–290. 2013.PubMed/NCBI
|
|
7
|
Ren C, Yan Z, Wei D, Gao X, Chen X and
Zhao H: Limb remote ischemic postconditioning protects against
focal ischemia in rats. Brain Res. 1288:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu
M, Li J, Zhu W and Redington AN: Repeated remote ischemic
postconditioning protects against adverse left ventricular
remodeling and improves survival in a rat model of myocardial
infarction. Circ Res. 108:1220–1225. 2011. View Article : Google Scholar
|
|
9
|
Ren J: Acetaldehyde and alcoholic
cardiomyopathy: lessons from the ADH and ALDH2 transgenic models.
Novartis Found Symp. 285:69–79. 198–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li SY and Ren J: Cardiac overexpression of
alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced
myocardial dysfunction and hypertrophy: role of insulin signaling
and ER stress. J Mol Cell Cardiol. 44:992–1001. 2008. View Article : Google Scholar
|
|
11
|
Hu XY, Fang Q, Wang JS, Xie JQ, Chai BS,
Li FQ, Cui X and Yang Y: Over-expression of aldehyde
dehydrogenase-2 protects against H2O2-induced
oxidative damage and apoptosis in peripheral blood mononuclear
cells. Acta Pharmacol Sin. 32:245–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart MJ, Malek K and Crabb DW:
Distribution of messenger RNAs for aldehyde dehydrogenase 1,
aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human
tissues. J Investig Med. 44:42–46. 1996.PubMed/NCBI
|
|
13
|
Chen CH, Budas GR, Churchill EN, Disatnik
MH, Hurley TD and Mochly-Rosen D: Activation of aldehyde
dehydrogenase-2 reduces ischemic damage to the heart. Science.
321:1493–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Q, Wang HJ, Wang XM, Kang PF, Yu Y, Ye
HW, Zhou H and Li ZH: Activation of ALDH2 with ethanol attenuates
diabetes induced myocardial injury in rats. Food Chem Toxicol.
56:419–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang J, Chen L, Wu L and Li W:
Intra-cardiac remote ischemic post-conditioning attenuates
ischemia-reperfusion injury in rats. Scand Cardiovasc J.
43:386–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hausenloy DJ and Yellon DM: Reperfusion
injury salvage kinase signalling: taking a RISK for
cardioprotection. Heart Fail Rev. 2:217–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwartz LM and Lagranha CJ: Ischemic
postconditioning during reperfusion activates Akt and ERK without
protecting against lethal myocardial ischemia-reperfusion injury in
pigs. Am J Physiol Heart Circ Physiol. 290:H1011–H1018. 2006.
View Article : Google Scholar
|
|
18
|
Dong XX, Wang YR, Qin S, Liang ZQ, Liu BH,
Qin ZH and Wang Y: p53 mediates autophagy activation and
mitochondria dysfunction in kainic acid-induced excitotoxicity in
primary striatal neurons. Neuroscience. 207:52–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YQ, Liu CH, Zhang JQ, Zhu DN and Yu
BY: Protective effects and active ingredients of yi-qi-fu-mai
sterile powder against myocardial oxidative damage in mice. J
Pharmacol Sci. 122:17–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lang XE, Wang X, Zhang KR, Lv JY, Jin JH
and Li QS: Isoflurane preconditioning confers cardioprotection by
activation of ALDH2. PLoS One. 8:e524692013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Contractor H, Støttrup NB, Cunnington C,
Manlhiot C, Diesch J, Ormerod JO, Jensen R, Bøtker HE, Redington A,
Schmidt MR, Ashrafian H and Kharbanda RK: Aldehyde dehydrogenase-2
inhibition blocks remote preconditioning in experimental and human
models. Basic Res Cardiol. 108:3432013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li CM, Zhang XH, Ma XJ and Luo M: Limb
ischemic postconditioning protects myocardium from
ischemia-reperfusion injury. Scand Cardiovasc J. 40:312–317. 2006.
View Article : Google Scholar
|
|
23
|
Andreka G, Vertesaljai M, Szantho G, Font
G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS,
Ashrafian H, Frenneaux MP and Andreka P: Remote ischaemic
postconditioning protects the heart during acute myocardial
infarction in pigs. Heart. 93:749–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin P, Zhu W, Li J, Ma S, Wang L, Liu M,
Wei M and Redington AN: Combined local ischemic postconditioning
and remote perconditioning recapitulate cardioprotective effects of
local ischemic preconditioning. Am J Physiol Heart Circ Physiol.
298:H1819–H1831. 2010. View Article : Google Scholar
|
|
25
|
Bøtker HE, Kharbanda R, Schmidt MR,
Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen
TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen
SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN and
Nielsen TT: Remote ischaemic conditioning before hospital
admission, as a complement to angioplasty, and effect on myocardial
salvage in patients with acute myocardial infarction: a randomised
trial. Lancet. 375:727–734. 2010.PubMed/NCBI
|
|
26
|
Thielmann M, Kottenberg E, Boengler K,
Raffelsieper C, Neuhaeuser M, Peters J, Jakob H and Heusch G:
Remote ischemic preconditioning reduces myocardial injury after
coronary artery bypass surgery with crystalloid cardioplegic
arrest. Basic Res Cardiol. 105:657–664. 2010. View Article : Google Scholar
|
|
27
|
Loukogeorgakis SP, Williams R,
Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM,
Deanfield JE and MacAllister RJ: Transient limb ischemia induces
remote preconditioning and remote postconditioning in humans by a
K(ATP)-channel dependent mechanism. Circulation. 116:1386–1395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Churchill EN, Disatnik MH and Mochly-Rosen
D: Time-dependent and ethanol-induced cardiac protection from
ischemia mediated by mitochondrial translocation of varepsilonPKC
and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol.
46:278–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He L, Liu B, Dai Z, Zhang HF, Zhang YS,
Luo XJ, Ma QL and Peng J: Alpha lipoic acid protects heart against
myocardial ischemia-reperfusion injury through a mechanism
involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol.
678:32–38. 2012. View Article : Google Scholar
|
|
30
|
Wang HJ, Kang PF, Wu WJ, Tang Y, Pan QQ,
Ye HW, Tang B, Li ZH and Gao Q: Changes in cardiac mitochondrial
aldehyde dehydrogenase 2 activity in relation to oxidative stress
and inflammatory injury in diabetic rats. Mol Med Rep. 8:686–690.
2013.PubMed/NCBI
|
|
31
|
Li ZH, Jiang CR, Xia ML, Ye HW, Guan SD
and Gao Q: Activation of mitochondrial aldehyde dehydrogenase 2 and
inhibition of mitochondrial permeability transition pore involved
in cardioprotection of ethanol postconditioning. Zhejiang Da Xue
Xue Bao Yi Xue Ban. 39:566–571. 2010.(In Chinese).
|
|
32
|
Xu D, Guthrie JR, Mabry S, Sack TM and
Truog WE: Mitochondrial aldehyde dehydrogenase attenuates
hyperoxia-induced cell death through activation of ERK/MAPK and
PI3K-Akt pathways in lung epithelial cells. Am J Physiol Lung Cell
Mol Physiol. 291:L966–L975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang SP, Wang ZH, Peng DY, Li SM, Wang H
and Wang XH: Therapeutic effect of mesenchymal stem cells in rats
with intracerebral hemorrhage: reduced apoptosis and enhanced
neuroprotection. Mol Med Rep. 6:848–854. 2012.PubMed/NCBI
|
|
34
|
Sun H, Zhou F, Wang Y, Zhang Y, Chang A
and Chen Q: Effects of beta-adrenoceptors overexpression on cell
survival are mediated by Bax/Bcl-2 pathway in rat cardiac myocytes.
Pharmacology. 78:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YB, Qin J, Zheng XY, et al: Diallyl
trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via
downregulation of Akt phosphorylation in human T24 bladder cancer
cells. Phytomedicine. 17:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breivik L, Helgeland E, Aarnes EK, Mrdalj
J and Jonassen AK: Remote postconditioning by humoral factors in
effluent from ischemic preconditioned rat hearts is mediated via
PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic
Res Cardiol. 106:135–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tamareille S, Mateus V, Ghaboura N,
Jeanneteau J, Croué A, Henrion D, Furber A and Prunier F: RISK and
SAFE signaling pathway interactions in remote limb ischemic
perconditioning in combination with local ischemic
postconditioning. Basic Res Cardiol. 106:1329–1339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mocanu MM, Bell RM and Yellon DM: PI3
kinase and not p42/p44 appears to be implicated in the protection
conferred by ischemic preconditioning. J Mol Cell Cardiol.
34:661–668. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Skyschally A, van Caster P, Boengler K,
Gres P, Musiolik J, Schilawa D, Schulz R and Heusch G: Ischemic
postconditioning in pigs: no causal role for RISK activation. Circ
Res. 104:15–18. 2009. View Article : Google Scholar : PubMed/NCBI
|