Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignancy worldwide. In males, it is the fifth
most common cancer and the second leading cause of
cancer-associated mortality (1).
The low rate of early diagnosis and rapid tumor development lead to
a poor prognosis and high mortality in HCC patients (2). Therefore, investigating the mechanism
of hepatocarcinogenesis and further examining effective therapeutic
targets are important for HCC prevention and treatment.
The abnormal metabolism of cancer cells has been
broadly investigated and is considered a potential target to treat
cancer (3,4). The Warburg effect, which is the
switch from oxidative phosphorylation to aerobic glycolysis, is
frequently observed in various types of cancer cells and is known
as a hallmark of cancer development (5,6).
Enhanced aerobic glycolysis affords a steady supply of metabolic
production essential for biosynthesis and energy generation and may
also affect various related metabolic pathways that are associated
with numerous biological processes, including cell proliferation,
apoptosis and invasion (7). HCC
treatments, which target aerobic glycolysis have been receiving
increasing attention.
2-deoxy-D-glucose (2-DG), a glucose analogue able to
suppress glycolysis by competitively inhibiting hexokinase 2 (HK2),
has an impact on HCC cell lines (8) and is reported to inhibit the growth
of transplanted HCCs (9,10). However, whether it has effects on
the development of primary liver tumors remains to be elucidated.
In the present study, the effects of 2-DG on the
N-diethylnitrosamine (DEN)-induced rat hepatocarcinoma model and
its mechanisms were investigated.
Materials and methods
DEN and 2-DG model in the rat
Male 10–12 week old Sprague Dawley rats, weighing
220–240 g were purchased from Shanghai Slaccas Laboratory Animal
Company Limited (Shanghai, China) and were maintained at an animal
facility under pathogen-free conditions. All rats received humane
care according to the guidelines of the Chinese Association for
Laboratory Animal Sciences (Beijing, China). The present study was
approved by the ethics committee of Medical College of Qingdao
University, (Qingdao, China).
To induce HCC, DEN at a dilution of 1/10,000 (95
mg/l; Sigma-Aldrich, St. Louis, MO, USA), was added to the drinking
water of rats for 13 weeks. Subsequently, the water was replaced by
normal sterile water and certain rats received 0.75 g/kg 2-DG
(Sigma-Aldrich), dissolved in normal saline solution via
intraperitoneal (IP) injection once every 3 days until the end of
the 17th week after initial DEN administration. A total of 30 rats
(control group, n=5; 2-DG group, n=5; DEN group, n=10; DEN+2-DG
group, n=10) were kept and assessed for survival time and 20 rats
(DEN group, n=10; DEN+2-DG group, n=10) were sacrificed through
cervical dislocation at 17 weeks in order to observe their primary
liver tumors. The tumors were measured with a micrometer up to a
maximum diameter of 3 mm. The tumor volumes were calculated using
the following formula: Volume=axb2/2 (‘a’ is the maximal
diameter and ‘b’ was the minimal diameter).
Liver sections were soaked in 10% neutral-buffered
formalin for hematoxylin and eosin (H&E) and
immunohistochemical (IHC) staining, preserved in RNA Later (Qiagen
GmbH, Hilden, Germany) for detecting mRNA expression and
snap-frozen in liquid nitrogen for detecting protein
expression.
H&E and IHC staining
The paraffin-embedded liver sections were subjected
to H&E staining for histopathological analysis. The primary IHC
antibodies included: Ki67 (polyclonal rabbit anti-rat; 1:1,000;
ab16667; Abcam, Cambridge, UK), Actived-Caspase-3 (polyclonal
rabbit anti-rat; 1:100; BS7004; Bioworld Technology, St. Louis
Park, MN, USA) Beclin-1 (polyclonal rabbit anti-rat; 1:100; 3738;
Cell Signaling Technology, Inc., Beverly, MA, USA) and
microtubule-associated protein 1A/1B-light chain 3 (LC3; polyclonal
rabbit anti-rat; 1:200; 4108; Cell Signalling Technology,
Inc.).
Western blotting
The lysates of liver tumors were subjected to
SDS-PAGE. The protein blots were incubated with specific primary
antibodies, including anti-proliferating cell nuclear antigen
(PCNA; polyclonal rabbit anti-rat; 1:1,000; Bs6438; Bioworld
Technology), anti-cyclin D1 (polyclonal rabbit anti-rat; 1:1,000;
BS6532; Bioworld Technology), anti-activated-caspase 3 (polyclonal
rabbit anti-rat; 1:1,000; BS7004; Bioworld Technology), anti-LC3
(polyclonal rabbit anti-rat; 1:1,000; catalogue number, 4108; Cell
Signalling Technology) and anti-P62 (polyclonal rabbit anti-rat;
1:1,000; 5114; Cell Signaling Technology, Inc.) and subsequently
incubated with polyclonal goat anti-rabbit IgG horseradish
peroxidase-conjugated (heavy and light chain) secondary antibody
(1:20,000; BS10350; Bioworld Technology) and chemiluminescent
substrates. Anti-β-actin (polyclonal rabbit anti-rat; 1:10,000;
AP0060; Bioworld Technology) was used to confirm equal protein
loading.
Biochemical analysis
The levels of glucose-6-phosphate (G-6-P),
acetyl-CoA, citrate, isocitrate (Biovision, Mountain View, CA,
USA), pyruvate, lactic acid, ATP (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China), 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG-CoA; Shanghai Enzyme-linked Biotechnology, Co., Ltd, Shanghai,
China) and malonyl-CoA (Shanghai Yu Ping Biotechnology Limited
Company, Shanghai, China) were measured using assay kits, according
to the manufacturer’s instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs of HCC tissues in the rats were extracted
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) and were further treated with RNase-free DNase (Promega
Corporation, Madison, WI, USA). The cDNA was synthesized using the
RevertAid First-Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania). RT-qPCR was performed using a Roche LightCycler 480
system (Roche Applied Science, Indianapolis, IN, USA). The specific
primers used to analyze gene expression were as follows: HK2,
forward 5′-TTTGGTCTCGTGGACTAAGGG-3′ and reverse
5′-ACCACGGCCACAATGTCAAT-3′; 6-phosphofructo-2-kinase (6PF2K),
forward 5′-AAAGGCATTGCCGCCCGGAAGTG-3′ and reverse
5′-TGTAATACGACTCACTATA-3′; pyruvate kinase M2 (PKM2), forward
5′-GGTCATCTGTGCCAACCAGA-3′ and reverse 5′-AGGGACAGGGGCTAGAA GAG-3′
and lactate dehydrogenase A (LDHA), forward
5′-GGTCATCTGTGCCAACCAGA-3′ and reverse 5′-AGGGACAGGGGCTAGAAGAG-3′.
Fold change in gene expression was determined by normalizing to
endogenous β-actin, with the following primer sequence: Forward
5′-CTCTATCCTGGCCTCACT GTCCACC-3′ and reverse
5′-AAACGCAGCTCAGTAACAGTCCGC-3′.
Cell culture
Human HCC cell lines SMMC7721 and HepG2 were
cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal
bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, which
were all purchased from Gibco-BRL (Carlsbad, CA, USA), at 37°C
under humidified air containing 5% CO2. For the hypoxic
condition, cells were cultured in a tri-gas incubator (Sanyo,
Osaka, Japan) maintained at 3% O2, 5% CO2 and
92% N2. 2-DG was dissolved in tri-distilled water as
stock solution (1 M) and was then added into the media to the final
concentration (10 mM).
Cell viability detection
Cell viability was evaluated using the Cell Counting
kit-8 (CCK-8; Dojingo Laboratories, Kumamoto, Japan). The cells in
exponential growth period were plated in 96-well culture plates
(1,000 cells/well) for different culture periods. Subsequently,
CCK-8 solution was added to each well. Incubation was performed at
37°C for 1 h. The optical density of each well was measured at 450
nm using an ELISA reader (ELx808; Bio-Tek Instruments, Winooski,
VT, USA).
Cell cycle detection
SMMC7721 and HepG2 cells were plated in 6-well
culture plates (5×105 cells/well) and treated as
described above. After 24 h treatment, the cells were centrifuged
at 500 × g for 5 min. The cells were washed with pre-cooled
phosphate-buffered saline (pH 7.4) twice and then fixed in 70%
alcohol and stained with propidium iodide (Sigma-Aldrich). Analysis
of cell cycle distribution was performed using a flow cytometer
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) in
accordance with the manufacturer’s instructions.
Colony formation assay
Exponentially growing cells were plated in 6-well
culture plates (200 cells/well) and treated using the indicated
methods. The plates were maintained at 37°C in 20% O2 or
3% O2 for 2 weeks. Following fixation in
paraformaldehyde, the colonies were stained with crystal violet for
10 min. Subsequently, images were captured with a digital camera
(Sony H7; Sony Corporation, Tokyo, Japan) and the visible colonies
were recorded.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical significance was determined by Student’s
t-test and one-way analysis of variance. Tumor incidence was
analyzed by Fisher’s exact test. Survival curves were analyzed by
log-rank test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
GraphPad Prism 6.04 software (GraphPad Software Inc., La Jolla, CA,
USA).
Results
2-DG inhibits DEN-induced
hepatocarcinogenesis in the rat
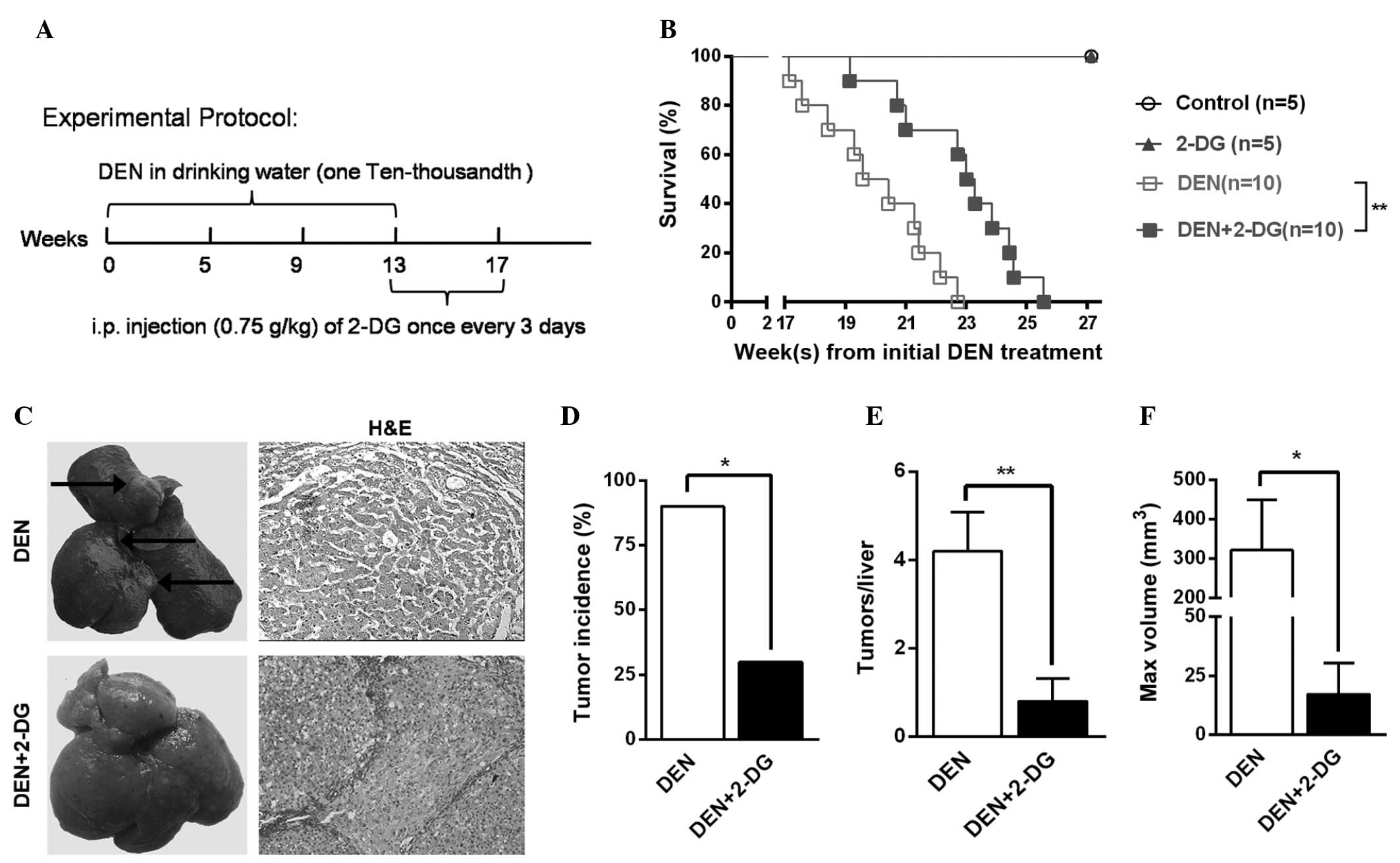
To examine the effects of 2-DG on primary liver
tumor development, a classical DEN rat model was used, which
resembles human hepatocarcinoma in rats. In this model, the
majority of the rats (>90%) developed liver tumor nodules by the
end of the 17th week after initial DEN administration (11,12).
The rats in the DEN+2-DG group were administered 2-DG by IP
injection from the 14th-17th week after the initial DEN
administration (Fig. 1A).
In the cohort of DEN-treated rats monitored for
survival time, the DEN+2-DG group exhibited a longer mean survival
time compared with that of the DEN group (Fig. 1B). Further investigation revealed
that, at 17 weeks, 90% of the rats in the DEN group had typical HCC
nodules, however, the HCC incidence in the DEN+2-DG group was only
30% (Fig. 1C and D). In addition,
the DEN group exhibited a 5-fold higher HCC multiplicity (4.2±0.9
versus 0.8±0.5) and an 18.7-fold higher maximum tumor volume
(321.5±127.4 versus 17.2±13.1 mm3) as compared with
those of the DEN+2-DG group (Fig. 1E
and F). The present results demonstrated that 2-DG
significantly suppressed hepatocarcinogenesis in the DEN-treated
rats.
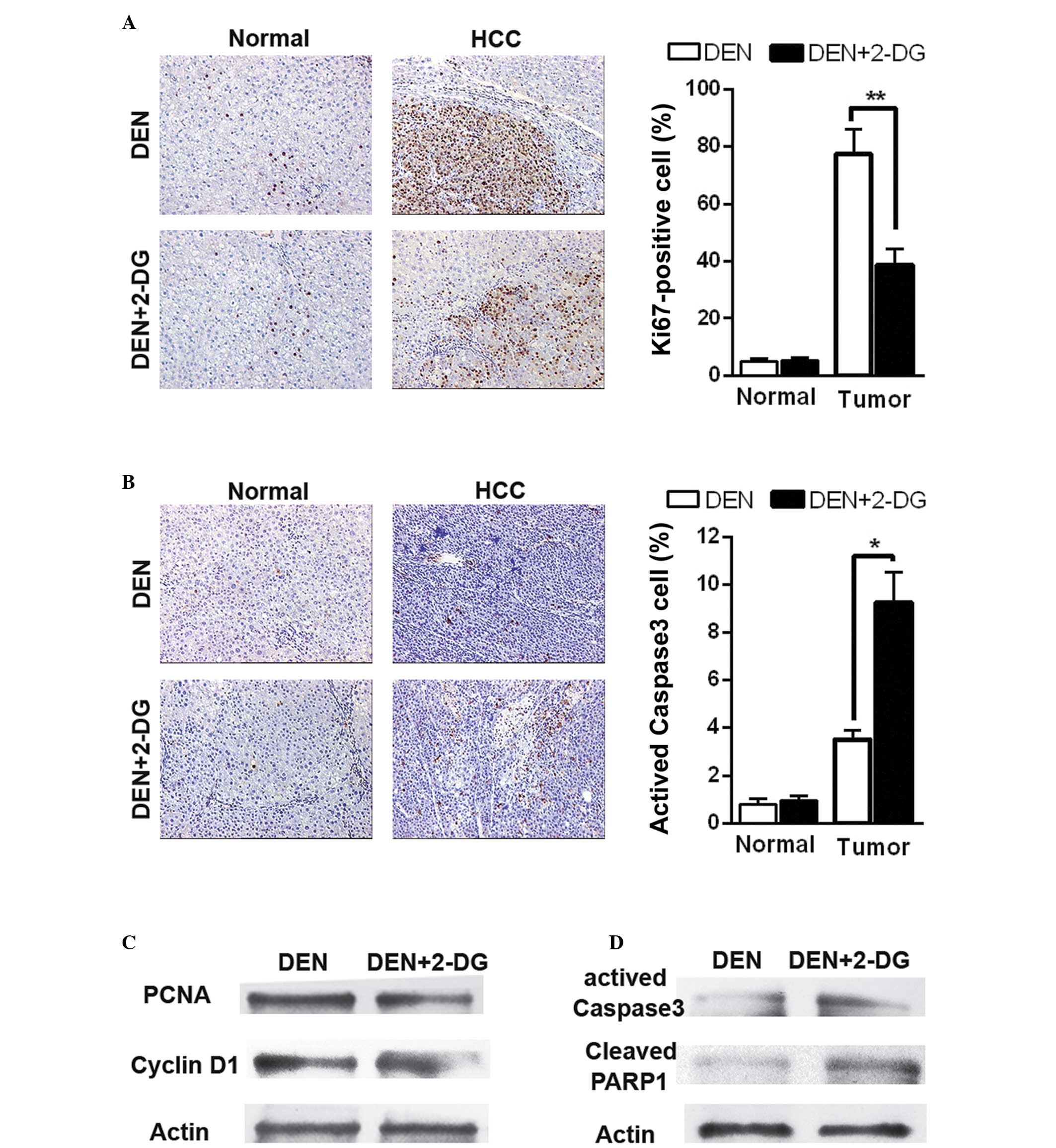
2-DG suppresses cell proliferation and
promotes cell apoptosis in DEN-induced HCC
Subsequently, the present study aimed to examine how
2-DG inhibits DEN-induced hepatocarcinogenesis in the rat. Cell
proliferation and apoptosis were detected in the DEN-treated rat
livers at 17 weeks. IHC staining revealed that 2-DG markedly
reduced cell proliferation (Ki67-positive cells) in the HCC
tissues, but had no significant effect on the normal liver cells in
the pericarcinous tissues (Fig.
2A). Additionally, treatment with 2-DG resulted in increased
cell apoptosis (activated caspase 3-positive cells) in the HCC
tissues, but not in the pericarcinous tissues (Fig. 2B). Immunoblot analyses also
revealed similar results. The data demonstrated that HCC tissues of
the DEN+2-DG group had lower levels of PCNA and cyclin D1,
indicators of cell proliferation and higher levels of activated
caspase-3 and cleaved poly ADP-ribose polymerase 1 (PARP1), another
cell apoptosis indicator, than those of the DEN group (Fig. 2C and D). These data demonstrated
that 2-DG led to a decrease in cell proliferation and an increase
in cell apoptosis in the DEN-induced HCC tissues in the rats.
2-DG efficiently inhibits glycolysis in
the HCC tissues of DEN-treated rats
The mechanisms by which 2-DG decreased tumor cell
proliferation and survival time in hepatocarcinogenesis were
further investigated. Initially, it was examined whether 2-DG
effectively suppressed tumor cell glycolysis during HCC development
in DEN-treated rats. The results demonstrated that HCC tissues of
the DEN+2-DG group had lower levels of glycolysis products, G-6-P,
pyruvate and lactic acid, as compared with those of the DEN group.
However, 2-DG had no notable effect on the levels of glycolysis
products of pericarcinous tissues in the DEN-treated rat livers
(Fig. 3A–C). By contrast,
treatment with 2-DG resulted in a compensatory increase in HK2 mRNA
expression and decrease in mRNA expression of other glycolysis
enzymes, including 6PF2K, PKM2 and LDHA, in the DEN-induced HCC
tissues (Fig. 3D–G). The present
results demonstrated that 2-DG prominently inhibited glycolysis in
the HCC tissues of DEN-treated rats.
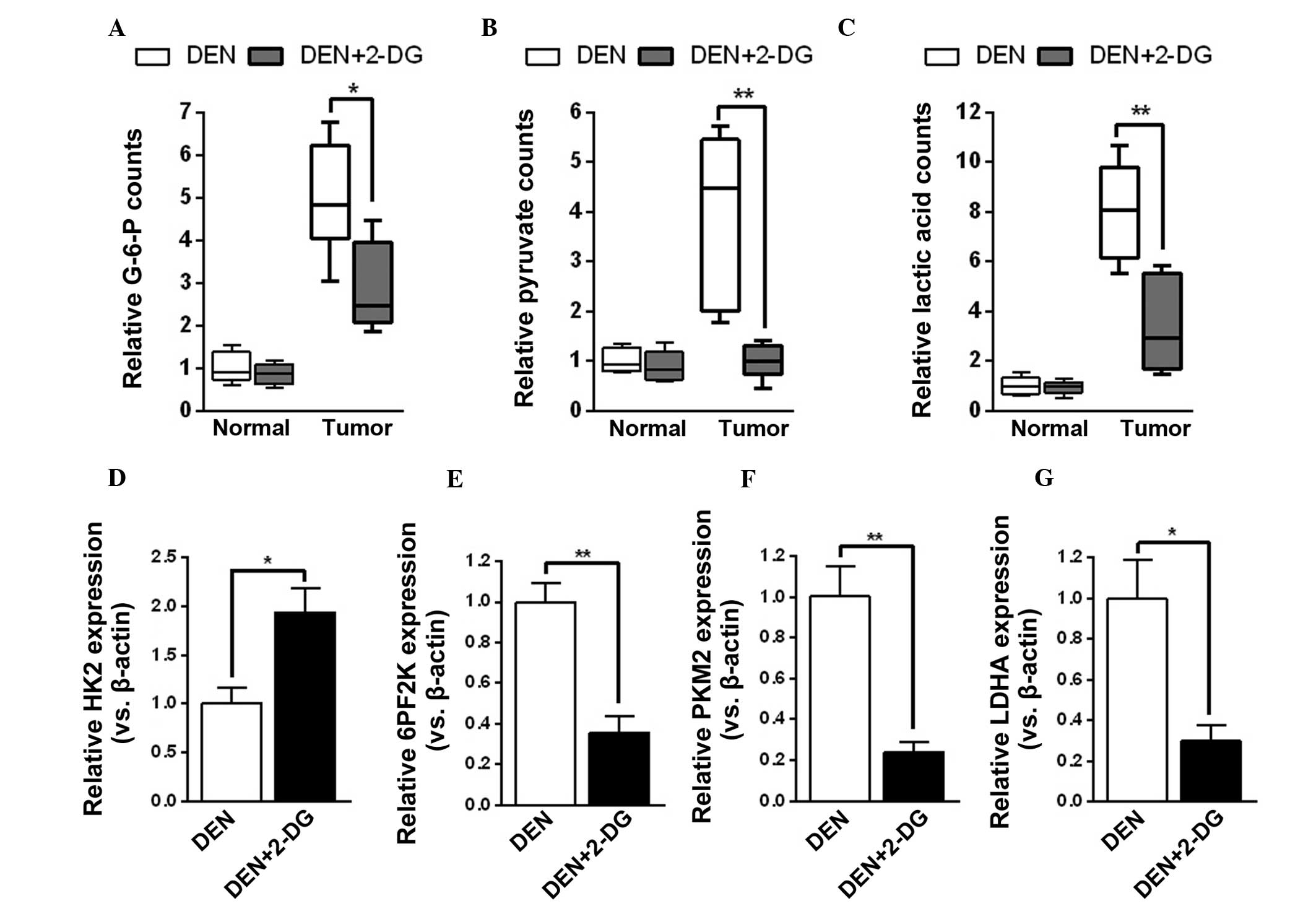 | Figure 32-DG inhibits tumor cell glycolysis in
DEN-treated rat livers. (A–C) Levels of (A) G-6-P, (B) pyruvate and
(C) lactic acid in the pericarcinous and HCC tissues of DEN and
DEN+2-DG groups. Data are presented as the mean ± standard error of
the mean (n=3; *P<0.05 and **P<0.01 vs.
DEN group). (D–G) Relative mRNA expression (versus β-actin) of (D)
HK2, (E) 6PF2K, (F) PKM2 and (G) LDHA in the HCC tissues of DEN and
DEN+2-DG groups. Data are presented as the mean ± standard error of
the mean (n=3; *P<0.05 and **P<0.01 vs.
DEN group). DEN, N-diethylnitrosamine; 2-DG, 2-deoxy-D-glucose;
HCC, hepatocellular carcinoma; HK2, hexokinase 2; 6PF2K,
6-phosphofructo-2-kinase; PKM2, pyruvate kinase M2; LDHA, lactate
dehydrogenase A; G-6-P, glucose-6-phosphate. |
2-DG suppresses cell metabolism and
promotes autophagic activation in HCC tissues
Subsequently, it was investigated whether glycolysis
inhibition resulting from 2-DG had an impact on the other cell
metabolism pathways. It was found that the levels of acetyl-CoA,
citrate and isocitrate, the three intermediates of the
tricarboxylic acid (TCA) cycle, malonyl-CoA, the crucial
intermediate of fatty acid biosynthesis, HMG-CoA, the crucial
intermediate of cholesterol biosynthesis and ATP in the HCC tissues
of the DEN+2-DG group were lower than those of the DEN group
(Fig. 4A–F). The present results
demonstrated that 2-DG led to inhibition of the TCA cycle, fatty
acid and cholesterol biosynthesis and energy generation in the
DEN-induced rat HCC tissues.
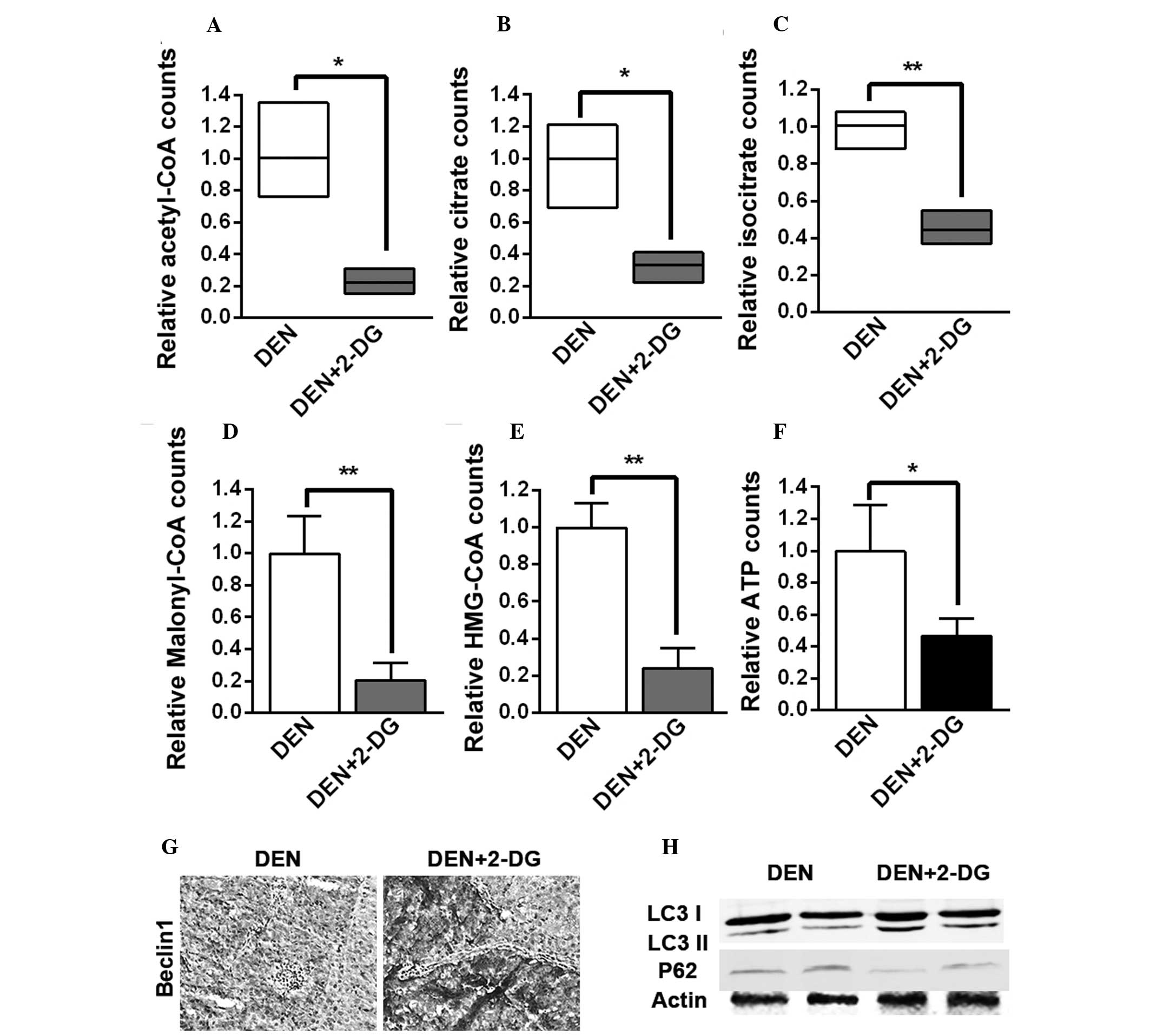 | Figure 42-DG inhibits TCA cycle, fatty acid
and cholesterol biosynthesis and energy generation and enhances
autophagy activation in the HCC tissues of DEN-treated rat livers.
(A–F) Levels of (A) acetyl-CoA, (B) citrate, (C) isocitrate, (D)
malonyl-CoA, (E) HMG-CoA and (F) ATP in the HCC tissues of DEN and
DEN+2-DG groups. Data are presented as the mean ± standard error of
the mean (n=3; *P<0.05 and **P<0.01 vs.
DEN group). (G) Immunohistochemistry staining for Beclin-1 in the
HCC tissues of DEN and DEN+2-DG groups at 17 weeks (magnification,
×200). (H) Cell lysates obtained from HCC tissues of DEN and
DEN+2-DG groups at 17 weeks were immunoblotted with the indicated
antibodies. DEN, N-diethylnitrosamine; 2-DG, 2-deoxy-D-glucose;
HCC, hepatocellular carcinoma; HMG-CoA,
3-hydroxy-3-methylglutaryl-coenzyme A; TCA, tricarboxylic acid;
LC3, microtubule-associated protein 1A/1B-light chain 3; ATP,
adenosine triphosphate. |
Several studies have reported that autophagy, the
degradation and recycling system, is activated to provide the
substrates of biosynthesis and energy generation in response to
nutritional deficiency and other stresses in cancer cells (13,14,15).
It was observed that 2-DG led to a higher expression of Beclin-1, a
crucial component of the autophagy pathway, in the HCC tissues of
DEN-treated rats (Fig. 4G).
Immunoblot analysis of HCC tissues suggested that the level of LC3
II was increased following treatment with 2-DG, while the level of
p62 was decreased, indicating that autophagic activation was
enhanced (Fig. 4H). These results
demonstrated that 2-DG resulted in the suppression of cell
metabolism and the promotion of autophagic activation in the HCC
tissues of DEN-treated rats. These severe metabolic blocks may be
the reason that 2-DG led to a decrease in tumor cell proliferation
and ultimately inhibited DEN-induced rat hepatocarcinogenesis.
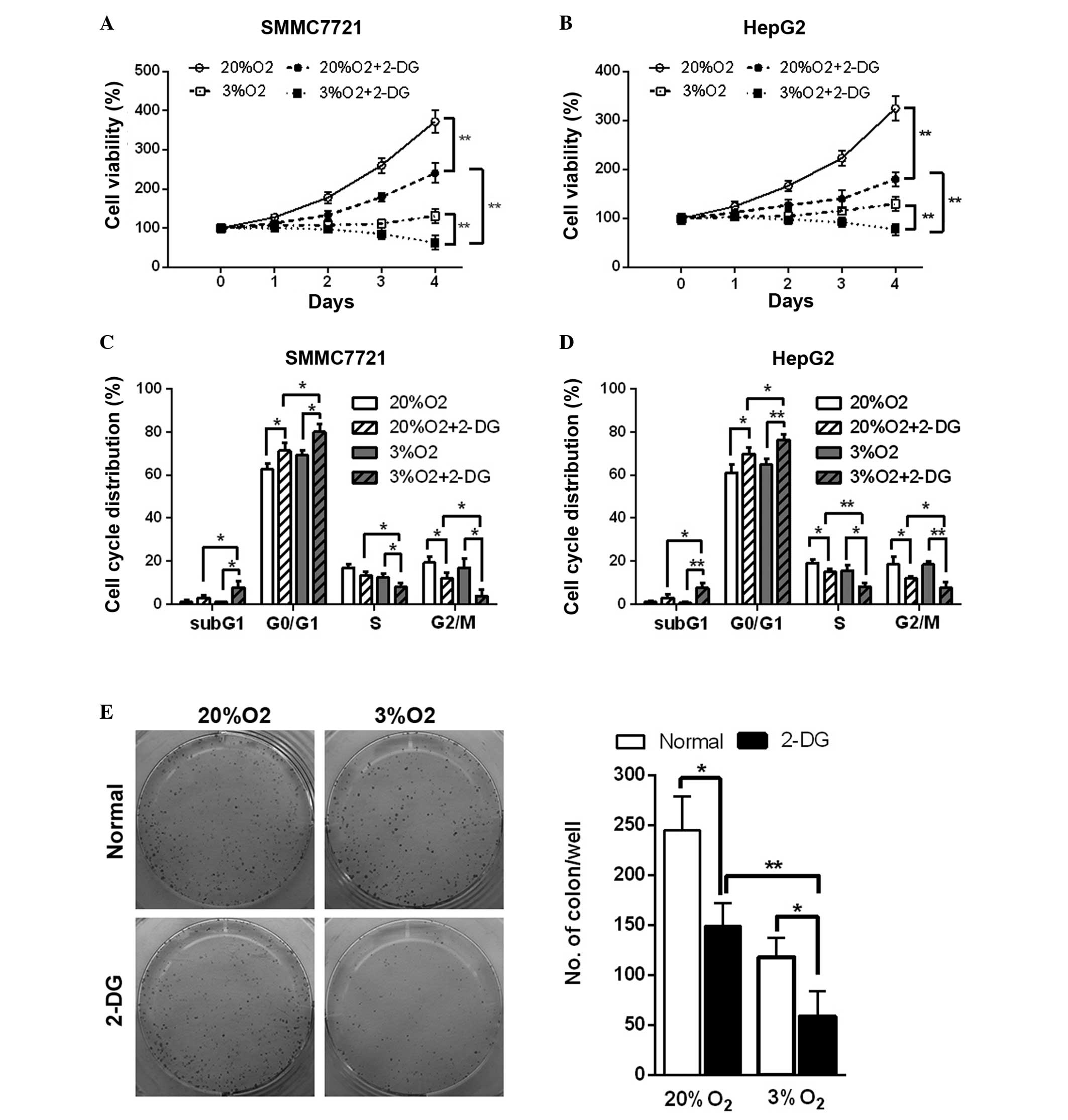
Hypoxia enhances inhibition of cell
viability, the cell cycle and tumor formation ability resulting
from 2-DG in HCC cell lines
Hypoxia is an important factor in the tumor
microenvironment and is present in 90% of solid tumors (16). The glycolysis pathway of cells is
enhanced in the hypoxic microenvironment. The in vitro study
revealed that in normal and hypoxic conditions, 2-DG led to a
decrease in cell viability in the HCC cell lines (Fig. 5A and B). In addition, cell cycle
analysis also revealed that 2-DG resulted in cell cycle arrest at
the G0/G1 phase and an increase in the
percentage of cells in the sub-G1 phase, an indicator of
cell apoptosis, in the HCC cell lines in normal and hypoxic
conditions (Fig. 5C and D).
Additionally, hypoxia enhanced these effects (Fig. 5A–D). The colony formation assay
also suggested that 2-DG reduced the colony number by 40 and 50% in
normal and hypoxic conditions in SMMC7721 cells, respectively.
Hypoxia further led to a marked decrease (~60%) of the colony
number in 2-DG-treated SMMC7721 cells (Fig. 5E). These results demonstrated that
hypoxic conditions further enhance 2-DG-induced cell viability
inhibition, cell cycle arrest and the reduction in tumor formation
ability in the HCC cell lines.
Discussion
In the present study, it was found that 2-DG
significantly delayed hepatocarcinogenesis by decreasing cell
proliferation and increasing cell apoptosis in the tumor. Further
investigation revealed that 2-DG not only reduced glycolysis but
also suppressed the TCA cycle, fatty acid and cholesterol
biosynthesis, ATP production and activated autophagy. In addition,
a hypoxic microenvironment, an inevitable factor during
tumorigenesis, may assist in improving 2-DG-induced cell viability
inhibition, cell cycle retardation and a decrease in colony
formation ability in hepatoma cells. These findings suggested that
2-DG may efficiently inhibit hepatocarcinogenesis in the
DEN-treated rats via suppression of cancer cell metabolism.
Uncontrolled cell growth forces cancer cells to
adjust their cellular metabolism. Even in the presence of oxygen,
cancer cells reprogram their glucose metabolism by limiting it
predominantly to glycolysis (17).
This alteration is apparently counterintuitive from the angle of
the efficiency of energy production, but it efficiently assists
cancer cells in overcoming the obstacles of uncontrolled cell
proliferation and the nutritional deficiency of the tumor
microenvironment. In addition, this glycolysis-dependent metabolism
was further enhanced under hypoxic conditions (5).
The current study revealed that the glycolysis
inhibitor 2-DG not only affects HCC cell lines in vitro and
transplanted tumor formations, but also has an inhibitory effect on
primary hepatocarcinogenesis. In the DEN-induced HCC tissues, 2-DG
suppressed G-6-P production by competitively occupying HK2, the
first enzyme of the glycolysis pathway and subsequently led to the
decrease of other glycolysis intermediates. Additionally, these
alterations also led to the compensatory expression of HK2 and the
reduction of other enzymes of the glycolysis pathway. Several
studies have reported that enhanced glycolysis supports various
biosynthetic pathways by supplying glycolysis intermediates
(18,19,20).
It was also identified that glycolysis inhibition delayed numerous
crucial biosynthetic pathways, including the TCA cycle, fatty acid
and cholesterol biosynthesis and ATP production. The HCC cells may
have activated autophagy in response to the inhibition of
metabolism. Furthermore, 2-DG had more significant anti-tumor
effects in the hypoxic environment, a crucial component in tumor
development. These mechanisms are the reason that 2-DG led to a
decrease of tumor cell proliferation and survival time and
ultimately inhibited hepatocarcinogenesis. Notably, although 2-DG
has prominent anti-tumoral effects, it had no significant effect on
normal liver tissues. The reason for this may be that normal cells
are not dependent on glycolysis. This finding supports a promising
treatment for liver diseases, which are at risk of
hepatocarcinogenesis.
In conclusion, the glycolysis inhibitor 2-DG may
efficiently suppress DEN-induced hepatocarcinogenesis in the rat by
reducing tumor cell proliferation and survival time via inhibition
of tumor cell metabolism. This finding provides a promising
approach for the prevention and treatment of HCC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Diaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi R and Perkins G: Challenges in
targeting cancer metabolism for cancer therapy. EMBO Rep.
13:1034–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Birsoy K, Sabatini DM and Possemato R:
Untuning the tumor metabolic machine: Targeting cancer metabolism:
a bedside lesson. Nat Med. 18:1022–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg’s contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang CV: Links between metabolism and
cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takemura A, Che XF, Tabuchi T, Moriya S,
Miyazawa K and Tomoda A: Enhancement of cytotoxic and pro-apoptotic
effects of 2-aminophenoxazine-3-one on the rat hepatocellular
carcinoma cell line dRLh-84, the human hepatocellular carcinoma
cell line HepG2, and the rat normal hepatocellular cell line RLN-10
in combination with 2-deoxy-D-glucose. Oncol Rep. 27:347–355.
2012.
|
|
9
|
Cay O, Radnell M, Jeppsson B, Ahren B and
Bengmark S: Inhibitory effect of 2-deoxy-D-glucose on liver tumor
growth in rats. Cancer Res. 52:5794–5796. 1992.PubMed/NCBI
|
|
10
|
Mack P, Ahren B, Jeppsson B, Kan Z and
Bengmark S: Influence of 2-deoxy-D-glucose and arterial ischaemia
on glucose oxidation and growth of liver cancer in the rat. Eur J
Cancer Clin Oncol. 24:1433–1437. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajewsky MF, Dauber W and Frankenberg H:
Liver carcinogenesis by diethylnitrosamine in the rat. Science.
152:83–85. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun K, Guo XL, Zhao QD, et al: Paradoxical
role of autophagy in the dysplastic and tumor-forming stages of
hepatocarcinoma development in rats. Cell Death Dis. 4:e5012013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun K, Deng W, Zhang S, et al: Paradoxical
roles of autophagy in different stages of tumorigenesis: protector
for normal or cancer cells. Cell Biosci. 3:352013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gewirtz DA: The four faces of autophagy:
implications for cancer therapy. Cancer Res. 74:647–651. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lorin S, Hamai A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hockel M and Vaupel P: Tumor hypoxia:
definitions and current clinical, biologic and molecular aspects. J
Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar
|
|
17
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
18
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez CD, Alvarez S, Ropolo A,
Rosenzvit C, Bagnes MF and Vaccaro MI: Autophagy, Warburg, and
Warburg reverse effects in human cancer. Biomed Res Int.
2014:9267292014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenific CM and Debnath J: Cellular and
metabolic functions for autophagy in cancer cells. Trends Cell
Biol. Sept 30–2014.(Epub ahead of print). PubMed/NCBI
|