Introduction
Since it was first described in 1968, IgA
nephropathy (IgAN) has become the most commonly diagnosed form of
primary glomerular disease worldwide (1) and remains a significant cause of end
stage renal disease (2–4). IgAN is characterized by an
overrepresentation of IgA1 molecules in the serum and mesangial
deposition of IgA immune complexes, accompanied by mesangial
proliferative glomerulonephritis. Clinically, the main
manifestations of IgAN are hematuria and/or proteinuria which
frequently emerge or worsen following upper respiratory tract
infection. Mesangial deposition of IgA, particularly polymeric IgA1
(pIgA1), was hypothesized to be an initiating event in the
pathogenesis of IgAN (5). However,
the mechanism of IgA1 production remains to be determined.
Activation of toll-like receptor 9 (TLR9) in the
mucosal tissue, including tonsils, stimulated by upper respiratory
infection or bacteria, which contain CPG-DNA, have an important
role in the occurrence and progression of IgAN (6). B-cell activating factor (BAFF) is an
important immune regulatory factor which is involved in B cell
proliferation, differentiation and secretion of IgA1
immunoglobulin. A previous study found that tonsil mononuclear
cells (TMCs) of IgAN patients stimulated by IFN-γ in vitro
induced increased BAFF secretion and proliferation of TMCs
(7). However, research on the
expression of BAFF and TLR9 in blood circulation and their
association has rarely been reported. In the present study, the
levels of serum BAFF as well as levels of TLR9 mRNA and protein in
PBMCs were evaluated, and correlation and regression analyses were
performed in order to investigate the pathogenesis of IgAN.
Materials and methods
Subjects
IgAN patient group: 30 patients were admitted to the
Second Xiangya Hospital (Changsha, China) between August 2011 and
February 2012 for a renal biopsy check. IgAN patients (14 men and
16 women; age range, 13–63 years; mean age, 33.63±14.04 years;
course of disease, one month to six years) were identified by
immunohistopathological examination of renal biopsy specimens. IgAN
was diagnosed on the basis of mesangial cell proliferation and
mesangial matrix expansion under light microscopy and detection of
predominantly granular mesangial IgA deposits by
immunofluorescence. Renal frozen sections were cut into 5-μm thick
sections and then incubated with rabbit anti-human antibody (Dako,
Denmark, Copenhagen) for direct immunofluorescence examination. The
range of IgA fluorescence intensity was between 1+ and 5+. Systemic
lupus erythematosus, Henoch-Schnlein purpura and hepatic diseases
were excluded by clinical history, physical examination and
negative laboratory test results. All IgAN patients exhibited
hematuria and normal renal function, while 11 exhibited
proteinueria. Prior to biopsy, patients that were selected were not
undergoing drug therapy which influences PBMCs, including
corticosteroids, immunosuppressants and antibiotics.
Minimal glomerular abnormalities (MGA) group: 30
patients (14 men and 16 women; age range, 23–55 years; mean age,
36.73±13.99 years) were admitted to the Second Xiangya Hospital
(Changsha, China) for renal diseases between August 2011 and
February 2012. Glomerular minor lesion was diagnosed on the basis
of light microscopy and immunofluorescence studies, and patients
with IgA deposits were excluded. All patients had normal renal
function.
Normal control group: 30 individuals (14 men and 16
women; age range, 15–60 years; mean age, 33.87±10.47 years) were
selected from individuals admitted to the Health Examination Center
of the Second Xiangya Hospital (Changsha, China) between November
2011 and February 2012. Based on laboratory tests and clinical
manifestations, viral hepatitis, renal disease, diabetes, heart
disease and other diseases were excluded and all individuals did
not present with respiratory, gastrointestinal tract or other
mucosal infections.
The study was performed in accordance with ‘Ethical
Principles for Medical Research Involving Human Subjects’ (World
Medical Association Declaration of Helsinki, 2004) and approved by
the Ethics Review Committee of the Second Xiangya Hospital of
Central South University and the Hunan Government Medical Research
Council. Oral informed consent was obtained from all the
subjects.
Specimen collection
Morning fasting blood of all subjects was collected,
serum was isolated within 2 h and preserved for IgA1 and BAFF
evaluation. Mononuclear cells were isolated by density gradient
centrifugation using Lymphocyte Separation Medium (Sigma-Aldrich,
St. Louis, MO, USA). The cells were washed twice with a minimal
essential medium supplemented with 7.5 mol/l hydroxyethyl
piperazineethanesulfonic acid and 2% heat-inactivated fetal calf
serum, and TLR9 mRNA and protein were evaluated by quantitative
polymerase chain reaction (qPCR) and western blot analysis,
respectively. The first morning urine of IgAN patients was
collected for urine sediment test within 2 h. According to the
results of the urinary sediment artificial red blood cell (RBC)
count, the degree of hematuria was divided into four ranks: <8
(0), 8–50 (1), 50–100 (2) and >100,000 (3).
Determination of serum IgA1 and BAFF
Serum IgA1 and BAFF levels were determined by
specific ELISA using horseradish peroxidase (HRP)-labeled goat
anti-human IgA1 and BAFF as the detector antibody. A commercial
ELISA kit (R&D Systems, Minneapolis, MN, USA) was used to
measure BAFF and IgA1 and the amount of BAFF and IgA1 was measured
by spectrophotometry at 450 nm using a microplate reader (Molecular
Devices, Silicon Valley, CA, USA).
qPCR analysis for TLR9 mRNA
expression
Total RNA was extracted from freshly isolated cells
using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), and was reverse-transcribed into cDNAs with
SuperScript™ II Reverse Transcriptase. PCR amplification of TLR9
was performed using two primers: TLR9 forward,
(5′-CTCACCCACCTGTCACTCAAG) and reverse, (5′-AGTTTGACGATGCGGTTGTAG),
which generated a specific 104 bp PCR product following 30 cycles
of PCR reaction (30 sec at 95°C, 30 sec at 56°C, and 60 sec at
72°C). β-actin was amplified as a control.
Western blot analysis of TLR9 protein
expression
Cells were lysed with Nonidet P-40 (NP-40) lysis
buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, and 1% NP-40) in the
presence of protease inhibitors at 4°C. Following incubation for 30
min, the lysates were centrifuged at 4°C for 10 min at 10,000 × g.
The protein concentrations of the lysates were measured using a BCA
method (BCA Protein Assay kit; Bio-Rad, Hercules, CA, USA). Soluble
lysates were boiled for 5 min with 2× SDS sample buffer. Samples
(40 mg per lane) from the total protein fraction were loaded and
separated by SDS-PAGE. Protein was transferred to polyvinylidene
difluoride membranes (Pharmacia Corporation, Peapack, NJ, USA) and
probed with primary antibodies against human TLR9 (goat polyclonal
antibody; R&D Systems). The primary antibodies that bound to
the target proteins were detected using HRP-conjugated anti-goat
immunoglobulin G (Promega Corporation, Madison, WI, USA). The
antibodies were visualized with enhanced chemiluminescent detection
(Pierce Biotechnology Inc., Rockford, IL, USA).
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for
Windows was used for data analysis. Continuous variables were
expressed as mean ± standard deviation. Comparisons were based on
the χ2 test for categorical data, and analysis of
variance for quantitative data and data at different time-points
were performed. Correlations between the parameters were analyzed
by the Spearman’s rank correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
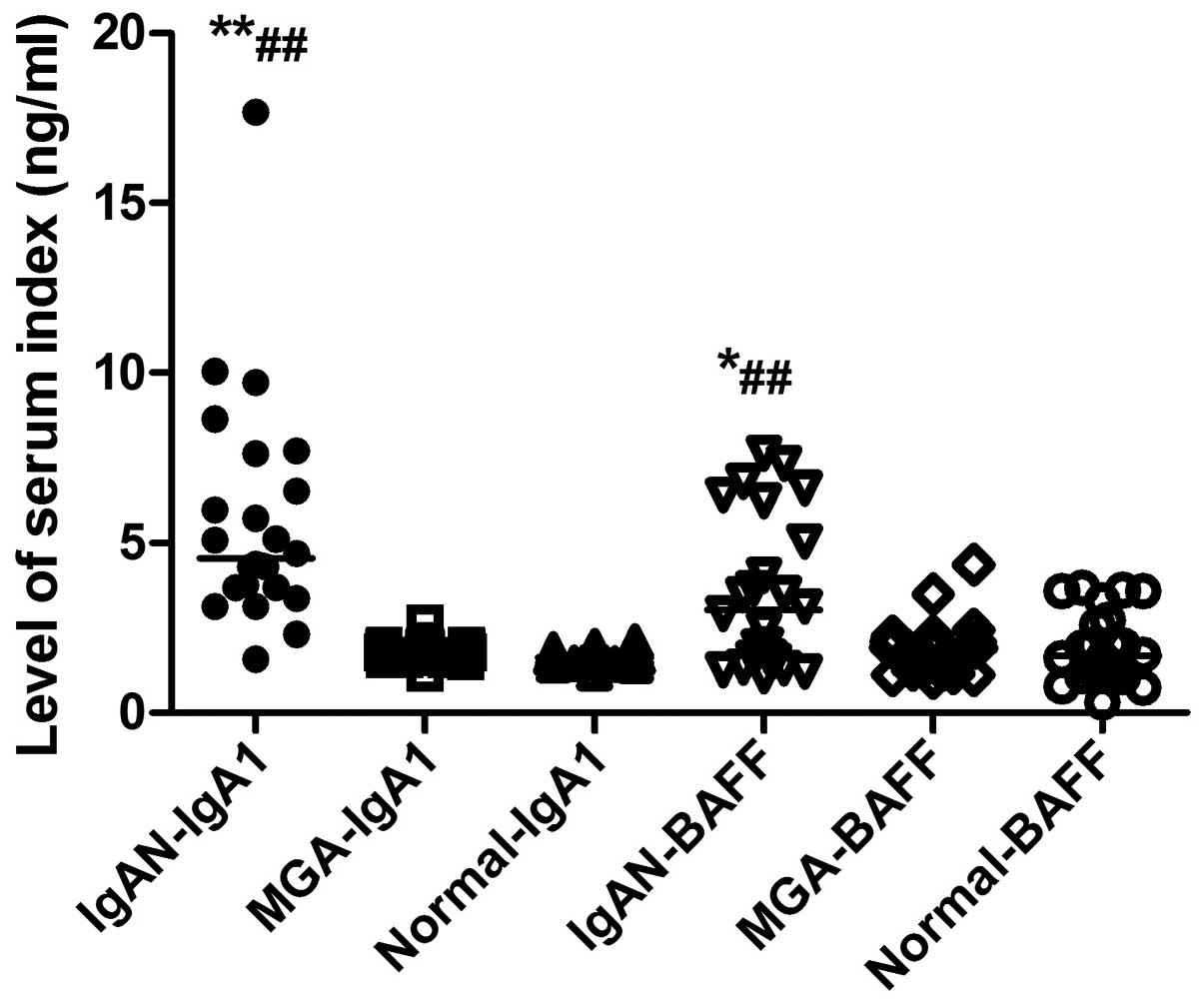
Serum levels of BAFF are increased in
IgAN
The serum BAFF levels of all subjects in the
different groups are shown in Fig.
1. Serum levels of BAFF in the IgAN, MGA and normal control
groups were 3.41±2.13, 2.05±1.018, and 1.86±0.99 ng/ml,
respectively. The serum levels of BAFF in the IgAN group were
significantly increased compared with the MGA (P<0.05) and
normal control (P<0.01) groups, while there was no difference
between the MGA and normal control groups (P>0.05).
Serum levels of IgA1 are increased in
IgAN
The serum IgA1 levels of all subjects in the
different groups are presented in Fig.
1. Serum levels of IgA1 in the IgAN, MGA and normal control
groups were 6.21±3.89, 1.78±0.26, and 1.66±0.23 ng/ml,
respectively. Serum levels of IgA1 in the IgAN group were
significantly increased compared with the MGA (P<0.01) and
normal control (P<0.01) groups, while there was no difference
between the MGA and normal control groups (P>0.05).
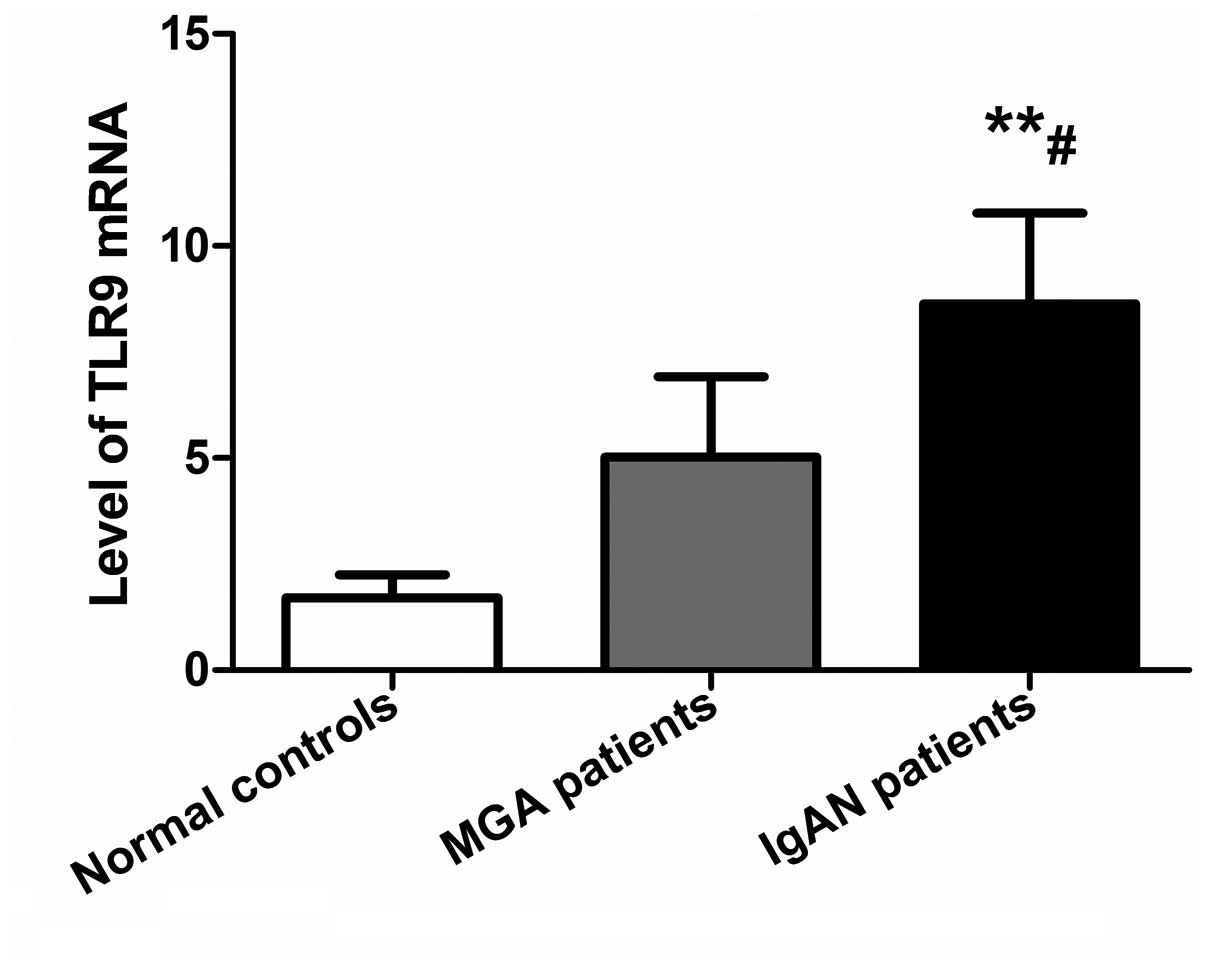
TLR9 mRNA levels in PBMCs are increased
in IgAN
Levels of TLR9 mRNA in PBMCs in the IgAN, MGA and
normal control groups were 8.65±2.12, 5.03±1.89, and 1.71±0.54,
respectively. qPCR data were analyzed by the 2−ΔΔCt
method and are shown in Fig. 2.
TLR9 mRNA levels in PBMCs in IgAN patients were significantly
higher compared with the MGA (P<0.05) and normal control
(P<0.01) groups, while there was no significant difference
between the MGA and normal control groups (P>0.05).
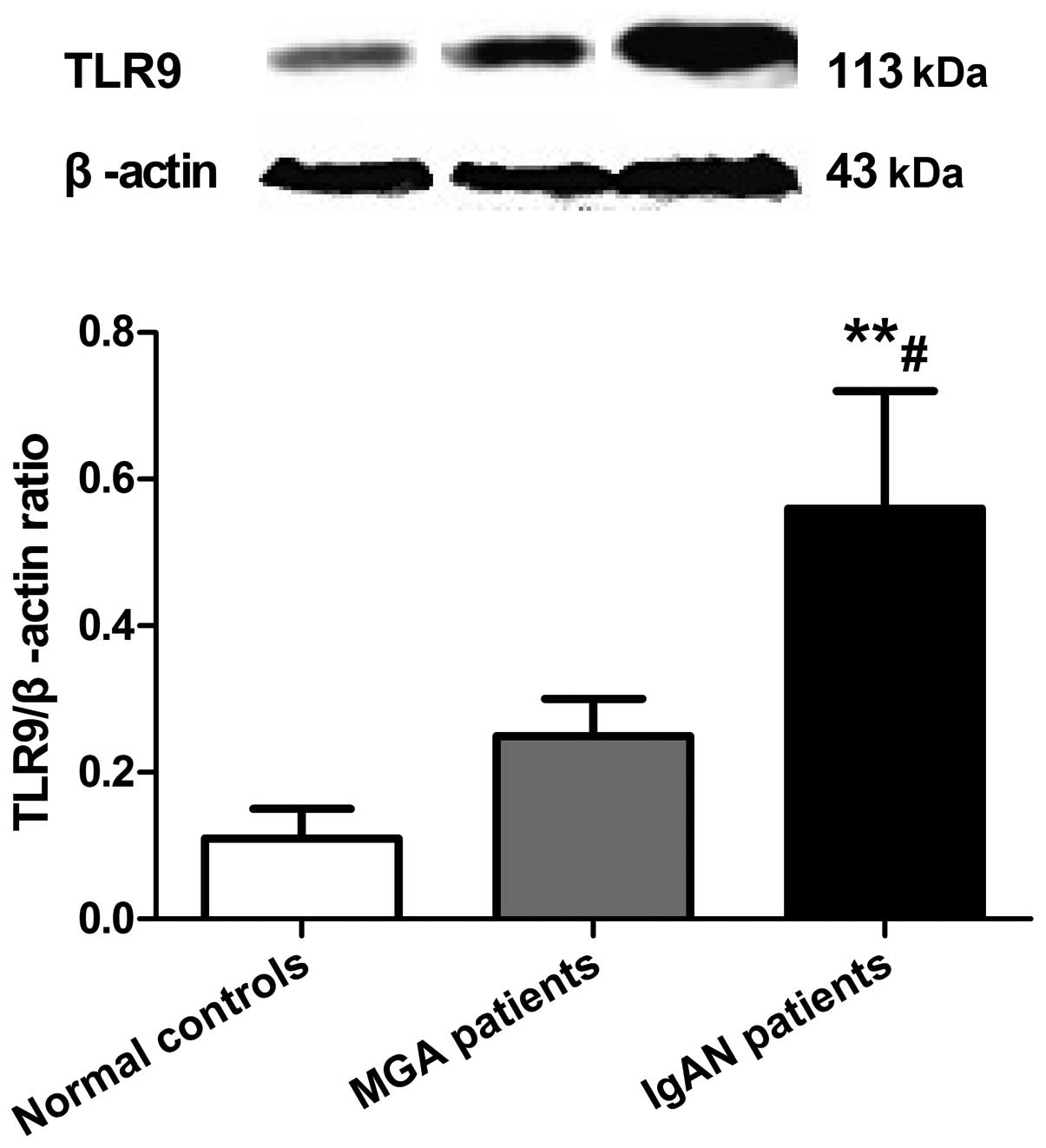
TLR9 protein levels in PBMCs are
increased in IgAN
Expression levels of TLR9 protein in PBMCs in the
IgAN, MGA and normal control groups were 0.56±0.16, 0.25±0.05, and
0.11±0.04, respectively (Fig. 3).
TLR9 protein expression in PBMCs in the IgAN group was
significantly higher compared with the MGA (P<0.05) and normal
control (P<0.01) groups, while there was no difference between
the MGA and normal control groups (P>0.05).
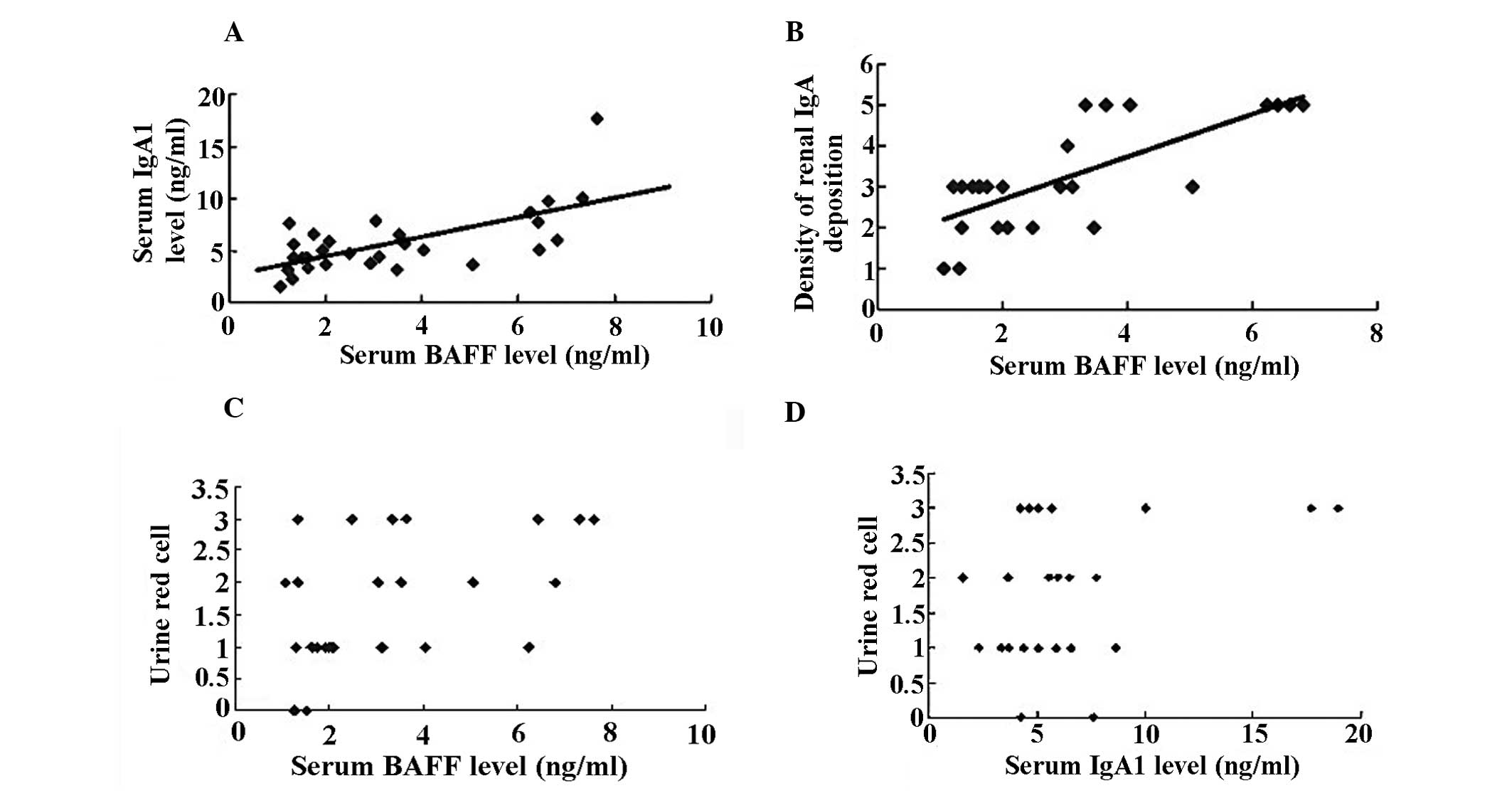
Correlation analysis
By correlation and regression analysis, it was
observed that serum levels of BAFF were positively correlated with
serum levels of IgA1 (rp, 0.515, P<0.01; Fig. 4A) and mesangial IgA deposition
density (P<0.01, rp, 0.746; Fig.
4B), while there was no correlation between rank of urinary RBC
count and serum levels of BAFF (Fig.
4C) and IgA1 (Fig. 4D) in IgAN
patients (P<0.05, respectively).
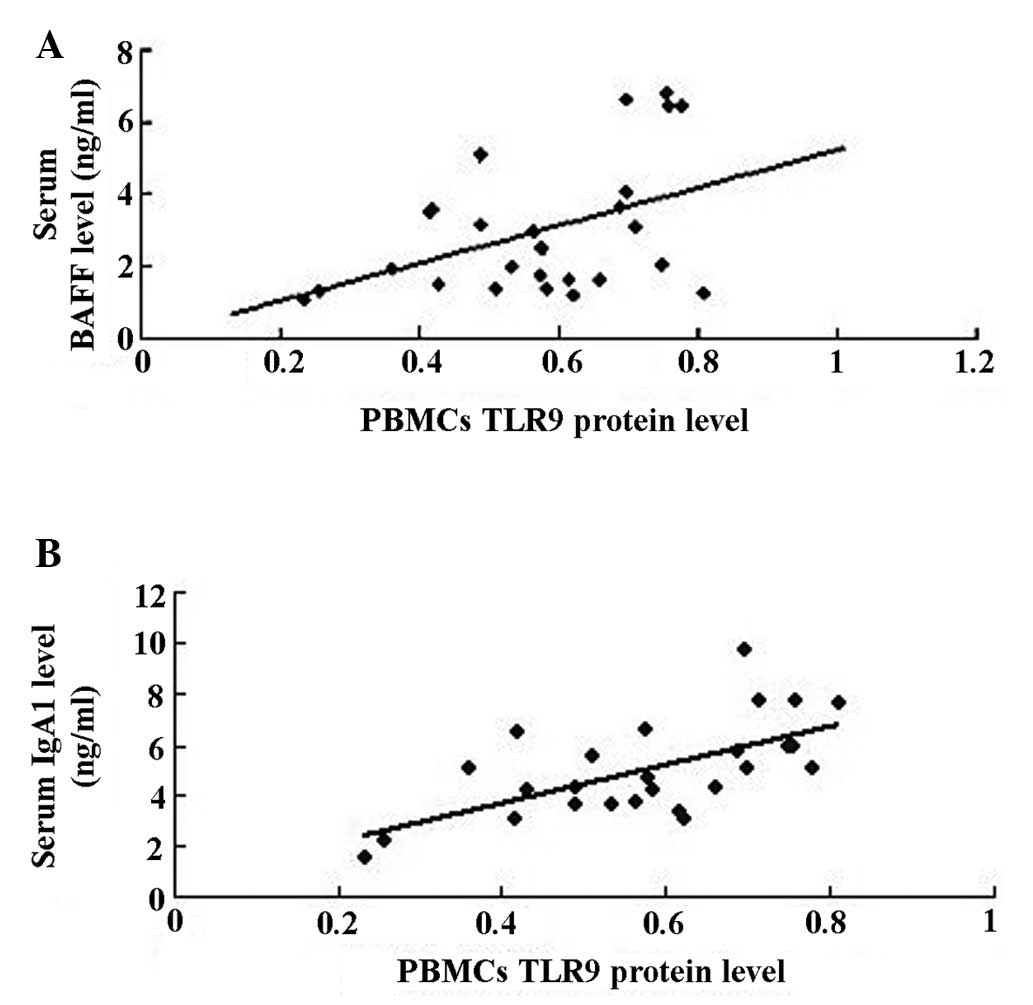
The correlation and regression analysis suggested
that the expression levels of TLR9 protein in PBMCs were positively
correlated with serum levels of BAFF (rp, 0.444, P<0.05;
Fig. 5A) and IgA1 (rp, 0.633,
P<0.01; Fig. 5B) in the IgAN
group.
Discussion
Polymeric IgA1 (pIgA1) has been suggested to be the
initiating event in the pathogenesis of IgAN; however, the
mechanism of polymeric IgA1 formation is obscure (5). Previous studies revealed that the
serum and tonsil tissue levels of IgA1 were elevated (8,9) and
the number of IgA-producing lymphocytes was increased in the serum
of patients with IgANs. In the present study, the serum levels of
IgA1 were observed to be significantly higher in patients with IgAN
compared with the MGA and normal control groups (P<0.01), which
is consistent with a previous study (9). Clinically, gross hematuria frequently
emerges following upper respiratory infections; thus, it is
hypothesized that mucosal immunity stimulated by cytosine-guanine
dinucleotide (CpG)-DNA is important in the pathogenesis of IgAN
(7). In mouse models, intranasal
immunization with CpG-DNA induced marked systemic and local IgA
responses (10), and clinically
presented with increased levels of serum IgA, hematuria,
proteinuria and renal IgA deposition intensity (11). In previous studies by our group,
the levels of IgA1 in tonsil mononuclear cells stimulated by
lipopolysaccharide or hemolytic streptococcus in the IgAN group
were significantly increased compared with the unstimulated group
(12).
TLR9 is a member of the TLR family (12) and mainly recognizes the CpG motif
in unmethylated bacterial DNA. Oligodeoxynucleotides (ODNs) with
CpG (CpG-ODN) are capable of mimicking the immunostimulatory
activity of microbial DNA (14).
Kajiyama et al (11)
observed that three CpG-ODNs, which were inoculated nasally in
mice, may result in different manifestations by stimulating TLR9 of
B cells and/or dendritic cells. Therefore, a number of researchers
hypothesized that the activation of TLR9 in the mucosa may be
involved in the pathogenesis of IgAN (15–17).
Suzuki et al (6) also
demonstrated that activation of the TLR9/myeloid differentiation
(MyD)88 pathway by common antigens may have a role in the
development of IgAN and affect its severity. In the present study,
the levels of TLR9 mRNA and protein in PBMCs of patients with IgAN
were higher than those in the MGA and normal control groups, which
further validated the role of TLR9 in IgAN.
BAFF is a member of the tumor necrosis factor
family, is critical for the maintenance of normal B-cell
development and homeostasis (18)
and is closely associated with autoimmune diseases (18,19).
The BAFF protein is mainly expressed by myeloid lineage cells,
including dendritic cells (20,21),
and its expression is upregulated by interferon-γ and
interleukin-10 (22). To date,
studies have shown that CpG-ODN may upregulate the expression and
secretion of BAFF and the recognized detailed processes are as
follows: Only TLR9 or B cells have a role in CpG-ODN-promoted
myeloid cell secretion of IL-12, IL-18, IFN-α. Furthermore, the
above cytokines promote NK cells secreting IFN-γ, which may
upregulate the secretion of BAFF. Finally, BAFF promotes
proliferation and antibody secretion of B cells by the interaction
between BAFF and the B cell surface receptor (20,23–25).
McCarthy et al (24)reported that BAFF-transgenic mice
showed an increase in serum IgA levels, as well as a deposition of
IgA immune complexes in the renal glomerular mesangium (26). Recently, Xin et al (27) showed that levels of serum BAFF were
elevated in patients with IgAN and were associated with clinical
and pathological features of the disease. These observations
indicate that BAFF may be involved in the development of IgAN. The
present study also revealed that the levels of serum BAFF were
significantly higher in the IgAN compared with the MGA and normal
control groups (P<0.05; P<0.01), and a significantly positive
correlation between serum BAFF levels and the IgA deposition in
density glomeruli (P<0.01; rp, 0.746) was observed, while no
correlation between levels of serum IgA1 and the rank of urinary
RBC count (P>0.05) was observed. Therefore, serum BAFF is
hypothesized to be closely associated with hyper-production of
IgA1, which has a key role in the pathogenesis of IgAN.
Previously, α-hemolytic streptococci have been
isolated and identified in all patients with IgAN (28). It has been confirmed that the
hemolytic streptococcus contains CpG-DNA, which may activate TLR9
in human B cells and plasmacytoid dendritic cells (pDCs) (29). The initial target of CpG-DNA is the
plasmacytoid dendritic cells, and it may upgrade the expression of
the TLR9 through stimulating pCDs. TLR9 activation of B cells may
increase the antigenic response sensitivity, promote B cell
differentiation into antibody-secreting plasma cells, and have a
role in the adaptive immune response. However, the mechanism of
TLR9 in the activation of B cells remains unknown. Fujieda et
al (30) showed that the
expression levels of IFN-γ and IgA in tonsil mononuclear cells were
significantly higher in patients with IgAN compared with patients
with chronic tonsillitis and it was hypothesized that IFN-γ has a
role in the pathogenesis of IgAN. Goto et al (7) demonstrated that when stimulated with
CpG-ODN, the production of IgA, BAFF and IFN-γ in TMCs were
significantly increased in patients with IgAN compared with a
control group. IgA production may be inhibited by the treatment
with anti-BAFF and/or anti-IFN-γ antibodies, while treatment with
IFN-γ significantly increased the expression of BAFF in CD1c cells
and TMCs in patients with IgAN compared with those in a control
group. Therefore, Goto et al (7) hypothesized that a hyper-immune
response to microbial DNA may be present in patients with IgAN and
may induce IFN-γ-mediated upregulation of BAFF, resulting in
overexpression of IgA in patients with IgAN.
Pontarollo et al (29) reported that the ability of CpG-ODN
to stimulate IFN-γ secretion was entirely abrogated by the
depletion of DH59B cells and that B-cell proliferation was
significantly reduced. Buchanan et al (31) showed that when purified bovine B
cells were co-cultured with CD14+ myeloid cells and/or
BAFF, there was a significant increase in CpG-specific B-cell
proliferation. Furthermore, Buchanan et al (31) found that stimulation with CpG
helper cells in mononuclear cells may secrete a number of
cytokines, which may activate antigens on B cells, including TLR9,
and may indirectly promote activation of B cells. A previous study
(32) also found that stimulation
by CpG-ODN bovine peripheral CD21+ B cells may express
TLR9 and proliferation in mixed cell populations. Based on a number
of studies, the present study confirms that stimulation with
CpG-ODN myeloid cells may secrete IL-12, IL-8 and IFN, which
promotes the secretion of IFN-γ by NK cells. IFN-γ upgrades BAFF
secretion and the excessive secretion of BAFF upregulates B cell
activity, including proliferation and differentiation into
antibody-secreting cells. Finally, induced abnormal secretion of
IgA and renal IgA deposition cause hematuria and proteinuria. In
the present study, the expression levels of TLR9 were significantly
correlated with the serum levels of BAFF and IgA1 in patients with
IgAN (P<0.05, P<0.01, respectively). Thus, it was
hypothesized that in the peripheral blood, TLR9 may increase the
expression levels of BAFF, which promotes B lymphocytes to secrete
immunoglobulins, particularly IgA1. A previous study also reported
that BAFF is a co-stimulator of TLR and B-1 cells (33). From these studies, it is
hypothesized that the high immune status induced by microorganism
DNA may result in the excessive secretion of BAFF, thus leading to
the excessive secretion of IgA through the activation of B-1
cells.
In conclusion, TLR9 mRNA and protein levels in PBMCs
as well as serum levels of BAFF were significantly increased and
may be associated with the overexpression of serum IgA1 in patients
with IgAN.
Acknowledgements
This study was supported by grants from the Key
Program (no. 81170663) of the National Natural Science Foundation
of China and the Program of the Natural Science Foundation of Hunan
province (no. 12JJ6094).
References
|
1
|
D’Amico G: The commonest
glomerulonephritis in the world: IgA nephropathy. Q J Med.
64:709–727. 1987.PubMed/NCBI
|
|
2
|
Appel GB and Waldman M: The IgA
nephropathy treatment dilemma. Kidney Int. 69:1939–1944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barratt J and Feehally J: IgA nephropathy.
J Am Soc Nephrol. 16:2088–2097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Julian BA and Novak J: IgA nephropathy: an
update. Curr Opin Nephrol Hypertens. 13:171–179. 2004. View Article : Google Scholar
|
|
5
|
Lai KN: Pathogenesis of IgA nephropathy.
Nat Rev Nephrol. 8:275–283. 2012. View Article : Google Scholar
|
|
6
|
Suzuki H, Suzuki Y, Narita I, et al:
Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc
Nephrol. 19:2384–2395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goto T, Bandoh N, Yoshizaki T, et al:
Increase in B-cell-activation factor (BAFF) and IFN-gamma
productions by tonsillar mononuclear cells stimulated with
deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in
patients with IgA nephropathy. Clin Immunol. 126:260–269. 2008.
View Article : Google Scholar
|
|
8
|
Suzuki S, Fujieda H, Sunaga H, et al:
Immune response of tonsillar lymphocytes to Haemophilus
parainfluenzae in patients with IgA nephropathy. Clin Exp
Immunol. 119:328–332. 2000. View Article : Google Scholar
|
|
9
|
Fujieda S, Suzuki H, Sunaga, et al:
Induction of IgA against Haemophilus parainfluenzae antigens
in tonsillar mononuclear cells from patients with IgA nephropathy.
Clin Immunol. 95:235–243. 2000.
|
|
10
|
McCluskie MJ, Weeratna RD and Davis HL:
Intranasal immunization of mice with CpG DNA induces strong
systemic and mucosal responses that are influenced by other mucosal
adjuvants and antigen distribution. Mol Med. 6:867–877. 2000.
|
|
11
|
Kajiyama T, Suzuki Y, Kihara M, et al:
Different pathological roles of toll-like receptor 9 on mucosal B
cells and dendritic cells in murine IgA nephropathy. Clin Dev
Immunol. 2011:8196462011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Peng Y, Liu F, Xiao W, Zhang Y and
Li W: Expression of IgA class switching gene in tonsillar
mononuclear cells in patients with IgA nephropathy. Inflamm Res.
60:869–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krieg AM and Vollmer J: Toll-like
receptors 7, 8, and 9: linking innate immunity to autoimmunity.
Immunol Rev. 220:251–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krieg AM, Yi AK, Matson S, et al: CpG
motifs in bacterial DNA trigger direct B-cell activation. Nature.
374:546–549. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaisho T and Akira S: Dendritic-cell
function in Toll-like receptor- and MyD88-knockout mice. Trends
Immunol. 22:78–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashkar AA and Rosenthal KL: Toll-like
receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2:545–556.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leadbetter EA, Rifkin IR and
Marshak-Rothstein A: Toll-like receptors and activation of
autoreactive B cells. Curr Dir Autoimmun. 6:105–122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schneider P, MacKay F, Steiner V, et al:
BAFF, a novel ligand of the tumor necrosis factor family,
stimulates B cell growth. J Exp Med. 189:1747–1756. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moore PA, Belvedere O, Orr A, et al: BLyS:
member of the tumor necrosis factor family and B lymphocyte
stimulator. Science. 285:260–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Craxton A, Magaletti D, Ryan EJ and Clark
EA: Macrophage- and dendritic cell - dependent regulation of human
B-cell proliferation requires the TNF family ligand BAFF. Blood.
101:4464–4471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scapini P, Nardelli B, Nadali G, Calzetti
F, Pizzolo G, Montecucco C and Cassatella MA: A G-CSF-stimulated
neutrophils are a prominent source of functional BLyS. J Exp Med.
197:297–302. 2003.PubMed/NCBI
|
|
22
|
Nardelli B, Belvedere O, Roschke V, et al:
Synthesis and release of B-lymphocyte stimulator from myeloid
cells. Blood. 97:198–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bergamin F, Vincent IE, Summerfield A and
McCullough KC: Essential role of antigen-presenting cell-derived
BAFF for antibody responses. Eur J Immunol. 37:3122–3130. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jego G, Pascual V, Palucka AK and
Banchereau J: Dendritic cells control B cell growth and
differentiation. Curr Dir Autoimmun. 8:124–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Litinskiy MB, Nardelli B, Hilbert DM, He
B, Schaffer A, Casali P and Cerutti A: DCs induce CD40-independent
immunoglobulin class switching through BLyS and APRIL. Nat Immunol.
3:822–829. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
McCarthy DD, Chiu S, Gao Y, Summers-deLuca
LE and Gommerman JL: BAFF induces a hyper-IgA syndrome in the
intestinal lamina propria concomitant with IgA deposition in the
kidney independent of LIGHT. Cell Immunol. 241:85–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xin G, Shi W, Xu LX, Su Y, Yan LJ and Li
KS: Serum BAFF is elevated in patients with IgA nephropathy and
associated with clinical and histopathological features. J Nephrol.
26:683–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang H, Peng Y, Liu H, Yang X and Liu F:
Decreased CD4+CD25+ cells and increased
dimeric IgA-producing cells in tonsils in IgA nephropathy. J
Nephrol. 23:202–209. 2010.
|
|
29
|
Pontarollo RA, Rankin R, Babiuk LA, et al:
Monocytes are required for optimum in vitro stimulation of bovine
peripheral blood mononuclear cells by non-methylated CpG motifs.
Vet Immunol Immunopathol. 84:43–59. 2002. View Article : Google Scholar
|
|
30
|
Fujieda S, Suzuki S, Sunaga H, Yamamoto H,
Seki M, Sugimoto H and Saito H: Production of interferon-gamma by
tonsillar mononuclear cells in IgA nephropathy patients. Acta
Otolaryngol. 120:649–654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buchanan RM, Popowych Y, Arsic N, et al:
B-cell activating factor (BAFF) promotes CpG ODN-induced B cell
activation and proliferation. Cell Immunol. 271:16–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buchanan R, Popowych Y, Dagenais C, et al:
Interferon-gamma and B-cell activating factor (BAFF) promote bovine
B cell activation independent of TLR9 and T-cell signaling. Vet
Immunol Immunopathol. 145:453–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng LG, Ng CH, Woehl B, et al: BAFF
costimulation of Toll-like receptor-activated B-1 cells. Eur J
Immunol. 36:1837–1846. 2006. View Article : Google Scholar : PubMed/NCBI
|