Introduction
Ketamine was first synthesized in 1962 (1) and introduced into clinical medicine
for dissociative anesthesia in 1970 (2). It is a non-competitive
N-methyl-d-aspartic acid receptor
antagonist and used as a short-acting general anesthetic in human
and veterinary clinical settings (3). Due to its low cost and the fact that
it induces hallucination and alters the state of consciousness,
ketamine soon emerged as a recreational drug (4). As the number of ketamine abusers
gradually increased, a new side effect on the bladder was first
reported in 2007 (5,6). The symptoms of ketamine-induced
cystitis include dysuria and urgency (7,8), and
cystoscopic examination of severe cases demonstrated hemorrhagic
cystitis, denuded mucosa and marked inflammation (9). The symptoms are similar to
interstitial cystitis (IC) (6),
therefore, certain treatment regimens, including oral pentosan
polysulphate and intravesical instillation of hyaluronic acid, are
used in ketamine-induced cystitis, to relieve the varying degrees
of symptoms (6,9,10).
Other medicines, including antibiotics, non-steroidel
anti-inflammatory drugs, steroid and anticholinergic drugs, are
also applied for therapy, but the benefit is limited (9).
To date, no specific treatment for patients with
ketamine-induced cystitis has been established. In spite of the
increase in the number of recreational users, investigating
ketamine-induced cystitis in humans is not simple. As a result, a
number of studies have used animal models for investigating the
mechanisms of action and effects of ketamine. Previously, to the
best of our knowledge, two mouse model (11,12)
and two rat model (13,14) in vivo studies have been
published. In Yeung et al’s mouse model (11), 30 mg/kg/day ketamine injection
induced submucosal infiltration of mononuclear inflammatory cells.
The urothelium became thinner and the number of nerve fibers was
reduced following one month of ketamine treatment. In Meng’s mouse
study (12), following 100
mg/kg/day ketamine injection for 2~4 months, a decrease in the
mouse body weight growth rate and bladder capacity, the increase of
adenosine triphosphate-evoked detrusor contraction and P2X1
receptor protein was observed in the animal bladders. In Gu’s rat
model (13), the whole rat bladder
proteins were analyzed by two-dimensional electrophoresis following
50 mg/kg/day ketamine injection for four months. The bladder
histological examination demonstrated hyperplastic urotheliums and
inflammatory cell infiltration. The phosphorylated transgelin of
bladder smooth muscle was increased by ketamine treatment, which
suggested that transgelin may have a role in decreasing bladder
contractility. In Chuang’s rat study (14), it was revealed that 25 mg/kg/day
ketamine injection for one month induced cyclooxygenase-2 and
inducible nitric oxide synthase gene expression in the rat
bladders.
Although the animal studies mentioned above provided
notable insight into the mechanisms of ketamine-induced bladder
damage, these effects remain to be fully elucidated. In the present
study, three urothelial cell lines were used to study the
cytotoxicity of ketamine and the barrier permeability affected by
ketamine. In the in vivo assay, a mouse animal model was
designed for global gene expression analysis in the bladders.
Materials and methods
Cell culture and ketamine treatment
Three different urothelial cell lines, purchased
from Bioresource Collection and Research Center (Hsinchu, Taiwan)
were used. The SV-HCU-1 cell line derived from normal human
urothelial cells immortalized by the SV40 virus. The RT4 cell line
is derived from a well-differentiated papillary tumor of the human
bladder (15). The 5637 cell line
is a grade II carcinoma of the human bladder (16). SV-HUC-1 cells were cultured in
Ham’s F-12 medium (Gibco Life Technologies, Grand Island, NY. USA)
supplied with 7% fetal bovine serum (FBS; Biological Industries,
M.P. Ashrat, Israel). RT4 cells were cultured in McCoy’s 5A medium
(Sigma-Aldrich, St. Louis, MO, USA) supplied with 10% FBS, 1%
penicillin and 1% streptomycin. 5637 cells were maintained in
RPMI-1640 medium (Gibco Life Technologies) supplied with 10% FBS,
1% penicillin and 1% streptomycin (Gibco Life Technologies). The
cells were incubated in a CO2 incubator at 37°C, with 5%
CO2 and 95% filtered air. Ketamine (Sigma-Aldrich) was
dissolved in normal saline. For the cultured cell assay, ketamine
was added to cells of the ketamine-treated groups, while the same
volume of normal saline was added to the control cells.
Cell viability assay
The cell number was determined by a colorimetric MTT
assay. The cells were seeded in 96-well plates for 24 h, then were
incubated with various concentrations of ketamine or normal saline
for another 24–48 h. MTT was added into the medium for 2 h, then
the medium was discarded and dimethyl sulfoxide was added to
dissolve the formazan product. Each well was measured by light
absorbance at 490 nm. The result was expressed as the percentage of
the normal saline-treated control group.
Cell cycle analysis
The cells were seeded in 100-mm dishes. Following 24
h incubation, ketamine or normal saline was added. Following
treatment for 24 and 48 h, the cells were trypsinized, centrifuged
at 800 × g for 5 min and fixed with ice-cold 75% ethanol overnight
at 4°C. Following removal of the ethanol, the cells were stained
with a DNA staining solution [containing 1 mg/ml propidium iodide
and 10 mg/ml RNase A dissolved in phosphate-buffered saline (PBS)]
for 30 min at room temperature. The DNA content of the stained
cells was measured using a FACScan flow cytometer. The cell
doublets were removed by gating the left area of the FL2-W/FL2-A
plot for analysis. The cell cycle data from flow cytometry were
analyzed using ModFit LTTM software (Verity Inc.
Sunnyvale, CA).
Urothelial barrier function assay
Approximately 4×104 SV-HUC-1 cells,
4×104 RT4 cells and 1×104 5637 cells were
seeded on an Transwell insert with 0.4 μm pore size filter membrane
(Millipore Corp. Billerica, MA, USA) and incubated for 24 h.
Ketamine was added into the upper and lower chamber media at the
same time. Following incubation for 19 or 43 h, green
fluorescence-labeled antibodies (Alexa Fluor® 488 goat
anti-mouse immunoglobulin G; Invitrogen Life Technologies) were
added into upper chamber medium (9.6 μg/insert) and continued
incubating for another 5 h. The total medium in the upper and lower
chambers were collected for fluorescence analysis by a fluorescence
microplate reader (excitation/emission: 488/519 nm).
Animals and ketamine treatment
Six-week-old male Balb/c mice were used in the
present study and purchased from the National Laboratory Animal
Center (Taipei, Taiwan). All of the animals were maintained at the
qualified animal care facility of Biotechnology and Health Hall in
National Chiayi University (Chiayi City, Taiwan, R.O.C) for one
week prior to intraperitoneal (i.p.) injection. At seven weeks of
age, the mice were divided into four groups (12 mice/group),
including control-30 days (i.p. normal saline for 30 days),
ketamine-30 days (i.p. 30 mg/kg/day ketamine for 30 days),
control-60 days (i.p. normal saline for 60 days) and ketamine-60
days (i.p. 30 mg/kg day ketamine for 60 days). The mice were housed
in polycarbonate cages, provided with food and water ad libitum and
maintained on a 12 h light-dark cycle at 22±2°C. All of the
experiments were approved by the Institutional Animal Care and Use
Committee of National Chiayi University.
Bladder tissue collection and hematoxylin
and eosin staining
Following the 30- or 60-day treatment, the mice were
euthanized and the bladder tissues were removed. A total of 20
bladders (five/group) were fixed in 10% neutral formalin for
histological examination, three bladders/group were homogenized
together and RNA was extracted, and the other bladders were stored
under liquid nitrogen for future use. The bladder tissues in 10%
neutral formalin were embedded in paraffin and then cut into 4-μm
sections on glass slides. One slide from each mouse was stained
with hematoxylin and eosin (H&E). Other slides were prepared
for immunohistochemical analysis.
Global gene expression analysis
Total RNA was isolated from three bladders in each
group using TRIzol reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. The quality of RNA was examined
using Agilent’s RNA LabChip kits on the 2100 Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). The RNA samples from the
four groups (control-30 days, ketamine-30 days, control-60 days and
ketamine-60 days) were transferred to fluorescence-labeled
antisense (a)RNA using OneArray Amino Allyl aRNA Amplification kit
(Phalanx Biotech Group, Hsinchu, Taiwan) and Cy5 dye labeling
(Amersham Pharmacia, Piscataway, NJ, USA). For global gene
expression analysis, the fluorescent targets were hybridized to the
Mouse Whole Genome OneArrayTM version MOA 2.0 (Phalanx
Biotech Group), containing 27,295 mouse genome probes. One mixture
sample was applied to two chips, and the normalized intensities
were calculated from raw intensities by median scaling. Microarray
image scanning and data analysis were achieved by Phalanx Biotech
Group.
Polymerase chain reaction (PCR)
analysis
Reverse transcription was performed on 2 μg of total
RNA by 5 μM random hexamer and RevertAidTM reverse
transcriptase (Thermo Fisher Scientific, Fermentas, Pittsburgh, PA,
USA), then 1/10 volume of reaction mixture was used for PCR with
specific primers (keratin 6a forward 5′-TGCCAGGGGCAAGCTGGAAG-3′ and
reverse 5′-ACGGGATTCTGCAGCCATGACA-3′; keratin 13 forward
5′-AGCTTGGAGGAGGCCGTAAT-3′ and reverse 5′-AAGCACTGTAGTCCCGCTCT-3′;
keratin 14 forward 5′-TGGTGCAGAGCGGCAAGAGTG-3′ and reverse
5′-TGCGGATCTGGCGGTTGGTGG-3′) and β-actin forward
5′-CCTAAGGCCAACCGTGAAAAG-3′ and reverse
5′-TCTTCATGGTGCTAGGAGCCA-3′). The PCR products (keratin 6a, 486 bp;
keratin 13, 375 bp; keratin 14, 399 bp; β-actin, 623 bp) were
analyzed by 1% agarose gel.
Immunohistochemical analysis
After being washed in PBS, the slides were incubated
in a blocking solution for 30 min and then with primary antibodies
against keratin 14 (Genetex, Taipei, Taiwan) at a 1:100 dilution at
4°C overnight. The slides were then washed and incubated with
secondary antibodies containing horseradish peroxidase at 25°C for
30 min. Following this treatment, the slides were washed with PBS
and further incubated with 3,3′-diaminobenzidine for 5 min.
Finally, the sections were rinsed in running water, treated with
hematoxylin for ~10–15 sec and mounted for evaluation.
Statistical analysis
Numerical data (except gene expression microarray
data) are expressed as the mean ± standard error. Statistical
differences were analyzed by one-way analysis of variance analysis
of variance followed by Tukey’s test. All statistics were
calculated using SigmaState version 3.5 (Systat Software, San Jose,
CA, USA)
Results
Cytotoxicity of ketamine in human
urothelial cell lines SV-HUC-1, RT4 and 5637
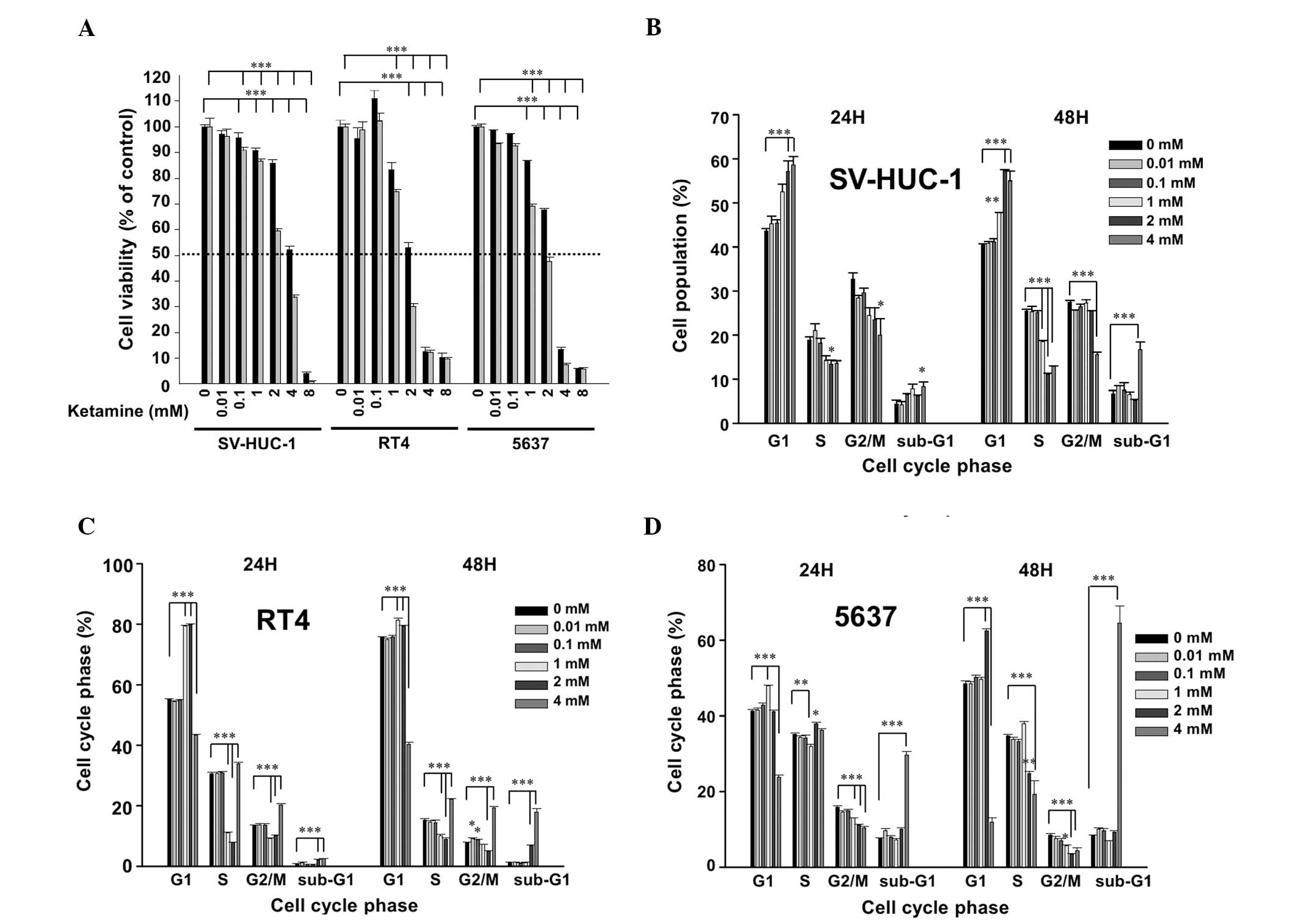
Following ketamine treatment for 24 h, the
IC50 value of ketamine was ~4, 2 and 3 mM in SV-HUC-1,
RT4 and 5637 cells, respectively. At 48 h, the IC50 was
~3, 1.5 and 2 mM in the SV-HUC-1, RT4 and 5637 cells, respectively
(Fig. 1A). These results suggested
that ketamine is cytotoxic to urotheliums in a dose-dependent and
time-dependent manner. Due to the identified cytotoxicity,
ketamine-induced cell cycle changes were analyzed. In the SV-HUC-1
cells, ketamine dose-dependently increased the G1 phase cells at a
dose higher than 1 mM and significantly increased the sub-G1 level
at 4 mM (Fig. 1B). In the RT4
(Fig. 1C) and 5637 (Fig. 1D) cells, ketamine also arrested the
cells in the G1 phase between 1 to 2 mM, and significantly
increased the sub-G1 level at 4 mM. All of the above data suggested
that ketamine induced G1 arrest and cytotoxicity in the human
urothelial cells.
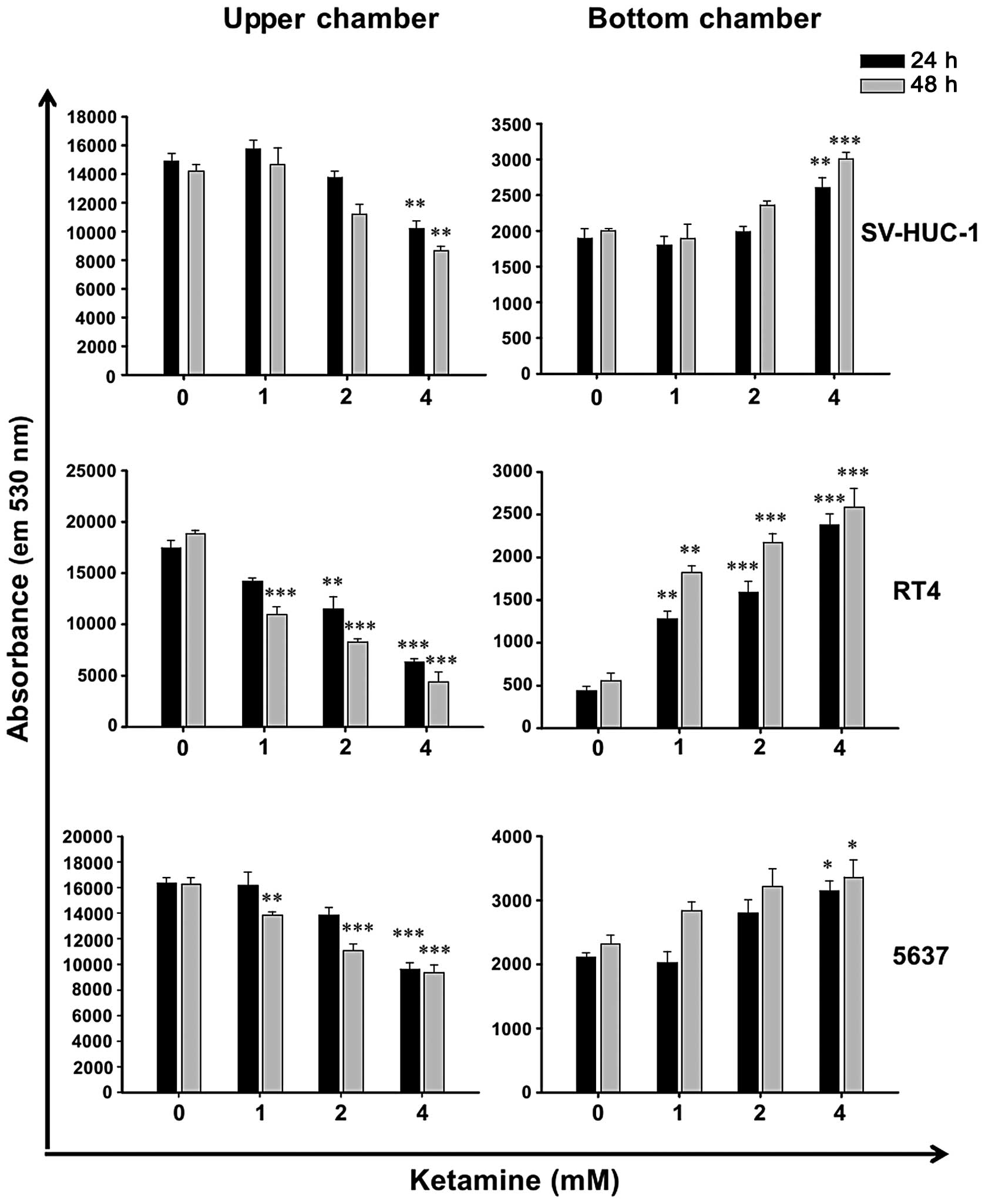
Ketamine increases barrier permeability
of human urothelial cells
Due to the cytotoxicity of ketamine (Fig. 1), it was hypothesized that ketamine
may decrease epithelial barrier function. Therefore, the urothelial
barrier permeability assay was employed. Following ketamine
treatment for 24 and 48 h, the permeability of green
fluorescence-labeled antibodies was increased dose-dependently in
SV-HUC-1, RT4 and 5637 cells (Fig.
2). When comparing the cytotoxicity of ketamine and its
enhancing effect on the barrier permeability, it was evident that
the dose causing cytotoxicity accompanied barrier function loss.
This suggested that the cytotoxic effect of ketamine may, at least
in part, cause the loss of barrier function in ketamine-treated
urotheliums.
Effect of daily ketamine injection on
mouse body weight, behavior and bladder tissue histology
In addition to the in vitro assay, the
present study aimed identify the gene expression in
ketamine-treated mouse bladder. Following daily ketamine injection
for 30 and 60 days, the growth rate of murine body weight was not
significantly different between the control and ketamine-treated
group (data not shown). This suggested that intraperitoneal
administration of 30 mg/kg/day ketamine for 60 days may not affect
the physiological properties of the mice. At this dosage, that the
mice displayed symptoms of excitation following ketamine injection
for 2–5 min, which lasted for ~40 min. During the injection period,
the onset of excitation was gradually delayed and its intensity was
also gradually decreased. This suggested that the mice developed a
tolerance to ketamine-induced excitation. At the 30th and 60th day,
the bladders were isolated for tissue examination. The histology of
bladder tissues demonstrated no evident differences between the
control and ketamine groups at 30 and 60 days of treatment (data
not shown).
Global gene expression analysis in the
bladders of ketamine-injected mice
Gene expression microarray analysis of bladder
tissue was applied to compare gene expression between the control
and ketamine-treated animals. Upregulated genes with differential
expression (fold change log 2 ≥ 1 and P<0.05) at 60 days and a
statistical difference (only P<0.05) at 30 days were selected.
Downregulated genes with differential expression (fold change log 2
≤ −1 and P<0.05) at 60 days and statistical difference (only
P<0.05) at 30 days were selected. Analysis revealed that 10
genes were upregulated (Table I)
and 36 genes were downregulated (Table II only reveals the top ten genes
and keratin 78). Among these 46 genes, two keratin genes which were
associated with cell-cell/basement membrane adhesion function were
found to be significantly decreased. Of note, the amount of type I
keratin was also decreased in the ketamine-treated rat bladders in
the study by Gu et al (13).
 | Table IUpregulated genes with differential
expression (fold-change log 2 ≥ 1 and P<0.05) at 60-day ketamine
treatment and statistical difference (P<0.05) at 30-day ketamine
treatment in mouse bladders. |
Table I
Upregulated genes with differential
expression (fold-change log 2 ≥ 1 and P<0.05) at 60-day ketamine
treatment and statistical difference (P<0.05) at 30-day ketamine
treatment in mouse bladders.
| | Normalized
intensity | Ratio of change
(%) |
|---|
| |
|
|---|
| | 30-day | 60-day | (K-C)/C × 100% |
|---|
| |
|
|---|
| Gene name | Accession number | C | K | C | K | 30-day | 60-day |
|---|
|
Hedgehog-interacting protein | NM_020259.4 | 296.8 | 602.8 | 183.8 | 847.5 | 103.1 | 361.1 |
| Fucosyl-transferase
9 | NM_010243.3 | 165.3 | 226.6 | 78.2 | 330.7 | 37.1 | 322.9 |
| Leucine rich repeat
containing G protein coupled receptor 5 | NM_010195.2 | 97.3 | 143.4 | 149.1 | 435.0 | 47.4 | 191.8 |
| Titin-cap | NM_011540.2 | 264.0 | 501.2 | 282.9 | 741.5 | 89.8 | 162 |
| Family with
sequence similarity 55, member C | NM_001134494.1 | 438.0 | 705.3 | 170.4 | 401.8 | 61.0 | 135.8 |
| Toll-like receptor
12 | NM_205823.2 | 127.2 | 263.7 | 131.7 | 306.6 | 107.3 | 132.8 |
| Transthyretin | NM_013697.5 | 8198.6 | 12323.3 | 5331.2 | 11324.8 | 50.3 | 112.4 |
| Ras-related
associated with diabetes | NM_019662.2 | 699.4 | 1682.9 | 427.9 | 836.2 | 140.6 | 95.4 |
| Transformation
related protein 53 inducible nuclear protein 1 |
NM_021897.3
NM_001199105.1 | 917.6 | 1624.4 | 528.7 | 1016.2 | 77.0 | 92.2 |
| Claudin 23 | NM_027998.4 | 2564.6 | 3367.4 | 2346.5 | 4466.1 | 31.3 | 90.3 |
 | Table IITop ten downregulated genes with
differential expression (fold change log 2 ≤ −1 and P<0.05) at
60-day ketamine treatment and statistical difference (P<0.05) at
30-day ketamine treatment in mouse bladders. |
Table II
Top ten downregulated genes with
differential expression (fold change log 2 ≤ −1 and P<0.05) at
60-day ketamine treatment and statistical difference (P<0.05) at
30-day ketamine treatment in mouse bladders.
| | Normalized
intensity | Ratio of change
(%) |
|---|
| |
|
|---|
| | 30-day | 60-day | (K-C)/C × 100% |
|---|
| |
|
|---|
| Gene name | Accession
number | C | K | C | K | 30-day | 60-day |
|---|
| WAP four-disulfide
core domain 3 | NM_027961.1 | 102.6 | 46.6 | 528.3 | 59.7 | −54.6 | −88.7 |
| Metallothionein
2 | NM_008630.2 | 1230.5 | 782.7 | 5276.6 | 751.2 | −36.4 | −85.8 |
| Tissue inhibitor of
metallo-proteinase 1 |
NM_001044384.1
NM_011593.2 | 1445.2 | 551.3 | 3648.6 | 845.7 | −61.9 | −76.8 |
| Solute carrier
family 7, member 11 | NM_011990.2 | 624.4 | 403.7 | 2992.5 | 708.8 | −35.3 | −76.3 |
| Keratin 14 | NM_016958.1 | 2972.3 | 1652.2 | 6385.7 | 1621.5 | −44.4 | −74.6 |
| Glutamine
fructose-6-phosphate transaminase 2 | NM_013529.3 | 329.1 | 224.3 | 806.2 | 211.3 | −31.8 | −73.8 |
| Macrophage
scavenger receptor 1 | NM_031195.2 | 457.3 | 283.3 | 891.6 | 281.0 | −38.0 | −68.5 |
| Interleukin 33 |
NM_001164724.1
NM_133775.2 | 1403.7 | 705.9 | 3731.8 | 1207.9 | −49.7 | −67.6 |
| C-type lectin
domain family 4, member d |
NM_00116316.1
NM_010819.4 | 151.6 | 73.6 | 201.6 | 66.5 | −51.5 | −67.0 |
| Neuregulin 1 | NM_178591.2 | 86.5 | 49.5 | 199.8 | 68.0 | −42.8 | −66.0 |
| Keratin 78 | NM_212487.4 | 241.5 | 166.6 | 857.4 | 365.9 | −31.0 | −57.3 |
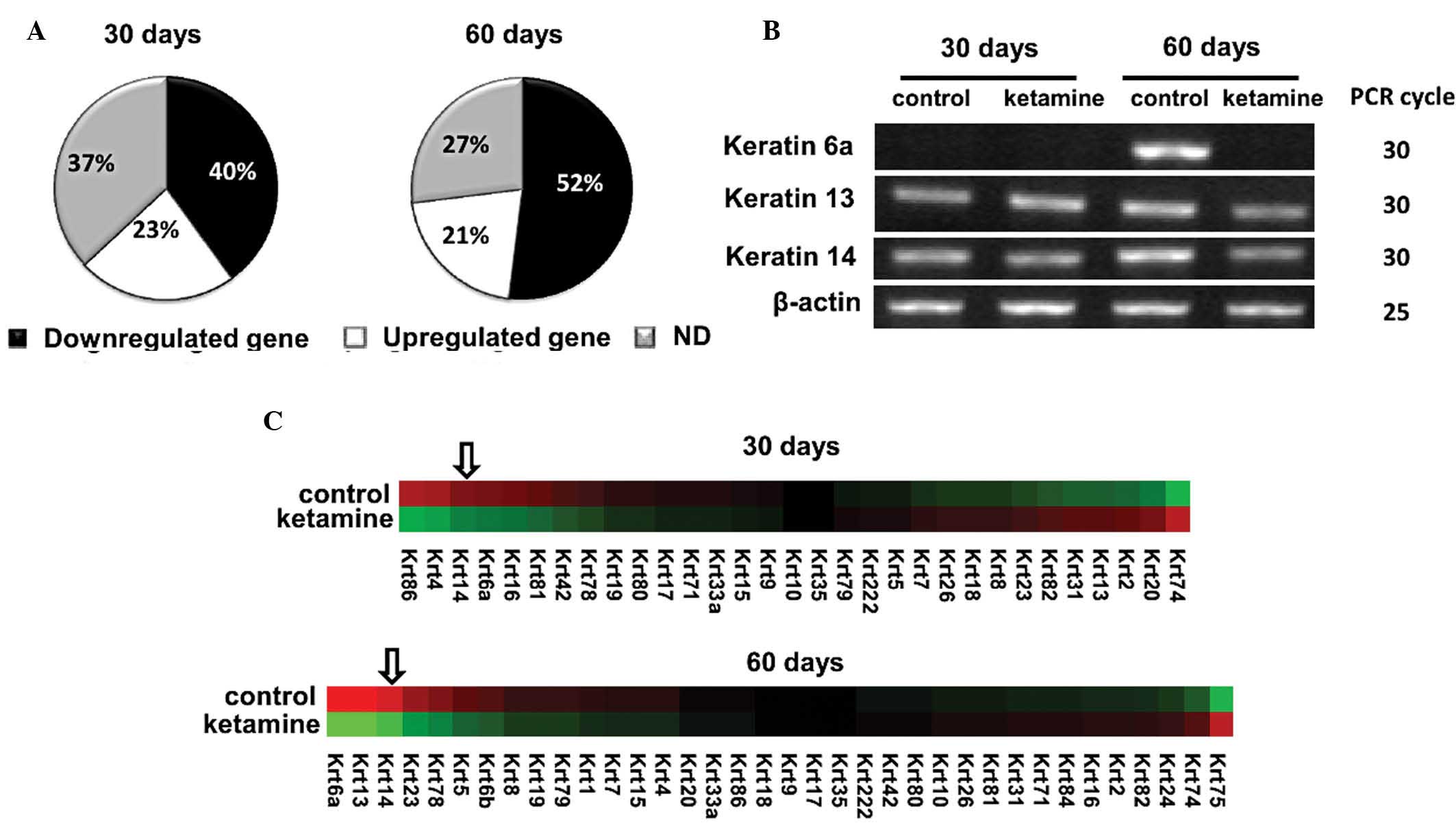
Keratin 14 gene expression is decreased
in ketamine-treated mouse bladders
Cytoskeletal keratins belong to intracellular
intermediate filaments that connect to epithelial cell adhesion
plaques in macula adherens and hemidesmosome sites. Numerous
inherited skin-blistering diseases are caused by keratin gene
mutations. There were 52 keratin family genes in the gene
expression microarray chip. The majority of the keratins were
downregulated by ketamine: 40% following 30 days and 52% following
60 days (Fig. 3A). The top ten
downregulated keratins in the 60-day treatment are listed in
Table III. The top three
downregulated keratins, including 6a, 13 and 14 were confirmed by
PCR analysis (Fig. 3B). Following
deleting the genes with no significant difference (P>0.05), a
heat map of residue keratin genes was constructed (Fig. 3C). Among the downregulated keratin
genes, keratin 14 gene was among the top three genes following 30-
and 60-day treatment. Keratin 14 was also among the selected top
ten downregulated genes in Table
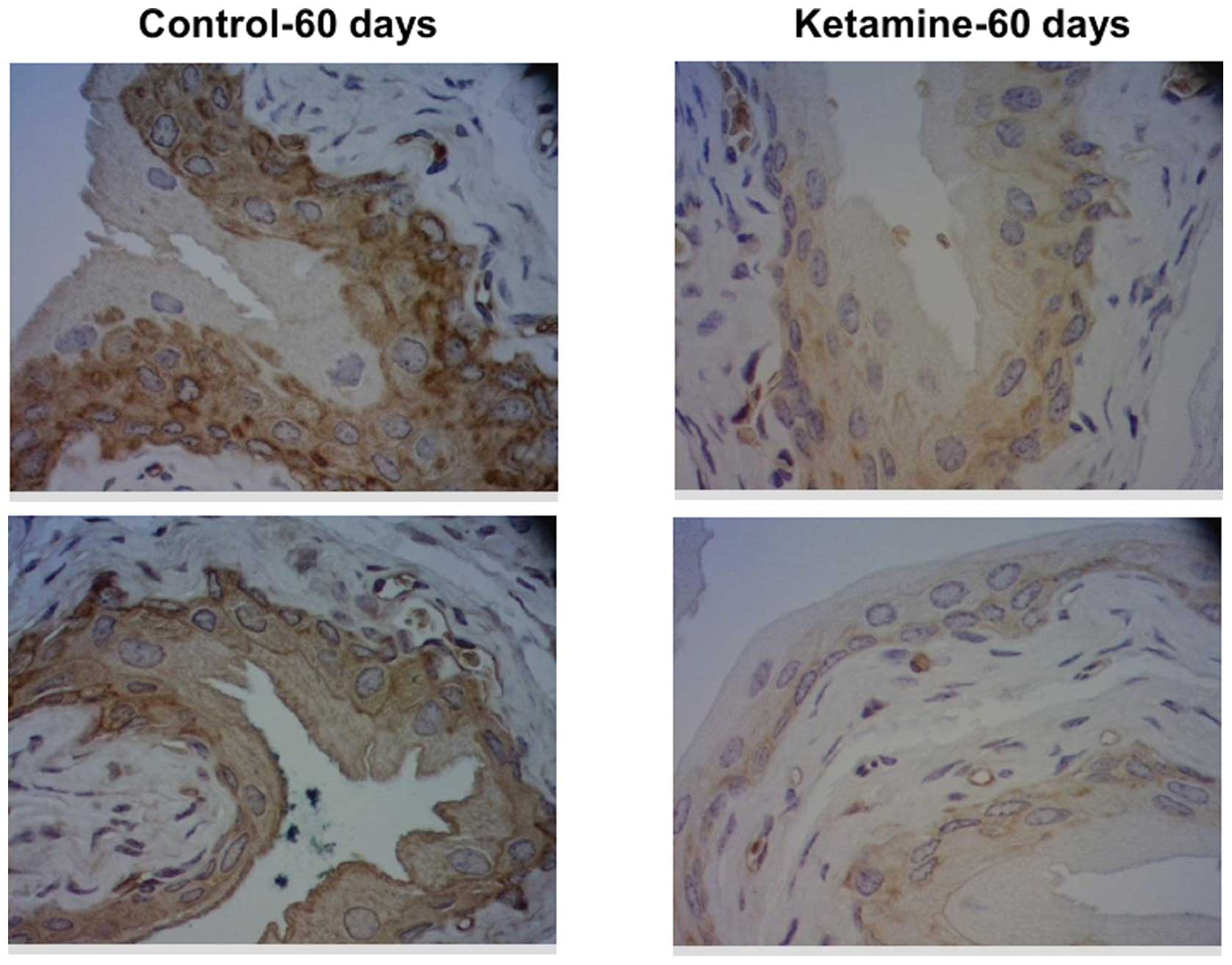
II. To confirm the protein expression change of keratin 14,
immunohistochemical analysis was applied. The results demonstrated
that keratin 14 protein expression was also decreased in the 60-day
murine urothelium (Fig. 4).
 | Table IIITop ten downregulated keratin genes
following ketamine treatment for 60 days. |
Table III
Top ten downregulated keratin genes
following ketamine treatment for 60 days.
| | Normalized
intensity | Ratio of change
(%) |
|---|
| |
|
|---|
| | 30-day | 60-day | (K-C)/C×100% |
|---|
| |
|
|---|
| Gene name | Accession
number | C | K | C | K | 30-day | 60-day |
|---|
| Keratin 6a | NM_008476.3 | 42.6 | 24.3 | 985.4 | 46.0 | −43.0 | −95.3a |
| Keratin 13 | NM_010662.1 | 130.9 | 170.0 | 1165.9 | 181.6 | 29.9 | −84.4a |
| Keratin 14 | NM_016958.1 | 2972.3 | 1652.2 | 6385.7 | 1621.5 | −44.4a | −74.6a |
| Keratin 23 | NM_033373.1 | 580.3 | 676.9 | 1926.2 | 722.6 | 16.6 | −62.5a |
| Keratin 78 | NM_212487.4 | 241.5 | 166.6 | 394.9 | 185.3 | −31a | −57.3a |
| Keratin 5 | NM_027011.2 | 6344.0 | 6327.0 | 9821.2 | 5129.2 | −0.3 | −47.8a |
| Keratin 6b | NM_010669.2 | 13.9 | 9.5 | 35.5 | 19.9 | (NA) | −44 |
| Keratin 8 | NM_031170.2 | 13729.3 | 15504.3 | 20223.6 | 13109.0 | 12.9 | −35.2a |
| Keratin 19 | NM_008471.2 | 9891.2 | 7572.7 | 15246.7 | 10048.5 | −23.4 | −34.1a |
| Keratin 79 | NM_146063.1 | 104.4 | 100.4 | 163.7 | 108.2 | −3.9 | −33.9a |
Discussion
In the present in vitro study, it was
identified that ketamine damaged urotheliums and decreased barrier
function in a dose-dependent manner. In the in vivo mouse
study, it was demonstrated that ketamine decreased the expression
of numerous keratin genes, including keratin 14. Keratin 14 protein
is also decreased in ketamine-treated mouse bladders. This
suggested that cytotoxicity may cause the loss of urothelial
barrier function at high doses of ketamine, while at low doses,
keratin gene downregulation may be a sign of urothelial
disorder.
Ketamine demonstrated toxicity (Fig. 1A) and induced sub-G1 formation in a
dose- and time-dependent manner (Fig.
1B–D). Therefore, it may be concluded that highly frequent and
repeated doses of ketamine may eventually cause urothelial damage
in abusive, recreational users. The cytotoxicity of ketamine has
been reported in neuroblastoma (17,18),
lymphoma Jurkat cells (18) and
hepatoma (19). In these three
studies, it was collectively suggested that ketamine induced cell
death via apoptosis and urothelial apoptosis was also found in
abusers (20). In addition, the
present study also analyzed the barrier function of urotheliums
in vitro. The results indicated that ketamine also increased
barrier permeability in a dose-dependent manner (Fig. 2).
According to Yeung’s ICR mouse model (30 mg/kg/day
for 1 and 3 months) (11),
ketamine injection induces urothelial degeneration and inflammatory
cell infiltration in bladders. In the present study, the same dose
of ketamine was used in Balb/c mice, and no inflammatory phenomenon
was observed by histological examination. Four inflammatory genes
(cyclooxygenase-2, nitric oxide synthase-2, interleukin-6 and -10)
from microarray data were selected for analysis by PCR and no
visual PCR products were observed on the gel (data not shown). This
result suggested that inflammation had not yet occurred in the
Balb/c mouse bladder tissue following 30 mg/kg/day ketamine
treatment for 30 and 60 days. These data were consistent with the
result of the histological analysis, using H&E staining, which
demonstrated no inflammatory cell infiltration in the bladder
tissue. In Meng’s mouse model (100 mg/kg/day for 1 to 4 months)
(12), another mouse strain
C57BL/6 was used with higher ketamine dosages. The authors
identified that ketamine reduced the mouse weight growth and
induced micturition following eight weeks. The bladder histology
also demonstrated urothelial degeneration and mononuclear cell
infiltration, while submucosal congestion was not present in
Yeung’s results. These results suggested that the strain and
ketamine dose may affect the level of ketamine-induced bladder
disorder. In addition to strain and ketamine dosage, gender may be
another reason for differences at the damage level. The same strain
of Sprague-Dawley rats but a different gender was used in Gu et
al’s study (50 mg/kg/day for 16 weeks, male rats) (13) and Chaung et al’s study (25
mg/kg/day for four weeks, female rats) (14). According to Gu et al’s
study, ketamine increased the urinary frequency and induced
hematuria, hyperplastic epithelium and inflammatory cell
infiltration in the bladder (13).
By contrast, according to Chaung et al’s study, using a
lower ketamine dose and short treatment time, ketamine induced
urothelial degeneration, red blood cell debris accumulation in
bladder cavity and mononuclear cell infiltration. The urothelial
mucosal damage of female rats appeared to be more severe than that
of male rats.
Keratins are the major component of the fibrous
intermediate filament in epithelial cells. Keratin 20 is a tumor
marker of urothelial dysplasia (21). One study found that keratin 20
expression decreased in the bladder of ketamine-abusing individuals
(22). The microarray data of the
present study also indicated that keratin 20 decreased within 60
days (Table IV). Different
keratins are expressed in different layers of the urothelium
(basal, intermediate and umbrella), keratin 20 is in the umbrella
layer and keratin 14 is in the basal/intermediate layers (23). Keratin 14 and 5, type I and type II
keratins, assemble to heterodimers, and thousands of them assemble
to 10-nm-wide intermediate filament cytoskeleton. According to the
present study, keratin 5 was also decreased in the bladders of
ketamine-treated mice. Mutations in keratin 14 or keratin 5 cause a
rare genetic disease called epidermolysis bullosa simplex (24). In addition to the representative
skin bullous lesions, patients with epidermolysis bullosa simplex
also demonstrated fragility of epithelial tissues in the
genitourinary tract, which caused voiding difficulty and urinary
retention (25). The cell
proliferation rate was reduced following knockdown of keratin 14
(26). It remains elusive whether
or not the downregulation of keratin genes induces urinary
disorders in ketamine abusers, and therefore, it is worthy of
further study.
 | Table IVFour gene expression change data in
normal saline and ketamine treatment for 30 and 60 days. |
Table IV
Four gene expression change data in
normal saline and ketamine treatment for 30 and 60 days.
| | Normalized
intensity | Ratio of change
(%) |
|---|
| |
|
|---|
| | 30-day | 60-day | (K-C)/C×100% |
|---|
| |
|
|---|
| Gene name | Accession
number | C | K | C | K | 30-day | 60-day |
|---|
| Integrin α6 | NM_008397.3 | 6661.3 | 9441.9 | 9028.3 | 7893.3 | 41.7a | −12.6 |
| Integrin β4 |
NM_133663.2
NM_001005608.2 | 802.7 | 739.2 | 1082.0 | 394.7 | −7.9 | −63.5a |
| Claudin-1 | NM_016674.4 | 1146.1 | 1002.7 | 3597.1 | 1209.6 | −12.5 | −66.4a |
| Keratin 20 | NM_023256.1 | 473.0 | 568.4 | 1954.1 | 708.4 | 20.2 | −63.7a |
In addition to decreases in the levels of various
types of keratin, the cDNA array data indicated further mechanisms
leading to the downregulation of urothelial barrier function.
Firstly, the hemidesmosome, consisting of intracellular keratins,
plectin plaque and adhesion molecules, such as the α6β4 integrin
(27), contributed to the firm
attachment between urothelium and extracellular matrix. In the
array data, integrin α6/β4 demonstrated a marked decrease as well
at 60 days (Table IV), which may
implicate the hemidesmosome was collapsing and cell is
progressively denuding from basal lamina. Secondly, it was
identified the claudin-1 expression was also downregulated at 60
days (Table IV). This suggested
that tight junctions of the urothelium may also have been affected.
Although bladder damages were not identified in the 30 mg/kg/day
ketamine-treated Balb/c mice, the microarray data demonstrated
certain molecular defects which correlated with urothelial barrier
function. Additional studies are required to further elucidate
these correlations.
Acknowledgements
This study was supported by grants from the National
Science Council of Taiwan (NSC101-2320-B-415-002-MY3) and from
Chiayi Christian Hospital, Taiwan (R100-9).
Abbreviations:
|
FBS
|
fetal bovine serum
|
|
H&E
|
hematoxylin and eosin
|
|
PI
|
propidium iodide
|
|
RNase A
|
ribonuclease A
|
References
|
1
|
Domino EF, Chodoff P and Corssen G:
Pharmacologic effects of Ci-581, a new dissociative anesthetic, in
man. Clin Pharmacol Ther. 6:279–291. 1965.PubMed/NCBI
|
|
2
|
Sinner B and Graf BM: Ketamine. Handb Exp
Pharmacol. 313–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivani G, Vercellino C and Tonetti F:
Ketamine: a new look to an old drug. Minerva Anestesiol.
69:468–471. 2003.PubMed/NCBI
|
|
4
|
Domino EF: Taming the ketamine tiger.
1965. Anesthesiology. 113:678–684. 2010.PubMed/NCBI
|
|
5
|
Chu PS, Kwok SC, Lam KM, et al: ‘Street
ketamine’-associated bladder dysfunction: a report of ten cases.
Hong Kong Med J. 13:311–313. 2007.PubMed/NCBI
|
|
6
|
Shahani R, Streutker C, Dickson B and
Stewart RJ: Ketamine-associated ulcerative cystitis: a new clinical
entity. Urology. 69:810–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng SH, Tse ML, Ng HW and Lau FL: Emergency
department presentation of ketamine abusers in Hong Kong: a review
of 233 cases. Hong Kong Med J. 16:6–11. 2010.PubMed/NCBI
|
|
8
|
Tsai TH, Cha TL, Lin CM, et al:
Ketamine-associated bladder dysfunction. Int J Urol. 16:826–829.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Middela S and Pearce I: Ketamine-induced
vesicopathy: a literature review. Int J Clin Pract. 65:27–30. 2011.
View Article : Google Scholar
|
|
10
|
Chen CH, Lee MH, Chen YC and Lin MF:
Ketamine-snorting associated cystitis. J Formos Med Assoc.
110:787–791. 2011. View Article : Google Scholar
|
|
11
|
Yeung LY, Rudd JA, Lam WP, Mak YT and Yew
DT: Mice are prone to kidney pathology after prolonged ketamine
addiction. Toxicol Lett. 191:275–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng E, Chang HY, Chang SY, et al:
Involvement of purinergic neurotransmission in ketamine induced
bladder dysfunction. J Urol. 186:1134–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu D, Huang J, Shan Z, et al: Effects of
long-term ketamine administration on rat bladder protein levels: a
proteomic investigation using two-dimensional difference gel
electrophoresis system. Int J Urol. 20:1024–1031. 2013.PubMed/NCBI
|
|
14
|
Chuang SM, Liu KM, Li YL, et al: Dual
involvements of cyclooxygenase and nitric oxide synthase
expressions in ketamine-induced ulcerative cystitis in rat bladder.
Neurourol Urodyn. 32:1137–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rigby CC and Franks LM: A human tissue
culture cell line from a transitional cell tumour of the urinary
bladder: growth, chromosone pattern and ultrastructure. Br J
Cancer. 24:746–754. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fogh J, Fogh JM and Orfeo T: One hundred
and twenty-seven cultured human tumor cell lines producing tumors
in nude mice. J Natl Cancer Inst. 59:221–226. 1977.PubMed/NCBI
|
|
17
|
Mak YT, Lam WP, Lu L, Wong YW and Yew DT:
The toxic effect of ketamine on SH-SY5Y neuroblastoma cell line and
human neuron. Microsc Res Tech. 73:195–201. 2010.
|
|
18
|
Braun S, Gaza N, Werdehausen R, et al:
Ketamine induces apoptosis via the mitochondrial pathway in human
lymphocytes and neuronal cells. Br J Anaesth. 105:347–354. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee ST, Wu TT, Yu PY and Chen RM:
Apoptotic insults to human HepG2 cells induced by S-(+)-ketamine
occurs through activation of a Bax-mitochondria-caspase protease
pathway. Br J Anaesth. 102:80–89. 2009. View Article : Google Scholar
|
|
20
|
Lee CL, Jiang YH and Kuo HC: Increased
apoptosis and suburothelial inflammation in patients with
ketamine-related cystitis: a comparison with non-ulcerative
interstitial cystitis and controls. BJU Int. 112:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harnden P, Eardley I, Joyce AD and
Southgate J: Cytokeratin 20 as an objective marker of urothelial
dysplasia. Br J Urol. 78:870–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oxley JD, Cottrell AM, Adams S and Gillatt
D: Ketamine cystitis as a mimic of carcinoma in situ.
Histopathology. 55:705–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castillo-Martin M, Domingo-Domenech J,
Karni-Schmidt O, Matos T and Cordon-Cardo C: Molecular pathways of
urothelial development and bladder tumorigenesis. Urol Oncol.
28:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coulombe PA, Kerns ML and Fuchs E:
Epidermolysis bullosa simplex: a paradigm for disorders of tissue
fragility. J Clin Invest. 119:1784–1793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arifi M, Arifi S, Demni K, et al:
Genitourinary complications as initial presentation of inherited
epidermolysis bullosa. Afr J Paediatr Surg. 8:72–74. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alam H, Sehgal L, Kundu ST, Dalal SN and
Vaidya MM: Novel function of keratins 5 and 14 in proliferation and
differentiation of stratified epithelial cells. Mol Biol Cell.
22:4068–4078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercurio AM: Laminin receptors: achieving
specificity through cooperation. Trends Cell Biol. 5:419–423. 1995.
View Article : Google Scholar : PubMed/NCBI
|