Introduction
Prostate cancer, as the second most prevalent cause
of cancer-associated mortality in males in the USA, accounts for
~30% of malignant tumors in males, affecting one in six and causing
mortality in 1 in 35 males (1).
Prostate cancer, if diagnosed in the early stages of the disease,
may be successfully treated using surgical resection and radiation
therapy; however, the majority of patients are diagnosed with
locally advanced or metastatic prostate cancer, which requires
hormone ablation therapy (2).
Following initiation of hormone ablation therapy, ~80–90% of
patients develop metastatic castration-resistant prostate cancer
(CRPC) within 12–33 months (3).
CRPC is currently treated using chemotherapeutic drugs; however,
these drugs are unspecific and have numerous adverse effects
(4). Therefore, novel therapeutic
strategies as well as prophylactic measures are required.
A diet rich in fruits and vegetables has been
reported to have protective effects against chronic degenerative
diseases, including numerous types of cancer and cardiovascular
diseases; the mechanisms underlying these beneficial effects were
attributed to plant-derived compounds, such as polyphenols
(5). Quercetin is a dietary
flavonoid found in tea, onions, grapes, wines and apples, and the
anti-cancer activities of this compound have been previously
explored in breast and colon cancer cells (6,7).
Tang et al (8) reported
that quercetin and epigallocathechin gallate synergistically
inhibited invasion, migration and epithelial mesenchymal transition
in prostate cancer stem cells; another study demonstrated that
quercetin and sulforaphane synergistically inhibited self-renewal
in pancreatic cancer stem cells (9). Hyperoside (quercetin-3-O-galactoside)
is a flavonoid compound, which is primarily extracted from
Hypericum perforatum L (10). Hyperoside was reported to exhibit
anti-cancer effects via inhibition of cell proliferation, induction
of apoptosis, decreased angiogenesis and induction of cell cycle
arrest in numerous cancer cell lines (11). Another previous study demonstrated
that the combination of hyperoside and quercetin exhibited
synergistic inhibitory effects on the growth of human leukemia
cells (12). In a study using
mouse skin tumors, a combination of hyperoside and tea polyphenols
also demonstrated synergistic anti-cancer effects (13).
Micro (mi)RNAs are small non-coding single-stranded
RNAs composed of 22 nucleotides, which regulate coding RNAs at the
post-transcriptional level. A single miRNA controls hundreds of
target messenger (m)RNAs and miRNAs are therefore powerful
transcription factors which may regulate whole cell proteomes
(14). miRNAs have become the
focus of an increasing number of studies due to the reported roles
of miRNAs in influencing cancer biology, including proliferation,
apoptosis and invasive capacity. miR-21 was reported to regulate
the growth of breast cancer MCF7 cells in vitro and in
xenograft mouse models in vivo (15). Subsequent studies demonstrated that
miR-21 regulated breast cancer metastasis via the downregulation of
tumor suppressor genes, including maspin and programmed cell death
protein 4 (PDCD4) (16). In
addition, miR-21 was reported to regulate glioblastoma
intravasation and metastasis through the targeted downregulation of
PDCD4 (17). Therefore, the aim of
the present study was to evaluate the combined treatment of
prostate carcinoma cells with hyperoside and quercetin in order to
investigate its effect on PDCD4 expression and the potential
involvement of miR-21 in the downregulation of PDCD4 transcription
factors.
Materials and methods
Botanical extract
Polyphenols were extracted from a standardized
hyperoside and quercetin dihydrate supplement (ratio, 1:1) in
capsule form, which was obtained from Jiangsu Suzhong
Pharmaceutical Group Co., Ltd (Jiangsu, China). Polyphenols were
extracted using 50 mg/ml methanol (Sigma-Aldrich, St. Louis, MO,
USA), followed by centrifugation at room temperature for 10 min at
1,100 × g in order to remove inactive and insoluble components.
Methanol was evaporated in a rotavapor (BÜCHI Labortechnik AG,
Flawil, Switzerland) at 40°C. Residual moisture was evaporated
using a speedvac concentrator (Thermo Scientific, Waltham, MA, USA)
at 43°C. The final mixture of hyperoside and quercetin in
combination (QH) was stored at −80°C and dissolved in dimethyl
sulfoxide (Sigma-Aldrich) prior to use.
High-performance liquid chromatography
photo-diode array (HPLC-PDA) analysis
The polyphenolic mixture was analyzed and quantified
by retention time and PDA spectra using HPLC-PDA. Chromatographic
separation was performed in an Alliance 2695 Seperations Module
(Waters Corp., Milford, MA, USA) using a Discovery® C18
column (Supelco; 250×4.6 mm, 5 μm; Sigma-Aldrich) at room
temperature. The chromatographic conditions used were as follows:
Mobile phase A, water/acetic acid (Sigma-Aldrich) 98:2; mobile
phase B, acetonitrile/water/acetic acid 68:30:2. A gradient program
with a flow rate of 1 ml/min was used as follows: 0 min, 100% A; 20
min, 60% A; 30 min, 30% A; 32 min, 0% A; and 35 min, 100% A.
Wavelengths were detected at 306 and 360 nm for hyperoside and
quercetin, respectively. Standard compounds for the identification
and quantitative analysis of hyperoside and quercetin were obtained
from Acros Organics (Morris Plains, NJ, USA).
Oxygen radical absorbance capacity
The antioxidant capacity was determined using an
oxygen radical absorbance capacity assay (ORAC) with fluorescein as
the fluorescent probe using a FLUOstar fluorescent microplate
reader (485 nm excitation and 538 nm emission; BMG Labtech Inc.,
Cary, NC, USA). Results are expressed in μmol of Trolox
equivalents/ml.
Cell culture
Hormone-independent PC3 prostate cancer epithelial
cell lines were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured at 37°C with 5%
CO2 in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL),
penicillin (100 IU/ml; Gibco-BRL) and streptomycin (100 μg/ml;
Gibco-BRL).
Generation of ROS
ROS production was determined using the
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Eastman Kodak,
Rochester, NY, USA) assay according to the manufacturer’s
instructions. In brief, cells were seeded in a clear-bottom,
96-well plate (1×104 cells/well), incubated for 24 h and
then treated with different concentrations of QH (0–60 μg/ml).
Following incubation, cells were washed twice with
phosphate-buffered saline (PBS) and incubated with 200 μM hydrogen
peroxide for 2 h at 37°C. Cells were then washed with PBS in order
to remove hydrogen peroxide and 10 μM DCFH-DA diluted in PBS was
added to cells, followed by incubation for 15 min at 37°C. DCFH-DA
was removed and fluorescence intensity was measured using a
FLUOstar fluorescent microplate reader as described above.
Cell viability assay
Prostate cancer cells were seeded in a 96-well plate
(3×103 cells/well) and incubated for 24 h. The growth
medium was then replaced with the experimental medium containing
various concentrations of QH extract (0–60 μg/ml). Cell viability
was assessed at 48 and 72 h using a Cell Titer 96®
AQueous One Solution Cell Proliferation assay kit (Promega Corp.,
Madison, WI, USA) according to manufacturer’s instructions, and a
FLUOstar microplate reader at 490 nm. The IC50-value was
calculated using sigmoidal nonlinear regression analyses of the
percentage of cell inhibition as a ratio of the control using
GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA,
USA).
Cell proliferation
Prostate cancer cells were cultured in a 24-well
plate (2×104 cells/well) for 24 h. The growth medium was
then replaced with the experimental medium containing numerous
concentrations of QH extract (0–60 μg/ml). Cell proliferation was
determined following 48 and 72 h incubation using a cell counter
(Beckman Coulter LH500, Brea, CA, USA). Cell counts were expressed
as a percentage of the control cells.
Cell apoptosis
The rate of apoptotic cell death was determined
using a fluorescein isothiocyanate (FITC)-Annexin V apoptosis
detection kit (BD Pharmingen, San Diego, CA, USA) according to the
manufacturer’s instruction and quantified using flow cytometry. In
brief, following treatment of prostate cancer cells with QH for 24
h, cells were washed once with PBS and harvested in a 0.5%
trypsin/EDTA solution at 37°C, centrifuged at 500 × g for 5 min and
then immediately re-suspended in 1X physiological buffer (provided
in the kit). Cells (1×105/500 μl) were then maintained
in the dark for 15 min at room temperature with 5 μl each of
propidium iodide and FITC conjugated Annexin V solution (Promega
Corp.). The samples were then analyzed using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA). Results were
quantified using CellQuest software (BD Biosciences, San Jose,
CA).
Cleaved caspase-3 activation
Cells (6×105/well) were cultured for 24 h
and then incubated with numerous concentrations of QH (0–40 μg/ml)
for 24 h. Cleaved caspase-3 activation was determined using an
ELISA kit (Cell Signaling Technology Inc., Danvers, MA, USA)
according to the manufacturer’s instructions and quantified using a
FLUOstar microplate reader at 450 nm.
Cell cycle analysis
Prostate cancer cells were seeded in a 12-well plate
(5×104 cells/well) with medium containing 2.5% FBS for
24 h. Cells were then treated with QH (0–40 μg/ml) for 24 h. Cells
were fixed with 90% ethanol and stored at −20°C. DNA was stained
using propidium iodide (PI; Promega Corp.) containing a 0.2 mg/ml
RNAse solution and analysis was performed at 488 nm excitation and
620 nm emission using a FACSCalibur flow cytometer. The percentage
of cells in each cell cycle phase was analyzed using ModFit LT
version 3.2 for Macintosh (Verity Software House Inc., Topsham, ME,
USA).
Wound-healing assay
Equal numbers of cells were seeded into each well of
a 12-well culture plate. Cells were incubated until they reached
70–80% confluence and a wound was created by scratching a line down
the middle of the well using a sterile white pipette tip. Cells
were then treated with different concentrations of QH and incubated
for 48 hours. Differential interference contrast images of the
wounded area were captured of three random fields per well using a
microscope (Nikon E-600 microscope; Nikon, Inc., Melville, NY, USA)
at 0 and 48 h post-wounding. Wound-healing was quantified by
measuring the area of the closing wound, which was normalized to
that of the vehicle-treated controls.
Invasion assay
Prostate cancer cell migration through
Matrigel-coated membranes was measured using a 24-well BD Biocoat
Matrigel® invasion chamber (BD Biosciences). Prostate
cancer cells (5×104) were suspended in culture media
without serum and then seeded onto the top compartment of the
invasion chamber, followed by respective QH treatments. Complete
media was added to the bottom chamber. Following 48 h, the cell
inserts were obtained and cells were removed from the top surface
of the membrane using a cotton swab. The invasive cells adhering to
the bottom surface of the membrane were stained using 100% methanol
(Sigma-Aldrich) and 1% toluidine blue (Sigma-Aldrich),
respectively. Images were captured under a light microscope using a
20× objective. The total number of invaded cells was manually
counted in four randomly selected fields per treatment per
insert.
Reverse transcription quantitative
polymerase chain reaction analysis (RT-qPCR) of miRNA and mRNA
Prostate cancer cells were cultured in a six-well
plate (2×105 cells/well) for 24 h prior to incubation
with different concentrations of QH (0–30 μg/ml). Total RNA,
containing mRNA and miRNA, was isolated using the mirVana™ miRNA
Isolation kit (Applied Biosystems, Foster City, CA, USA) according
to the manufacturer’s instructions. Extracted nucleic acid was
evaluated qualitatively and quantitatively using the
NanoDrop® ND-1,000 Spectrophotometer (NanoDrop
Technologies Inc., Wilmington, DE, USA) at 260 and 280 nm.
SuperScriptTM III First-Strand (Invitrogen Life
Technologies, Carlsbad, CA, USA) was used to reverse-transcribe
mRNA. GAPDH was used as a qPCR endogenous control. qPCR for mRNA
was performed using the SYBR Green ER qPCR SuperMix (Invitrogen
Life Technologies) on a 7900HT Fast Real-Time PCR System (Applied
Biosystems). Primers used for RT-qPCR were as follows: PDCD4 sense,
5′-CCAAAGAAAGGTGGTGCA-3′ and antisense, 5′-TGAGGTACTTCCAGTTCC-3′;
GAPDH sense, 5′-GGCATTGCTCTCAATGACAA-3′ and antisense,
5′-ATGTAGGCCATGAGGTCCAC-3′.
The TaqMan® MicroRNA Assay for miR-21 and
the control RNU6B (Applied Biosystems) was used according to the
manufacturer’s instructions, to reverse-transcribe mature miRNA in
a MasterCycler (Eppendorf, Hamburg, Germany). RT-qPCR for miRNA was
performed using the TaqMan® assay, which contained the
forward and reverse primers as well as the TaqMan® probe
and TaqMan® Universal PCR Master Mix No
AmpErase® uracil N-glycosylase (Applied
Biosystems). Quantification of mRNA and miRNA gene expression was
then evaluated using the comparative critical threshold method.
Mimic transfections with 50 and 100 nM miR-21 (Dharmacon,
Lafayette, CO, USA) were performed using Lipofectamine
2000® (Invitrogen Life Technologies) for 6 h. Following
transfection, cells were incubated with 20 μg/ml QH for 24 h.
Western blot analysis
Cells were cultured (2×106 cells/plate)
for 24 h and then incubated with different concentrations of QH for
48 h. Cells were lysed in lysis buffer containing protease
inhibitor. Protein concentration was determined using a Bio-Rad
protein assay system (Bio-Rad, Hercules, CA, USA). Equal amounts of
proteins were separated using SDS-PAGE on a 15% gel and then
transferred onto polyvinylidene difluoride membranes (Bio-Rad).
Following blocking in Tris-buffered saline containing 5% non-fat
milk, the membranes were incubated with specific primary antibodies
(mouse anti-PDCD4 polyclonal antibody, dilution 1:1,000, Abnova
Corporation, Walnut, CA, USA; and mouse anti-PARP polyclonal
antibody, dilution 1:1,000, Abcam, Cambridge, MA, USA) at 4°C for
12 h and then with horseradish peroxidase-conjugated anti-mouse
secondary antibody for 2 h at room temperature. Enhanced
chemiluminescence detection reagent (GE Healthcare, Little
Chalfont, UK) was used to evaluate the results.
Statistical analysis
Data from in vitro experiments were analyzed
by one-way analysis of variance followed by a Tukey-Cramer HSD
multiple comparison test using SPSS version 18.0 (International
Business Machines, Armonk, NY, USA). A Student t-test was used to
determine differences between miR-21 mimic transfections. Nonlinear
modeling of sigmoidal curves for cell viability was performed using
GraphPad Prism 5.01. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Chemical composition
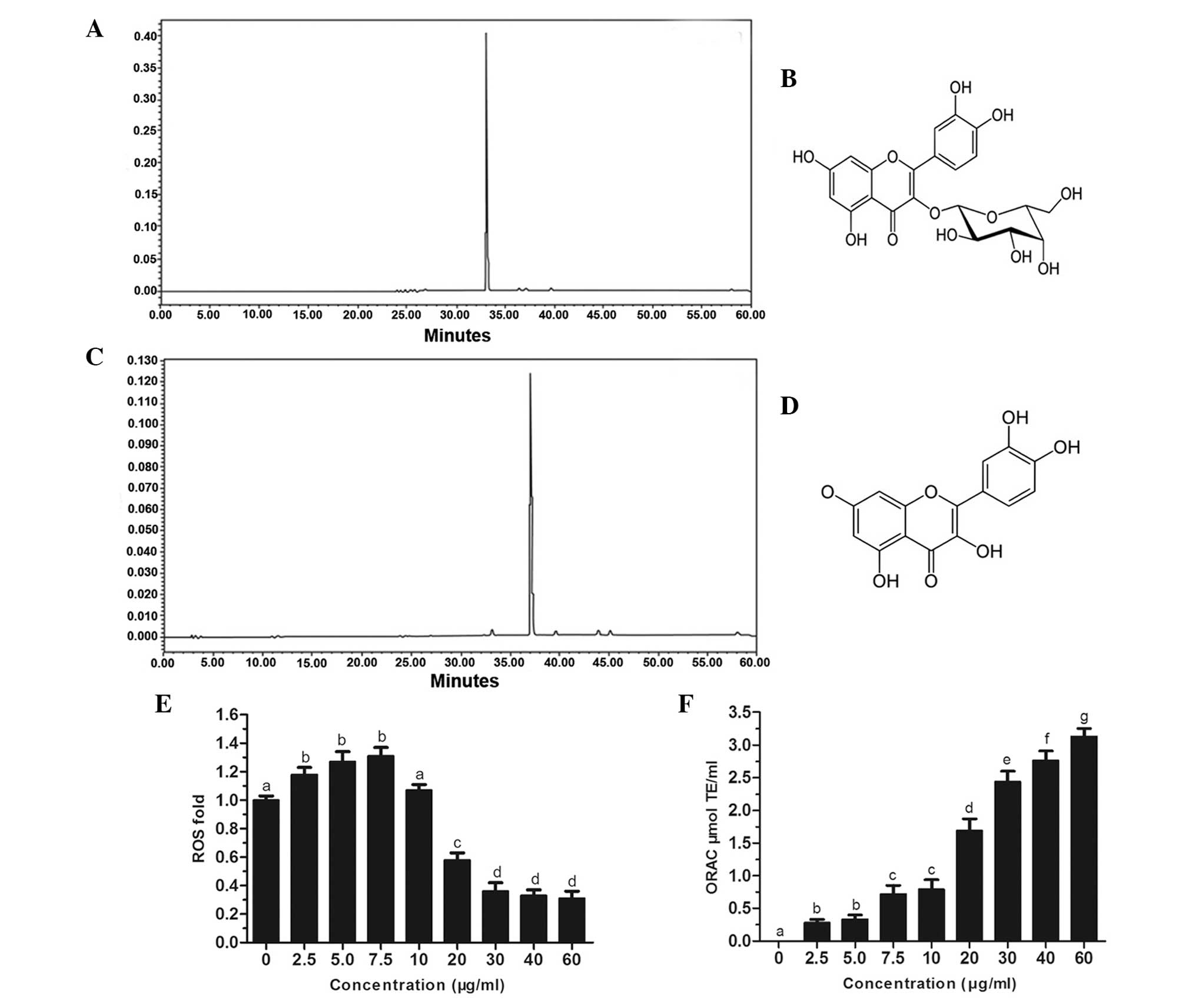
As shown in Fig. 1A and
C, the chromatographic stilbene and flavonal profiles of QH,
respectively, demonstrated the presence of two major polyphenols,
hyperoside (peak 1) and quercetin (peak 2) in this botanical
supplement. The chemical structures of hyperoside and quercetin are
shown in Fig. 1B and D,
respectively. In addition, quercetin and hyperoside were reported
to be among the most abundant flavonoids in a standard human diet
(5,18).
Production of intracellular ROS and
ORAC
Intracellular production of ROS was investigated in
PC3 cells following treatment with hydrogen peroxide. The results
revealed that at low concentrations of QH (0–10 μg/ml), there was a
slight increase in ROS production; however, at higher
concentrations of QH (20–60 μg/ml), ROS production was
significantly decreased by up to 69% compared to that of the
control cells (Fig. 1E). An ORAC
assay was then used to determine the antioxidant capacity of cells
following QH treatment (Fig. 1F).
The results demonstrated that all tested concentrations of QH
significantly increased the antioxidant capacity of PC3 cells in a
dose-dependent manner.
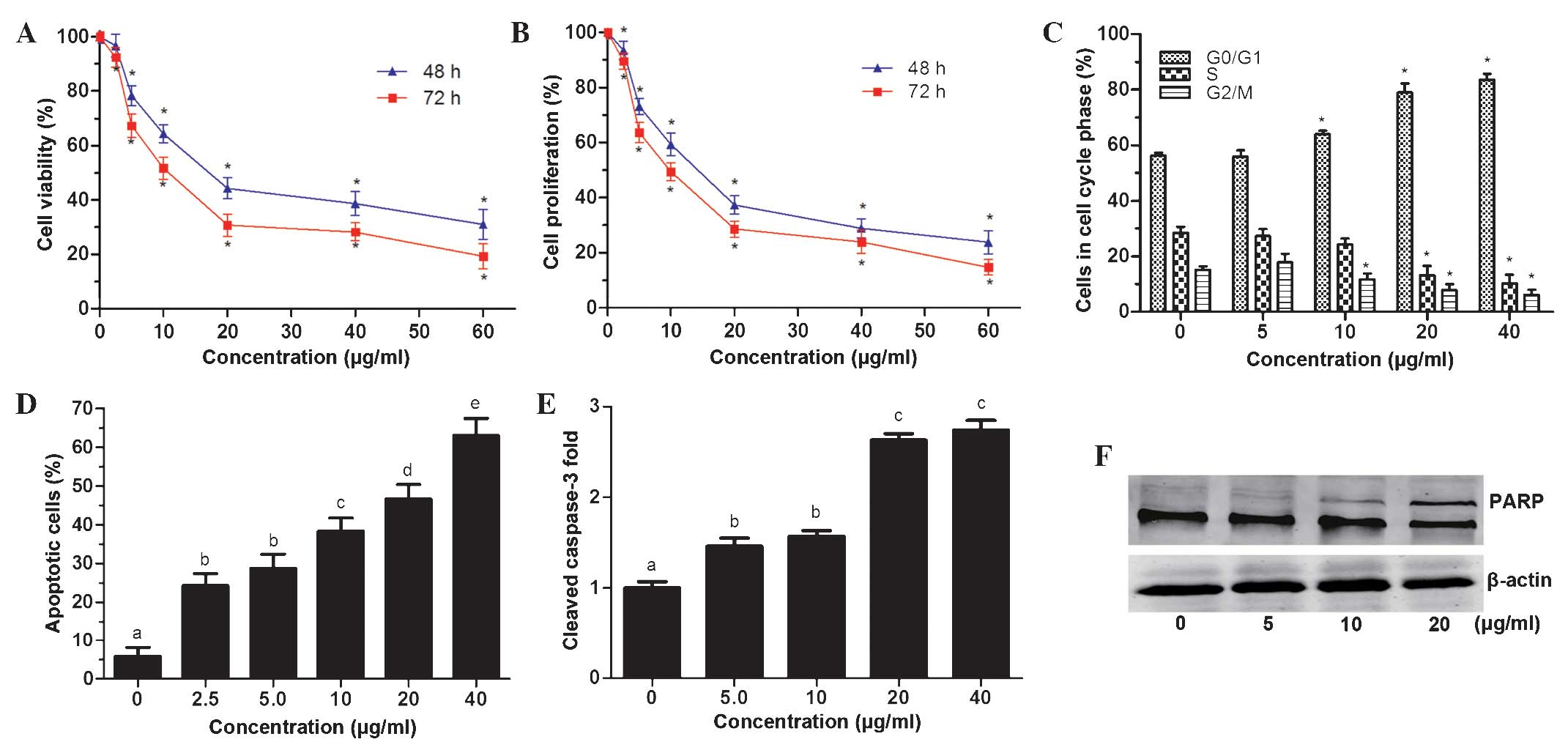
QH inhibits PC3 cell viability
A Cell Titer 96® cell proliferation assay
was used to determine cell viability following incubation with
different concentrations of QH for 48 and 72 h. The results
demonstrated that QH significantly decreased PC3 cell viability in
a dose- and time-dependent manner (Fig. 2A), with IC50-values of
19.7 and 12.4 μg/ml, for 48 and 72 h, respectively. In addition,
determination of the cell count of PC3 cells showed that QH
significantly inhibited the proliferation of PC3 cells following 48
and 72 h of treatment with QH in a dose- and time-dependent manner
(Fig. 2B). The total cell count
was significantly decreased at all tested concentrations (2.5–60
μg/ml) and at 60 μg/ml, QH cell proliferation was reduced by 76 and
85%, following 48 h and 72 h of incubation, respectively.
As shown in Fig.
2C, the effects of QH on cell-cycle progression were determined
by fluorescence-activated cell sorting analysis. No significant
changes were observed in the percentage of cells in different
phases of the cell cycle; however, there was a significant G0/G1 to
S phase block compared to control cells following treatment with
10, 20 and 40 μg/ml QH (Fig.
2C).
Annexin V-FITC and propidium iodide staining and
flow cytometric analysis determined that QH increased apoptosis in
PC3 cells at all tested concentrations (2.5–40 μg/ml) compared to
that in the control, with a maximum increase at 40 μg/ml QH by 63%
(Fig. 2D).
Furthermore, activated/cleaved caspase-3 levels were
found to be elevated at low concentration of QH (5 and 10 μg/ml) by
~1.5-fold and at higher concentrations (20 and 40 μg/ml) by
~2.7-fold (Fig. 2E).
Poly(adenosine diphosphate ribose) polymerase 1 (PARP-1) is a
substrate for caspase-3 cleavage, which produces cleaved PARP-1
(19). In the present study,
western blot analysis revealed an increase in PARP cleavage in PC3
cells following QH treatment (Fig.
2F). These results therefore indicated that cleaved caspase-3
was activated and had a role in the induction of apoptosis
following QH treatment.
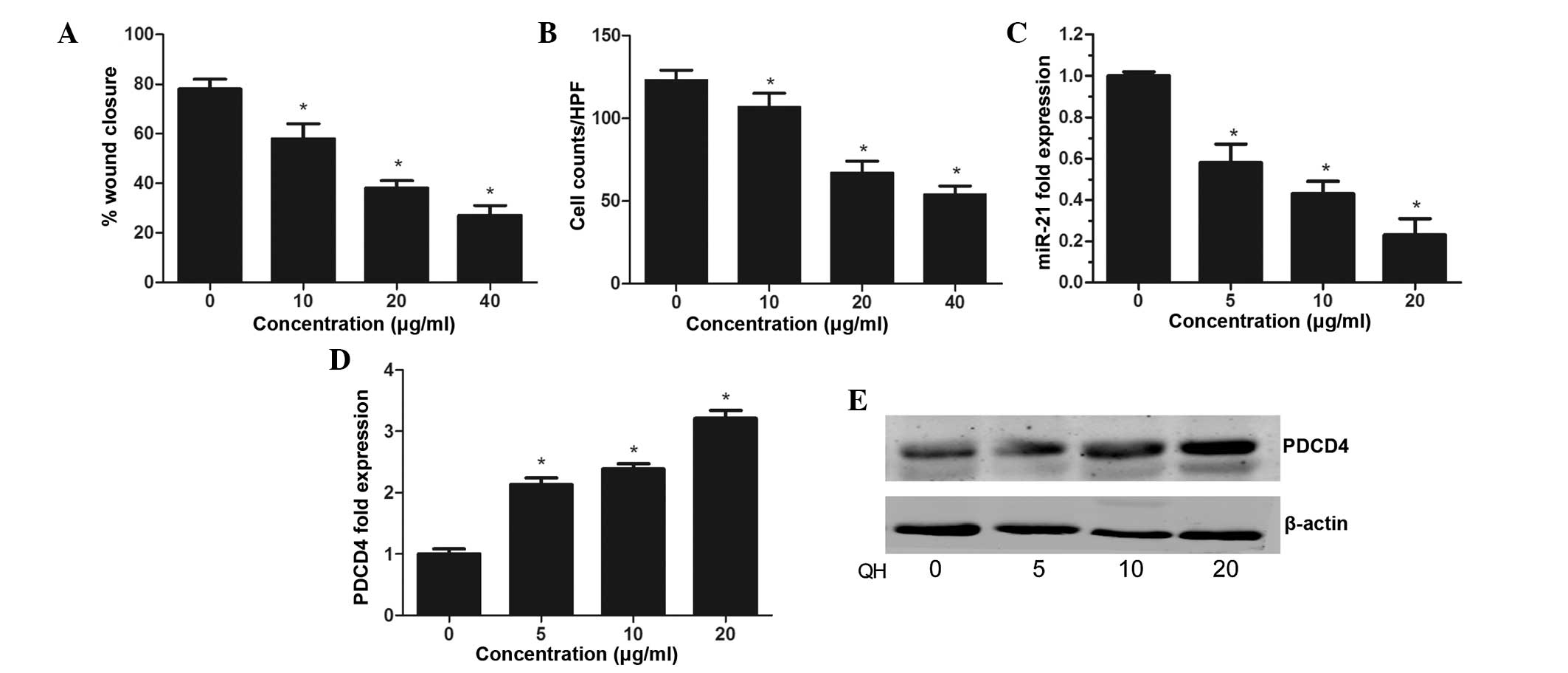
QH inhibits the invasive activity of PC3
cells
The present study aimed to investigate the effect of
QH on prostate cancer cell migration and invasion. The results of
the wound healing assay demonstrated that QH-treated PC3 cells had
significantly decreased migratory ability compared to that of the
control (P<0.05) (Fig. 3A). A
Matrigel® invasion assay determined that the average
cell counts crossing a Matrigel®-coated membrane in the
control group was significantly increased compared to that of the
QH treatment group, indicating that QH significantly suppressed the
invasive capacity of prostate cancer cells (P<0.05) (Fig. 3B).
QH regulates miR-21 expression in PC3
cells
A TaqMan® assay was performed in order to
evaluate the expression of miR-21 in PC3 cells following QH
treatment. The results showed a dose-dependent decrease in miR-21
expression, with inhibition rates of 42, 56 and 77% observed at 5,
10 and 20 μg/ml QH, respectively (Fig.
3C). In addition, a dose-dependent increase in PDCD4
expression, a target molecule for miR-21, was detected in PC3 cells
following treatment with QH (Fig. 3D
and E). This therefore indicated that the mechanisms underlying
the anti-cancer effect of QH in PC3 cells may proceed via the
suppression of the miR-21 gene.
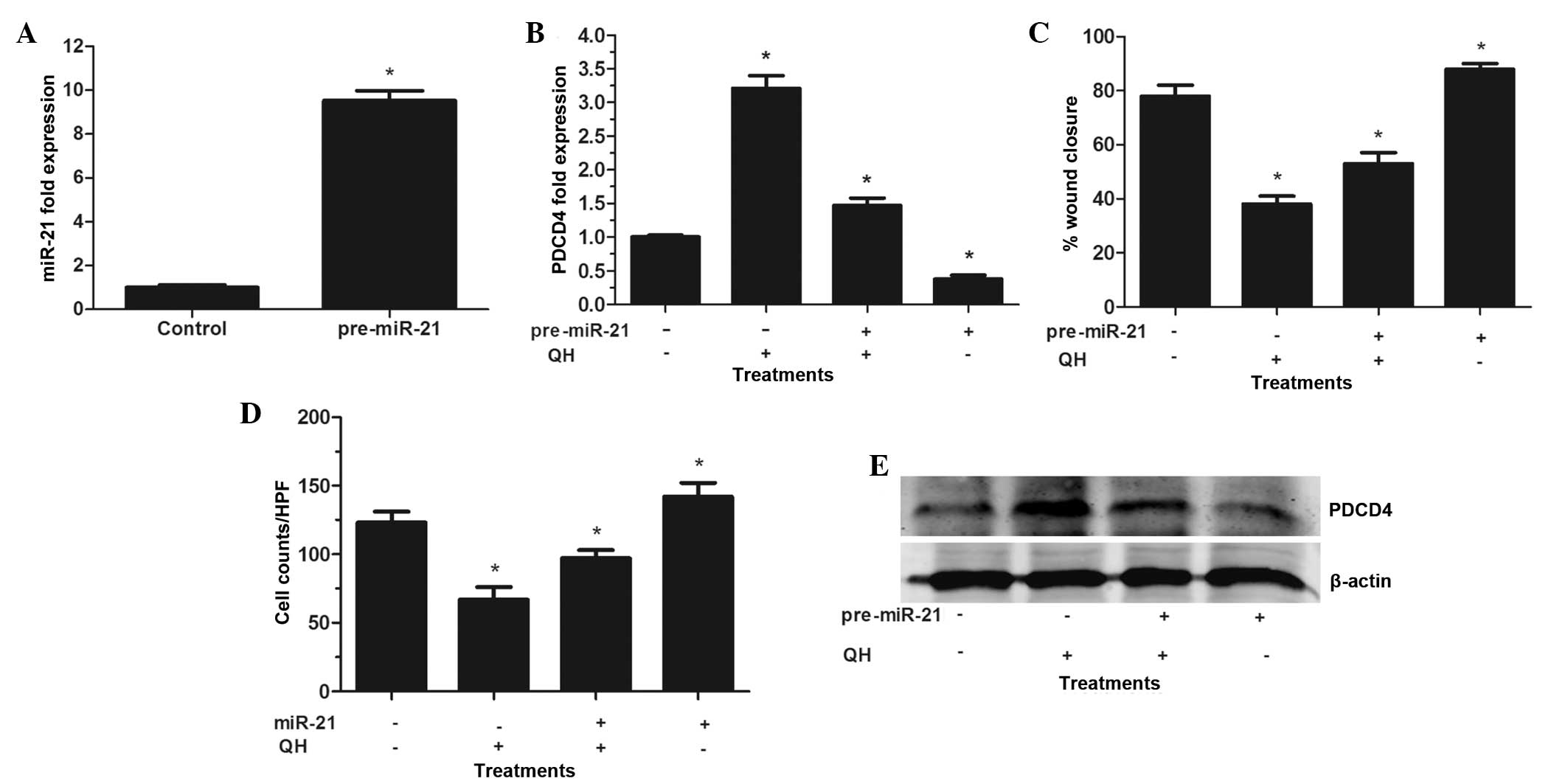
PC3 cells were then transfected with pre-miR-21 in
order to induce the overexpression of miR-21. Pre-miR-21 is
processed by the RNAse III enzyme Dicer, which results in a 22 base
pair double-stranded RNA with two nucleotide 39 overhangs; one of
these strands forms the mature miRNA, which is incorporated into an
RNA silencing complex (RISC), allowing it to functionally suppress
the expression and translation of its targeted RNAs (20). In the present study, increased
miR-21 levels compared to those in the control-transfected cells
confirmed the effectiveness of miR-21 transfection (Fig. 4A). As hypothesized, PDCD4 levels
were significantly decreased compared to those of the control,
indicating that miR-21 bound to the 3′ untranslated region on PDCD4
mRNA and enhanced its degradation (15). These results suggested that
pre-miR-21 was effectively processed in PC3 cells, resulting in the
functional overexpression of miR-21. Furthermore, the
overexpression of pre-miR-21 resulted in partial resistance of
transfected PC3 cells to QH-induced PDCD4 stimulation (Fig. 4B and E). However, QH retained its
ability to augment PDCD4 levels in cells via the suppression of the
high basal miR-21 expression. In addition, cells overexpressing
miR-21 exhibited an increased resistance to QH-induced suppression
of PC3 cells wound healing and invasive capacity (Fig. 4C and D). In conclusion, the results
of these experiments indicated that miR-21 was the target gene for
QH treatment, which mediated the growth and invasiveness of PC3
cells in vitro.
Discussion
The production of ROS is known to be associated with
the oxidative cellular damage involved in the development of
numerous pathological conditions (21). ROS have a complex role in
carcinogenesis; the majority of cancer cells have increased
constitutive levels of ROS compared to those of normal cells, due
to mutations in nuclear and mitochondrial genes responsible for the
electron transport chain as well as increased metabolic and
mitochondrial activity (22). It
was reported that elevated ROS levels may enhance cell
proliferation among other events associated with cancer progression
(23). In addition, ROS were
reported to induce DNA damage and oxidation of fatty acids in
cellular membrane structures, which may facilitate mutagenesis and
cancer development. However, numerous anti-cancer drugs are toxic
to mitochondria and induce ROS production, which may in turn lead
to cancer cell death (24).
Polyphenols, including hyperoside and quercetin, have the ability
to scavenge free radicals and induce the activation of antioxidant
and detoxifying enzymes, therefore protecting cells against
oxidative damage caused by carcinogenic compounds (25). In the present study, QH induced a
dose-dependent increase in the intracellular antioxidant capacity;
by contrast, low concentrations of QH increased ROS production in
PC3 cells, whereas high concentrations significantly reduced ROS
levels. One possible explanation for this biphasic effect may be
the toxic effects of polyphenols in mitochondria, which may have
induced ROS production, whereas at higher concentrations of QH,
their antioxidant properties became dominant (25). Previous studies have demonstrated
the protective effect of polyphenols against oxidative damage under
certain conditions (26). This may
indicate that in the present study, the lower concentrations of QH
were insufficient to have protective effects and therefore resulted
in the additional formation of radicals, whereas at higher
concentrations, QH inhibited ROS production, potentially through
scavenging ROS.
The anti-proliferative effects of quercetin were
previously demonstrated in MOLT-4 leukemia cells through the
combination of resveratrol and quercetin, which exhibited
synergistic anti-cancer effects (12). Based on previous studies, a
combination of hyperoside and quercetin (ratio, 1:1) was
investigated in the present study, resulting in significant
decrease in cell viability and proliferation following QH
treatment. However, it remains to be elucidated whether a different
ratio may be more effective.
A previous study demonstrated that polyphenols,
including resveratrol and quercetin, induced cell cycle arrest in
numerous cancer cell lines at different phases (27). Tan et al (28) studied the effect of quercetin on
HepG2 cells and reported that following treatment with quercetin
for 48 h, cells were arrested in G0/G1 phase. In MOLT-4 leukemia
cells, polyphenol-mediated cell cycle arrest was influenced by the
duration of treatment and the type of polyphenol. In the present
study, QH (20 μg/ml) decreased the percentage of cells in S-phase
and increased the percentage of cells in G0/G1-phase, which was
consistent with the inhibition of the progression from G0/G1 to
S-phase.
Caspase-3 is an important enzyme in apoptosis and a
commonly used indicator for the induction of apoptosis (28). PARP-1, an abundant
chromatin-associated protein, has a significant role in maintaining
genome integrity and is cleaved by caspase-3 during apoptosis
(29). Previous studies have
demonstrated the effects of quercetin, as well as other
polyphenols, on caspase-3 and PARP-1 activity (30,31).
In general, the results of the present study are in accordance with
those of previous studies, which showed that polyphenols induced
apoptosis via the activation of caspase-3 accompanied by cleavage
of PARP (28). These previous
studies also reported that resveratrol caused the induction of
caspase-3 and PARP cleavage in human articular chondrocytes and
myeloid leukemia cells.
The results of the present study indicated that the
miR-21 axis was an important target of QH for mediating the
survival and invasive capacity of PC3 prostate cancer cells. It was
found that the underlying mechanism of QH anti-cancer activity was
via the induction of miR-21 targeted genes, such as PDCD4. In
addition, the present study demonstrated that overexpression of
miR-21 antagonized the anti-tumor effects of QH, which further
highlighted the role of the miR-21 pathway in mediating the
anti-tumor actions of QH in prostate cancer cells.
miR-21 is an oncomir which has an important role in
regulating numerous cellular processes in order to enhance cancer
cell growth and invasion. Expression of miR-21 is high in
androgen-independent prostate cancer cell lines, including PC3 and
DU145, and low in androgen-dependent prostate cancer cells, such as
LNCaP cells (32). It was
hypothesized that the androgen/androgen receptor complex binds to
the promoter region of miR-21 in order to induce its expression
(33). Of note, the resultant high
expression of miR-21 was suggested to promote androgen resistance
via downstream gene regulation; miR-21-regulated genes include
myristoylated alanine-rich protein kinase c substrate (MARCKS),
PDCD4, maspin and tropomyosin-1 (20,34).
miR-21 has been reported to negatively regulate MARCKS, which was
suggested to control cell motility through interactions with the
actin cytoskeleton (32). It was
subsequently reported that cells treated with antisense miR-21
exhibited increased MARCKS expression and reduced invasive capacity
(35). Downregulation of MARCKS
using siRNAs increased the invasiveness of DU-145 prostate cancer
cells (32).
The anti-tumor activity of PDCD4 was suggested to
proceed through numerous mechanisms; PDCD4 was reported to suppress
protein translation via inhibition of the eukaryotic initiation
factor 4A activity (36). In
addition, PDCD4 suppressed the transactivation of the activator
protein (AP)-1 promoter via c-Jun (37), therefore inhibiting growth
promotion. These previous studies provided evidence to support the
conclusion that QH inhibited miR-21 expression in PC3 cells and
increased the expression of key target proteins, such as PDCD4;
furthermore, overexpression of miR-21 decreased the expression of
the miR-21 target PDCD4 and reduced the ability of QH to mediate
the invasive capacity of PC3 cells.
In conclusion, the results of the present study
indicated that a combination of quercetin and hyperoside exhibited
anti-tumor activities in prostate cancer cells, resulting in
apoptosis, cell cycle arrest and reduced invasive capacity.
QH-induced regulation of the miR-21-PDCD4 axis was identified as
one possible underlying mechanism of the anti-cancer effects of QH.
Further studies are required in order to assess the role and
clinical relevance of miRNA-21 in the anti-cancer effects exhibited
by botanicals.
Acknowledgements
This work was partially supported by grants from the
National Natural Science Foundation of China (nos. 81000311 and
81270831).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mottet N, Bellmunt J, Bolla M, et al: EAU
guidelines on prostate cancer. Part II: Treatment of advanced,
relapsing, and castration-resistant prostate cancer. Actas Urol
Esp. 35:565–579. 2011.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuu CP, Kokontis JM, Hiipakka RA, et al:
Androgens as therapy for androgen receptor-positive
castration-resistant prostate cancer. J Biomed Sci. 18:632011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harzstark AL and Small EJ:
Castrate-resistant prostate cancer: therapeutic strategies. Expert
Opin Pharmacother. 11:937–945. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Theodoratou E, Kyle J, Cetnarskyj R, et
al: Dietary flavonoids and the risk of colorectal cancer. Cancer
Epidemiol Biomarkers Prev. 16:684–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi JA, Kim JY, Lee JY, et al: Induction
of cell cycle arrest and apoptosis in human breast cancer cells by
quercetin. Int J Oncol. 19:837–844. 2001.PubMed/NCBI
|
|
7
|
Kim MJ, Kim YJ, Park HJ, Chung JH, Leem KH
and Kim HK: Apoptotic effect of red wine polyphenols on human colon
cancer SNU-C4 cells. Food Chem Toxicol. 44:898–902. 2006.
View Article : Google Scholar
|
|
8
|
Tang SN, Singh C, Nall D, Meeker D,
Shankar S and Srivastava RK: The dietary bioflavonoid quercetin
synergizes with epigallocathechin gallate (EGCG) to inhibit
prostate cancer stem cell characteristics, invasion, migration and
epithelial-mesenchymal transition. J Mol Signal. 5:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srivastava RK, Tang SN, Zhu W, Meeker D
and Shankar S: Sulforaphane synergizes with quercetin to inhibit
self-renewal capacity of pancreatic cancer stem cells. Front Biosci
(Elite Ed). 3:515–528. 2011. View
Article : Google Scholar
|
|
10
|
Zou Y, Lu Y and Wei D: Antioxidant
activity of a flavonoid-rich extract of Hypericum perforatum L. in
vitro. J Agric Food Chem. 52:5032–5039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao LL, Feng L, Yao ST, et al: Molecular
mechanisms of celery seed extract induced apoptosis via s phase
cell cycle arrest in the BGC-823 human stomach cancer cell line.
Asian Pac J Cancer Prev. 12:2601–2606. 2011.
|
|
12
|
Mertens-Talcott SU, Talcott ST and
Percival SS: Low concentrations of quercetin and ellagic acid
synergistically influence proliferation, cytotoxicity and apoptosis
in MOLT-4 human leukemia cells. J Nutr. 133:2669–2674.
2003.PubMed/NCBI
|
|
13
|
George J, Singh M, Srivastava AK, et al:
Resveratrol and black tea polyphenol combination synergistically
suppress mouse skin tumors growth by inhibition of activated MAPKs
and p53. PLoS One. 6:e233952011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar :
|
|
15
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
16
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Liu W, Chao T, et al: MicroRNA-21
down-regulates the expression of tumor suppressor PDCD4 in human
glioblastoma cell T98G. Cancer Lett. 272:197–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frémont L: Biological effects of
resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brauns SC, Dealtry G, Milne P, Naudé R and
Van de Venter M: Caspase-3 activation and induction of PARP
cleavage by cyclic dipeptide cyclo(Phe-Pro) in HT-29 cells.
Anticancer Res. 25:4197–4202. 2005.PubMed/NCBI
|
|
20
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37(Pt 4): 918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carew JS and Huang P: Mitochondrial
defects in cancer. Mol Cancer. 9:92002. View Article : Google Scholar
|
|
22
|
Fresco P, Borges F, Diniz C and Marques
MP: New insights on the anticancer properties of dietary
polyphenols. Med Res Rev. 26:747–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biasutto L, Szabo’ I and Zoratti M:
Mitochondrial effects of plant-made compounds. Antioxid Redox
Signal. 15:3039–3059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu SC, Lu JH, Kuo CL, et al: Crude
extracts of Solanum lyratum induced cytotoxicity and apoptosis in a
human colon adenocarcinoma cell line (colo 205). Anticancer Res.
28(2A): 1045–1054. 2008.PubMed/NCBI
|
|
27
|
Schlachterman A, Valle F, Wall KM, et al:
Combined resveratrol, quercetin, and catechin treatment reduces
breast tumor growth in a nude mouse model. Transl Oncol. 1:19–27.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan J, Wang B and Zhu L: Regulation of
survivin and Bcl-2 in HepG2 cell apoptosis induced by quercetin.
Chem Biodivers. 6:1101–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shakibaei M, John T, Seifarth C and
Mobasheri A: Resveratrol inhibits IL-1 beta-induced stimulation of
caspase-3 and cleavage of PARP in human articular chondrocytes in
vitro. Ann NY Acad Sci. 1095:554–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZQ, Stingl L, Morrison C, et al: PARP
is important for genomic stability but dispensable in apoptosis.
Genes Dev. 11:2347–2358. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu CP, Lin YH, Chou CC, et al: Mechanisms
of grape seed procyanidin-induced apoptosis in colorectal carcinoma
cells. Anticancer Res. 29:283–289. 2009.PubMed/NCBI
|
|
32
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ribas J, Ni X, Haffner M, et al: miR-21:
an androgen receptor-regulated microRNA that promotes
hormone-dependent and hormone-independent prostate cancer growth.
Cancer Res. 69:7165–7169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pang Y, Young CY and Yuan H: MicroRNAs and
prostate cancer. Acta Biochim Biophys Sin (Shanghai). 42:363–369.
2010. View Article : Google Scholar
|
|
35
|
Arbuzova A, Schmitz AA and Vergères G:
Cross-talk unfolded: MARCKS proteins. Biochem J. 362(Pt 1): 1–12.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang HS, Jansen AP, Komar AA, et al: The
transformation suppressor Pdcd4 is a novel eukaryotic translation
initiation factor 4A binding protein that inhibits translation. Mol
Cell Biol. 23:26–37. 2003. View Article : Google Scholar :
|
|
37
|
Bitomsky N, Böhm M and Klempnauer KH:
Transformation suppressor protein Pdcd4 interferes with
JNK-mediated phosphorylation of c-Jun and recruitment of the
coactivator p300 by c-Jun. Oncogene. 23:7484–7493. 2004. View Article : Google Scholar : PubMed/NCBI
|