Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver malignancy (1).
Although the incidence of certain types of cancer have been
declining, that of HCC has been increasing worldwide (2,3). In
the United States between 1975 and 2005, the 5-year survival rate
is <2% in patients with advanced stages of the disease (2). Radical surgery remains the most
effective strategy to treat HCC. However, ~70% of patients relapse
or develop metastases within 5 years of undergoing this treatment
(2). Therefore, more effective
interventions for targeting cancer metastasis are required in order
to reduce the morbidity and mortality rates associated with
HCC.
The initiation of cancer metastasis requires
migration and invasion of cells, which is enabled by
epithelial-mesenchymal transition (EMT) (4). EMT is a biological process in which
epithelial cancer cells in primary tumors lose their cell polarity
and cell-cell adhesion, and gain increased migratory and invasive
properties in order to become mesenchymal cells. The loss of
E-cadherin, the key marker of epithelial cells, is hypothesized to
be a crucial event in the initiation of EMT (5). Downregulation of E-cadherin is
mediated by overexpression of several EMT-inducing factors, such as
snail (6,7). The gain of mesenchymal markers (e.g.,
vimentin and N-cadherin) is another feature of EMT. Accumulating
data have demonstrated a correlation between EMT, and the
progression and metastasis of HCC (8,9).
Hypoxia is a common characteristic of solid tumor
microenvironments, and is caused by aberrant neovascularization of
the rapidly growing tumor mass (10). Evidence indicates that this hypoxic
microenvironment can facilitate tumor metastasis (11). The transcription factor
hypoxia-inducible factor-1α (HIF-1α), a key mediator of the
cellular response to hypoxia, is overexpressed in a wide variety of
solid tumors, including HCC (12).
HIF-1α consists of an oxygen-dependent degradation domain (ODDD)
that mediates oxygen-regulated stability (13). In normoxic conditions,
hydroxylation of two proline residues and acetylation of a lysine
residue at the ODDD of HIF-1α leads to HIF-1α degradation by the
ubiquitin-proteasome pathway. In hypoxia, the HIF-1α subunit
remains stable, accumulates and translocates to the nucleus, where
it regulates the expression of target genes. These genes in turn,
regulate a variety of cellular processes, such as angiogenesis
(14) and EMT (15). Recent evidence suggests that
stabilization of the HIF-1α transcription complex, caused by
intratumor hypoxia, promotes tumor progression and metastasis
(11).
Curcumin is a biological product obtained from the
ground rhizomes of Curcuma longa, which is a member of the
zingiberaceae plant family, and is widely cultivated in Southeast
Asian countries (16). Previous
studies have demonstrated medicinal qualities, including antiseptic
(17), anti-oxidative (18), anti-inflammatory (19), anticoagulative (20) and anti-atherosclerotic (21) properties. Moreover, curcumin has
been shown to suppress the transformation, proliferation and
metastasis of several types of cancer cells in vivo and
in vitro (22). There is
extensive literature suggesting that the potential anticancer
effects of curcumin may, in part, be mediated through its
regulation of various transcription factors, for example nuclear
factor (NF)-κB and signal transducers and activators of
transcription (23–25). However, previous studies have
focused on the effects of curcumin under normoxic conditions. The
aim of this study was to determine whether curcumin can inhibit the
HIF-1α-associated proliferation, migration, invasion and EMT in HCC
in a hypoxic microenvironment, which may more realistically model
tumor conditions.
Materials and methods
Cell culture and cell treatments
Cells from the human hepatoma cell line, HepG2
(American Type Culture Collection, Rockville, MD, USA), were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone,
South Logan, UT, USA) containing 10% fetal bovine serum (FBS;
HyClone). Cells were grown in a 5% CO2 atmosphere at
37°C. To establish hypoxic conditions, the culture medium was
supplemented with 10 mol/l CoCl2 dissolved in
ddH2O (26). Curcumin
was dissolved in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO,
USA). The concentration of curcumin (Wako, Tokyo, Japan) in the
medium of the HepG2 cells was 10 μmol/l. The cells in the control
group were treated with DMSO only, under identical conditions.
MTT proliferation assays
To determine the cell proliferation rate, 5,000
cells/well were plated in 96-well plates, cultured for 12 h and
then treated with CoCl2 and curcumin. Cells treated with
DMSO served as a control. At indicated time points (24, 48, 72 and
96 h), 20 μl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma) was added to each well and cells were incubated for an
additional 4 h at 37°C (27).
Following MTT incubation, 150 μl DMSO (Sigma) per well was added to
dissolve the crystals. Viable cells were counted by measuring
absorbance at 490 nm using a spectrophotometer (Bio-Rad, Hercules,
CA, USA).
Cell scratch-wound assays
For cell migration assessment, 5×105
cells/well were seeded in 6-well plates and starved for 24 h
immediately after cells had reached full confluency. Cells were
scratched with a 200 ml pipette tip. Non-adherent cells were
removed with phosphate-buffered saline (PBS), and baseline cell
layers were photographed (0 h point; Nikon TE300, Tokyo, Japan) and
placed into the growth medium. After 24 h, matched-pair wound
regions were photographed. The cell migration distance was
calculated as the initial scratch width (0 h point) plus the wound
healing width (24 h point) (27).
In vitro invasion assay
Matrigel invasion assays were performed with 24-well
transwell inserts (8 μm; Millipore co., Billerica, MA, USA) as
described previously (28).
Briefly, the lower surface of the membrane was coated with
Matrigel™ (BD Biosciences, Franklin Lakes, NJ, USA). HepG2 cells
were pre-treated in a 6-well plate for 24 h and then suspended in
the upper chamber in a serum-free medium. Lower compartments were
filled with DMEM containing 10% FBS. After 48 h, non-invading cells
were gently removed by scraping with a cotton swab, and invading
cells in the lower side of the membrane were fixed with 4%
paraformaldehyde and stained with crystal violet. The average
number of invasive cells in 10 random fields selected on each
membrane was calculated using a microscope (x20; Nikon Eclipse
TE2000-U; Nikon Instruments, Inc., Melville, NY, USA) in order to
quantify the extent of invasion. Each set of experiments was
performed in triplicate (29).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from monolayers of cultured
HepG2 cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA).
cDNA was synthesized from 5 μg RNA using Takara Reverse
Transcription Reagent (Takara, Tokyo, Japan) according to the
manufacturer’s instructions. The primer sequences were: Forward:
5′-CAAAACACACAGCGAAGC-3′ and reverse: 5′-TCAACCCAGACATATCCACC-3′
for HIF-1α; forward: 5′-CATCACTATCGGCAATGAGC-3′ and reverse:
5′-GACAGCACTGTGTTGGCATA-3′ for β-actin; forward:
5′-ATTCTGATTCTGCTGCTCTTG-3′ and reverse: 5′-AGTCCTGGTCCTCTTCTCC-3′
for E-cadherin; forward: 5′-CTTCTCCTCTACTTCAGTCTC TTC-3′ and
reverse: 5′-CGTGTG GCTTCGGATGTG-3′ for snail and forward:
5′-AATGACCGCTTCGCCAAC-3′ and reverse: 5′-CCGCATCTCCTCCTCGTAG-3′ for
vimentin.
The following cycling conditions were used: 94°C for
5 sec, 60°C for 30 sec and then 72°C for 30 sec. HIF-1α and β-actin
were amplified for 35 cycles. HIF-1α relative expression was
analyzed by the comparative CT method with β-actin as
the normalization control (30).
Western blotting
Western blot analyses were performed as described
previously (28). Briefly, total
protein was extracted using Mammalian Protein Lysis Buffer (Thermo
Scientific Waltham, MA, USA) after each group of cells was treated
for 72 h. Equal quantities of protein were added, separated by
SDS-polyacrylamide gel electrophoresis and electro-transferred onto
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
After blocking the membranes with non-fat milk in PBS with Tween-20
(PBST) for 1 h at room temperature, the membranes were incubated
with specific primary antibodies overnight at 4°C, and then washed
in PBST. The blots were incubated with horseradish peroxidase
conjugated-anti-rabbit or anti-mouse monoclonal IgG secondary
antibodies (Sigma) at a dilution of 1:10,000 for 1 h. Secondary
antibodies were detected by an enhanced chemiluminescence method
(Amersham, Piscataway, NJ, USA). The density of specific protein
bands were determined by Image-Pro Plus 5.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The E-cadherin antibody was
purchased from BD Bio-sciences (San Jose, CA, USA) and mouse
monoclonal HIF-1α antibody was purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). The mouse monoclonal β-actin,
rabbit monoclonal snail and rabbit polyclonal vimentin antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Statistical analysis
All experiments were repeated at least three times.
Results are expressed as the mean ± standard deviation. Differences
were evaluated using one-way analysis of variance with the least
significant difference post hoc test for multiple comparisons via
SPSS (version 15.0; SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin inhibits
CoCl2-induced HIF-1α protein expression
To determine the effect of curcumin on
hypoxia-induced HIF-1α expression, HepG2 cells were treated with
CoCl2 and curcumin, separately and in combination. The
HIF-1α expression was measured by qPCR and western blot analysis.
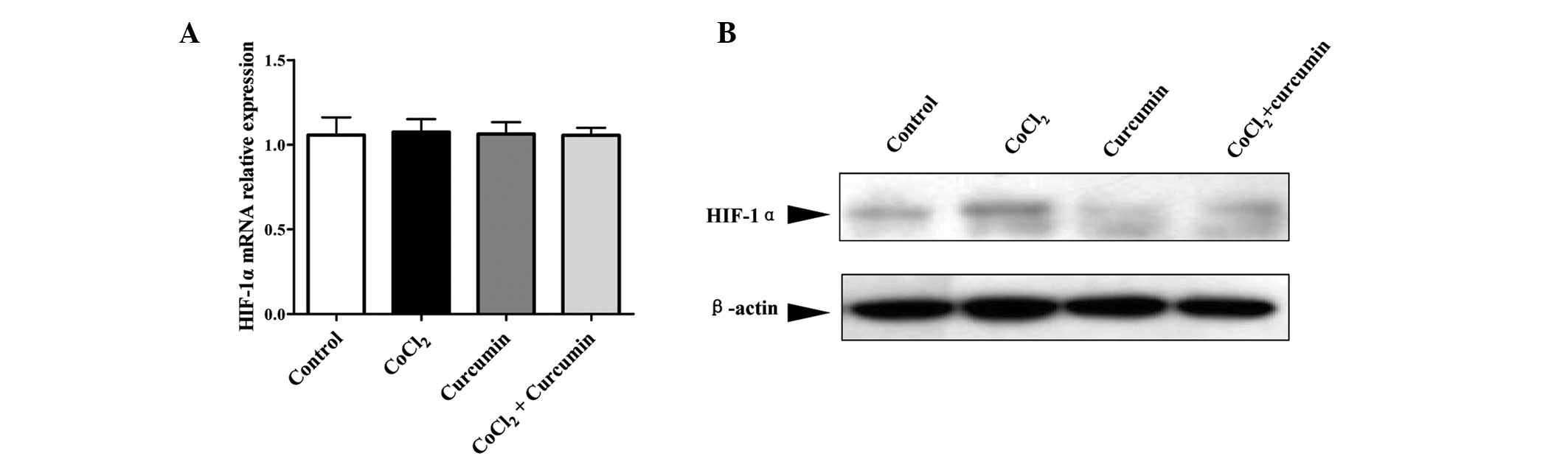
As shown in Fig. 1A, no
significant changes were found in the expression of HIF-1α mRNA in
HepG2 cells treated with CoCl2 and curcumin, alone or in
combination, as compared with the DMSO control group. However,
treatment with CoCl2 markedly increased HIF-1α protein
levels (Fig. 1B). HIF-1α protein
levels were reduced by 70% with curcumin treatment alone compared
with the control group. Moreover, accumulation of the HIF-1α
protein induced by CoCl2 was prevented by administration
of curcumin. These results confirmed that CoCl2 is an
effective inducer of HIF-1α at the posttranscriptional level. In
addition curcumin appeared to inhibit the HIF-1α protein
accumulation induced by CoCl2 without affecting
transcription.
Curcumin inhibits HepG2 cell
proliferation
A previous study demonstrated that
CoCl2-induced HIF-1α expression was correlated with
enhanced cell proliferation (31).
To explore the role of curcumin on proliferation, HepG2 cells were
seeded onto 96-well plates, and treated with CoCl2 and
curcumin alone or in combination. At the time points indicated in
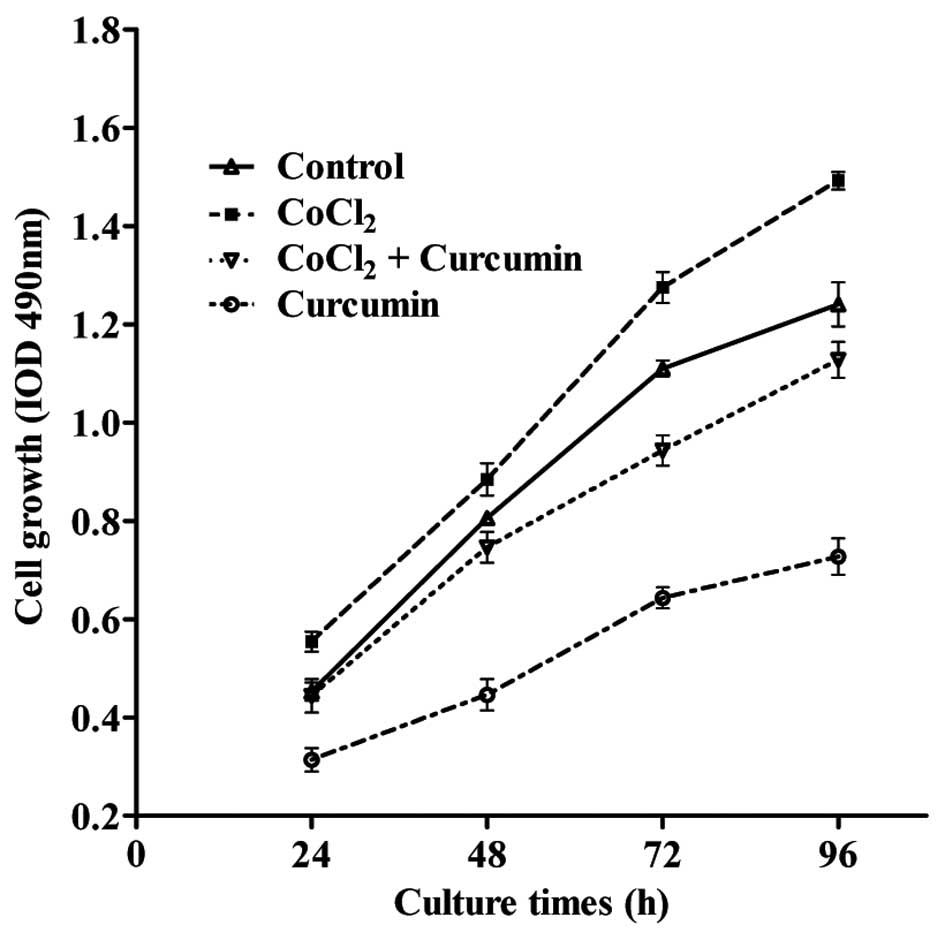
Fig. 2, the proliferative rate of
HepG2 cells in each group was determined by the MTT assay. The
results demonstrated that the proliferation of HepG2 cells
increased in response to the administration of CoCl2
compared with the DMSO control group (P<0.05). By contrast,
curcumin treatment decreased the rate of cell proliferation in a
dose-dependent manner. Furthermore, the increased rate of cell
proliferation induced by CoCl2 was reduced by ~30% in
the presence of curcumin at each time point measured.
Curcumin inhibits the migration and
invasion of HepG2 cells associated with HIF-1α accumulation
The effect of curcumin on HCC cell motility after
CoCl2 treatment was determined using a wound-healing
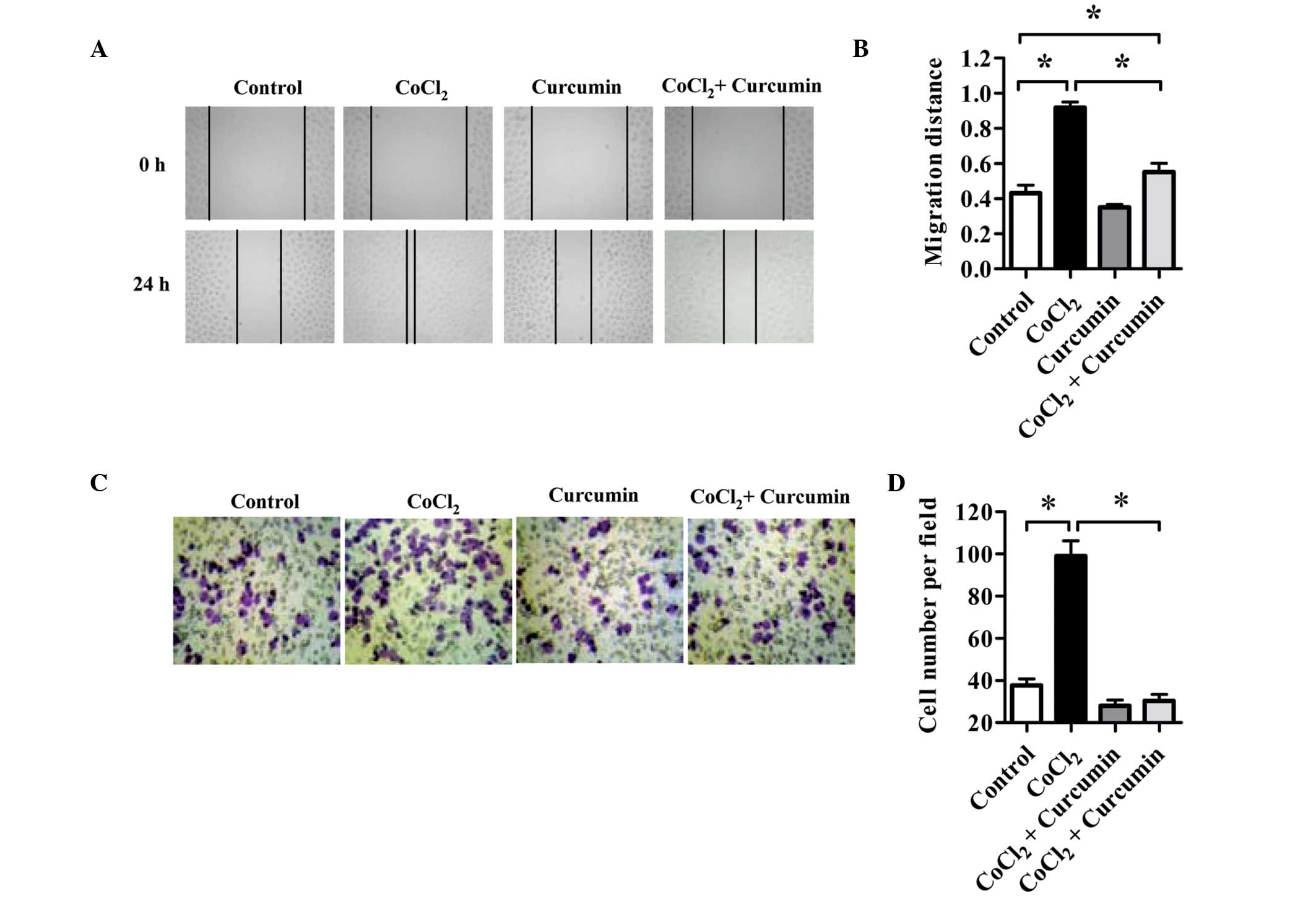
assay. As shown in Fig. 3A and B,
compared with normal controls, HepG2 cells treated with
CoCl2 alone exhibited increased migration into the wound
area 24 h after wounding occurred. The migration distance of cells
in the CoCl2 group was double that of the control group.
Cell migration was inhibited by curcumin. In addition, the
CoCl2-enhanced migration of HepG2 cells was reduced by
~40% with curcumin treatment. The migration distance of cells in
the CoCl2 plus curcumin group decreased by 30% compared
with that observed in the control group.
Invasiveness was determined by using Matrigel-coated
transwell chambers. As shown in Fig.
3C and D, without curcumin intervention, CoCl2
treatment markedly increased the invasiveness of HepG2 cells in
comparison with control cells.
As in the wound-healing assay, curcumin
significantly inhibited cell invasion. The average number of cells
that invaded the lower chamber in the curcumin-treated group was
50% lower than that of the control group. In addition, the enhanced
cell invasion observed in the CoCl2 group was also
inhibited by curcumin. The number of invasive cells in the
CoCl2 plus curcumin group decreased by ~60% compared
with that in the CoCl2 group. These results indicate
that curcumin inhibits migration and invasion of HCC cells under
hypoxic conditions.
Effects of curcumin on the expression of
EMT-related markers induced by HIF-1α in HepG2 cells
Enhanced cell migration and invasiveness is often
linked with EMT. As hypoxia has been shown to induce tumor cell
EMT, this study aimed to determine whether curcumin had an effect
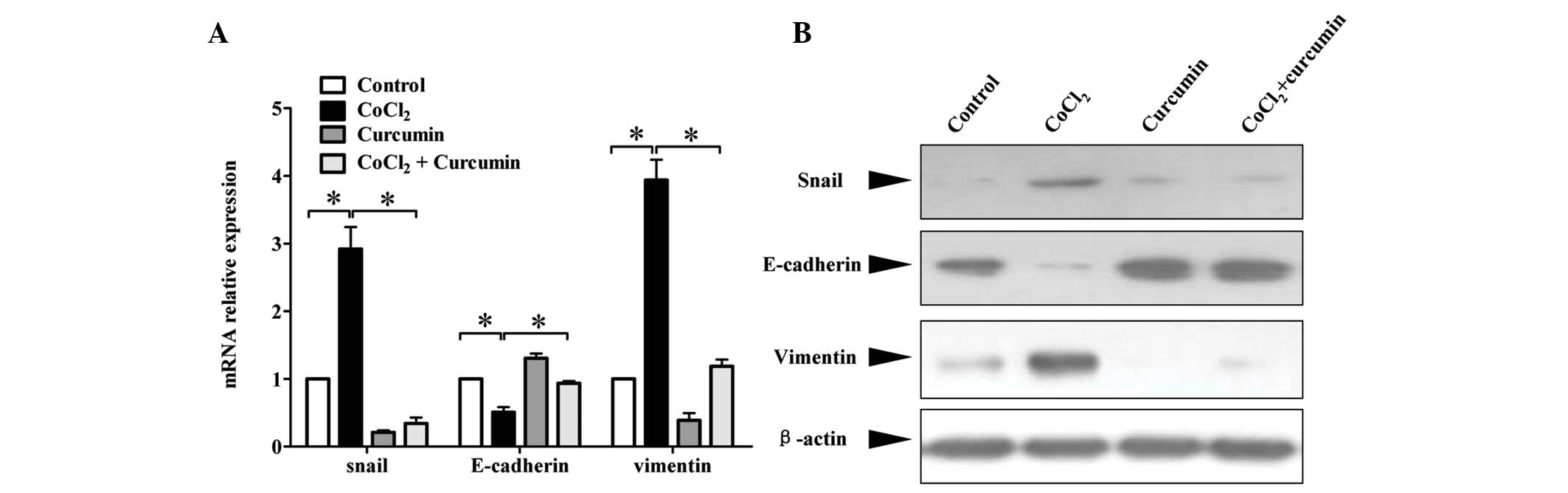
on EMT markers as measured by qPCR and western blotting. The
results showed that in response to CoCl2, HepG2 cells
had decreased mRNA expression of the epithelial marker, E-cadherin.
However, mRNA expression of the mesenchymal markers, vimentin and
snail, increased significantly compared with that in the control
cells. Treatment with curcumin markedly diminished the
CoCl2-induced downregulation of E-cadherin, and
upregulation of vimentin and snail mRNA levels (Fig. 4A). Measurement of protein levels
showed similar results (Fig. 4B).
These results demonstrated that curcumin suppressed EMT associated
with HIF-1α accumulation in HepG2 HCC cells.
Discussion
HCC is the one of the most common types of malignant
disorder, which runs a rapidly progressive clinical course. It is
rarely amenable to resection and does not generally respond well to
non-surgical treatments, resulting in a poor prognosis (32). The majority of solid tumors,
including HCC have been shown to have a hypoxic tumor
microenvironment (33). Although
hypervascularity and angiogenesis are important features of HCC,
due to the increased demand for blood and oxygen supply as a
consequence of rapid tumor growth, delivery of oxygen to the tumor
is often insufficient.
Recently, evidence from a number of studies has
suggested that hypoxic signaling is a central modulator of cellular
physiology in cancer cells (34–36).
Hypoxia is associated with a poor prognosis in several types of
malignancy. Hypoxia-mediated target gene expression has been shown
to stimulate proliferation (37),
angiogenesis (38), metastasis
(39), chemoresistance (40) and radio-resistance (41) of tumor cells. Suppression of tumor
cell differentiation (42) and
apoptosis (43) has been shown to
lead to tumor progression in several types of cancers. Thus,
therapeutic strategies aimed at improving the hypoxic intratumor
microenvironment, or blocking the signaling pathways activated by
hypoxia may be useful in the treatment of solid tumors, including
HCC.
It has been well established that HIF-1α is an
important transcription factor that is specifically activated
during hypoxia. In response to intratumor hypoxia, HIF-1α is
stabilized and translocates to the nucleus where it forms a
transcriptionally active complex, HIF-1, by coupling to HIF-1β
(also termed ARNT) (44). HIF-1
modulates the expression of a number of target genes, the products
of which control pathways involved in angiogenesis, cell survival
and metastasis (45).
The current study shows that HIF-1α accumulation in
HepG2 cells is associated with enhanced migration and invasiveness.
In addition, the alteration in levels of EMT-related molecules
suggests that cells underwent EMT programming in the hypoxia model
induced by CoCl2. This is in agreement with recent
studies showing that hypoxia-mediated HIF-1α accumulation leads to
β-catenin overexpression in HCC cells, and is accompanied by
enhanced invasiveness in vitro and metastasis in vivo
(46). Considering the central
role of HIF-1α in hypoxia and the crucial role of HIF-1α in cancer
progression, chemotherapeutic agents that target HIF-1α may be
attractive modalities to prevent tumor progression.
Research over the last few decades has indicated
that curcumin is a potent anti-inflammatory agent with strong
therapeutic potential against a variety of types of cancer
(22). Extensive studies have
demonstrated that curcumin has the ability to suppress
transformation, proliferation and metastasis of tumors by
regulation of certain molecules involved in cancer progression
(16,24,25).
The current study found that curcumin alone exhibited antitumor
effects under normoxic conditions. In addition, the results
indicated that curcumin may also inhibit HCC cell growth, migration
and invasion under hypoxic conditions induced by CoCl2.
A recent study has shown that curcumin suppresses proliferation and
induces apoptosis of human HCC cells in a concentration-dependent
manner by inhibiting the Wnt signaling pathway (47). In the current study, the antitumor
effect of curcumin was found to be partially mediated by inhibiting
HIF-1α stabilization in HepG2 cells, without affecting HIF-1α
transcription. These results suggest potential for the treatment of
HCC by altering the upregulation of HIF-1α observed in HCC.
In conclusion, the current study indicated that
curcumin inhibits hypoxia-induced HIF-1α accumulation in HepG2
cells in a hypoxia model induced by CoCl2. Moreover,
curcumin suppresses proliferation, migration, invasion and EMT of
HepG2 cells in this environment. These results suggest that
curcumin is a potential anticancer agent for the treatment of
HCC.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited for assisting in the preparation of this manuscript. This
study was financially supported by grants from the National Natural
Science Foundation of China (no. 81201824), the Fundamental
Research Funds for the Central Universities in Xi’an Jiaotong
University (no. 2013jdhz33) and the Scientific Research Program
Funded by Shaanxi Provincial Education Department (no.
11JK0704).
References
|
1
|
Jelic S and Sotiropoulos GC: ESMO
Guidelines Working Group: Hepatocellular carcinoma: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21:v59–v64. 2010. View Article : Google Scholar
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brabletz T, Jung A, Spaderna S, et al:
Opinion: migrating cancer stem cells - an integrated concept of
malignant tumour progression. Nat Rev Cancer. 5:744–749. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jou J and Diehl AM: Epithelial-mesenchymal
transitions and hepatocarcinogenesis. J Clin Invest. 120:1031–1034.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Li P, Chang Y, et al: The
SDF-1/CXCR4 axis induces epithelial–mesenchymal transition in
hepatocellular carcinoma. Mol Cell Biochem. 392:77–84. 2014.
|
|
10
|
Vaupel P and Mayer A: Hypoxia in cancer:
significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong ZZ, Yao M, Wang L, et al:
Hypoxia-inducible factor-1alpha: molecular-targeted therapy for
hepatocellular carcinoma. Mini Rev Med Chem. 13:1295–1304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan DA, Sutphin PD, Yen SE and Giaccia
AJ: Coordinate regulation of the oxygen-dependent degradation
domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol.
25:6415–6426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nyberg P, Salo T and Kalluri R: Tumor
microenvironment and angiogenesis. Front Biosci. 13:6537–6553.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao JH, Luo Y, Jiang YG, et al: Knockdown
of β-Catenin through shRNA cause a reversal of EMT and metastatic
phenotypes induced by HIF-1α. Cancer Invest. 29:377–382. 2011.
|
|
16
|
Singh S and Khar A: Biological effects of
curcumin and its role in cancer chemoprevention and therapy.
Anticancer Agents Med Chem. 6:259–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De R, Kundu P, Swarnakar S, et al:
Antimicrobial activity of curcumin against Helicobacter
pylori isolates from India and during infections in mice.
Antimicrob Agents Chemother. 53:1592–1597. 2009.PubMed/NCBI
|
|
18
|
Wang J, Du XX, Jiang H and Xie JX:
Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by
anti-oxidation and nuclear factor-kappa B modulation in MES23.5
cells. Biochem Pharmacol. 78:178–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jurenka JS: Anti-inflammatory properties
of curcumin, a major constituent of Curcuma longa: a review
of preclinical and clinical research. Altern Med Rev. 14:141–153.
2009.PubMed/NCBI
|
|
20
|
Kim DC, Ku SK and Bae JS: Anticoagulant
activities of curcumin and its derivative. BMB Rep. 45:221–226.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olszanecki R, Jawień J, Gajda M, et al:
Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout
mice. J Physiol Pharmacol. 56:627–635. 2005.PubMed/NCBI
|
|
22
|
Shishodia S, Chaturvedi MM and Aggarwal
BB: Role of curcumin in cancer therapy. Curr Probl Cancer.
31:243–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jobin C, Bradham CA, Russo MP, et al:
Curcumin blocks cytokine-mediated NF-kappa B activation and
proinflammatory gene expression by inhibiting inhibitory factor
I-kappa B kinase activity. J Immunol. 163:3474–3483.
1999.PubMed/NCBI
|
|
24
|
Prakobwong S, Gupta SC, Kim JH, et al:
Curcumin suppresses proliferation and induces apoptosis in human
biliary cancer cells through modulation of multiple cell signaling
pathways. Carcinogenesis. 32:1372–1380. 2011. View Article : Google Scholar
|
|
25
|
Yang CL, Liu YY, Ma YG, et al: Curcumin
blocks small cell lung cancer cells migration, invasion,
angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3
signalling pathway. PLoS One. 7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo M, Song LP, Jiang Y, et al:
Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce
leukemic cell apoptosis through different hypoxia-inducible
factor-1alpha independent mechanisms. Apoptosis. 11:67–77. 2006.
View Article : Google Scholar
|
|
27
|
Li W, Ma J, Ma Q, et al: Resveratrol
inhibits the epithelial-mesenchymal transition of pancreatic cancer
cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med
Chem. 20:4185–4194. 2013.PubMed/NCBI
|
|
28
|
Li X, Ma Q, Xu Q, et al: SDF-1/CXCR4
signaling induces pancreatic cancer cell invasion and
epithelial-mesenchymal transition in vitro through non-canonical
activation of Hedgehog pathway. Cancer Lett. 322:169–176. 2012.
View Article : Google Scholar
|
|
29
|
Han L, Peng B, Ma Q, et al: Indometacin
ameliorates high glucose-induced proliferation and invasion via
modulation of e-cadherin in pancreatic cancer cells. Curr Med Chem.
20:4142–4152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ardyanto TD, Osaki M, Tokuyasu N, et al:
CoCl2-induced HIF-1alpha expression correlates with
proliferation and apoptosis in MKN-1 cells: a possible role for the
PI3K/Akt pathway. Int J Oncol. 29:549–555. 2006.PubMed/NCBI
|
|
32
|
Kew MC: Hepatocellular carcinoma in
developing countries: Prevention, diagnosis and treatment. World J
Hepatol. 4:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anastasiadis AG, Bemis DL, Stisser BC, et
al: Tumor cell hypoxia and the hypoxia-response signaling system as
a target for prostate cancer therapy. Curr Drug Targets. 4:191–196.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kizaka-Kondoh S, Inoue M, Harada H and
Hiraoka M: Tumor hypoxia: a target for selective cancer therapy.
Cancer Sci. 94:1021–1028. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yasuda H: Solid tumor physiology and
hypoxia-induced chemo/radio-resistance: novel strategy for cancer
therapy: nitric oxide donor as a therapeutic enhancer. Nitric
Oxide. 19:205–216. 2008. View Article : Google Scholar
|
|
37
|
Gordan JD, Bertout JA, Hu CJ, et al:
HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc
transcriptional activity. Cancer Cell. 11:335–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao D and Johnson RS: Hypoxia: a key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalliomäki TM, McCallum G, Wells PG and
Hill RP: Progression and metastasis in a transgenic mouse breast
cancer model: effects of exposure to in vivo hypoxia. Cancer Lett.
282:98–108. 2009.PubMed/NCBI
|
|
40
|
Selvendiran K, Bratasz A, Kuppusamy ML, et
al: Hypoxia induces chemoresistance in ovarian cancer cells by
activation of signal transducer and activator of transcription 3.
Int J Cancer. 125:2198–2204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Zhang J, Wang X, et al: HIF-1 and
NDRG2 contribute to hypoxia-induced radioresistance of cervical
cancer Hela cells. Exp Cell Res. 316:1985–1993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim Y, Lin Q, Glazer PM and Yun Z: Hypoxic
tumor microenvironment and cancer cell differentiation. Curr Mol
Med. 9:425–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiche J, Rouleau M, Gounon P, et al:
Hypoxic enlarged mitochondria protect cancer cells from apoptotic
stimuli. J Cell Physiol. 222:648–657. 2010.PubMed/NCBI
|
|
44
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Powis G and Kirkpatrick L: Hypoxia
inducible factor-1alpha as a cancer drug target. Mol Cancer Ther.
3:647–654. 2004.PubMed/NCBI
|
|
46
|
Zhang Q, Bai X, Chen W, et al:
Wnt/β-catenin signaling enhances hypoxia-induced
epithelial-mesenchymal transition in hepatocellular carcinoma via
crosstalk with hif-1α signaling. Carcinogenesis. 34:962–973.
2013.
|
|
47
|
Xu MX, Zhao L, Deng C, et al: Curcumin
suppresses proliferation and induces apoptosis of human
hepatocellular carcinoma cells via the wnt signaling pathway. Int J
Oncol. 43:1951–1959. 2013.PubMed/NCBI
|