Introduction
Paroxysmal or persistent tachycardia is a common
condition in pediatric patients. Pediatric cardiac and non-cardiac
diseases, such as anoxia, hydropenia and electrolyte imbalance, can
induce arrhythmias (1). Persistent
tachycardia can result in serious and potentially fatal pathologies
such as heart failure; therefore, timely and effective treatment is
of great clinical significance. However, the subjective symptoms of
tachycardia, such as a choking sensation in the chest or
palpitations, are not as evident in pediatric patients,
particularly infants, as those in adult patients (2). This can lead to missed treatment
opportunities and can result in severe complications, including
arrhythmia, cardiomyopathy and sudden mortality. Thus, finding
novel, specific biomarkers of tachycardia has great implications
for the early prevention and treatment of this condition in
pediatric patients and may reduce the chance of sudden mortality
caused by malignant arrhythmias. At present, the treatment of
arrhythmias primarily involves drug therapy and radiofrequency
catheter ablation. However, this methodology is not preferred for
pediatric patients since the organs of the patients are still
undergoing development and vascular complications can occur
(3,4). Therefore, the use of radiofrequency
catheter ablation and drug therapy in the treatment of pediatric
arrhythmias is limited. In recent years, clinical studies have
begun attempts to control paroxysmal or persistent tachycardia in
pediatric patients by gene-targeted therapy, with the aim of
improving cardiac function in affected children (5,6).
microRNAs (miRNAs) are a class of single-stranded,
endogenous, non-coding RNA molecules containing 20–25 nucleotides.
miRNAs are formed by the miRNA-processing enzyme Dicer, from a
single-stranded 70–90-base-pair RNA precursor with a hairpin
structure. Through incomplete complementary base pairing with the
3′-untranslated region of target mRNA, miRNA can inhibit specific
protein translation and expression or induce the degradation of
target mRNA (7–9). miRNAs are involved in numerous key
processes, including early development, cell proliferation,
differentiation and apoptosis (10). To date, tissue-specific miRNAs
identified in the heart have included miR-1, miR-133a/b and miR-208
(11,12). Additionally, the specific
expression of circulating miRNAs has been found in various types of
cancer and cardiac diseases (13).
However, this study, to the best of our knowledge, is the first to
report the levels of miRNAs in the plasma of pediatric patients
with recurrent sustained tachycardia symptoms. Finding specific
markers of tachycardia is particularly important for the early
diagnosis and treatment of this disease in children.
Materials and methods
Blood specimen collection
This study was approved by the Ethics Committee of
the Institute of Cardiovascular Diseases, Guangdong General
Hospital (Guangzhou, China). The families of all pediatric patients
that were included in this study signed an informed consent form.
Blood specimens were collected from 40 pediatric patients between
October 2012 and April 2013. The patients included 16 normal,
healthy children with normal electrocardiograms (ECGs) and no
history of cardiovascular disease (control group) and 24 children
with recurrent sustained tachycardia who were not receiving
radiofrequency ablation or antiarrhythmic drug therapy
(experimental group). An ECG of the pediatric patients taken at the
onset of tachycardia was used as the diagnostic criterion. Blood
specimens were centrifuged within 2 h of collection (1,358 × g, 10
min, 4°C). The separated plasma and blood cells were dispensed into
Eppendorf tubes and stored at –80°C prior to use.
Primer design and synthesis
miR-1, miR-133 and U6 gene sequences were retrieved
from the miRBase database (http://www.mirbase.org/) and used as a reference for
designing the polymerase chain reaction (PCR) primers. The designed
primers were synthesized by Shanghai Invitrogen Biotechnology Co.,
Ltd (Shanghai, China). The primer sequences were as follows: miR-1
forward primer, 5′-ACACTCCAGCTGGGTGGAATGTAAAGAAGT-3′ and reverse
primer, 5′-TCAACTGGTGTCGTGGAGTCGGCAATTCTTGAGCAGCTGGT-3′; miR-133
forward primer, 5′-ACACTCCAGCTGGGTTTGGTCCCCTTCAAC-3′ and reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGC AATTCAGTTGAGCAGCTGGT-3′;
reverse universal primer URP, 5′-TGGTGTCGTGGAGTCG-3′; internal
reference U6 forward primer, 5′-CTCGCTTCGGCAGCACA-3′ and U6 reverse
primer, 5′-AACGCTTCACGAATTTGCGT-3′. The downstream primers of
miR-1, miR-133 and U6 were mixed in equal volumes to obtain a
concentration of 10 μM for each primer.
Reverse transcription (RT) and
fluorescent quantitative PCR (qPCR)
Total RNA was extracted from the plasma using
TRIzol® LS reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The total RNA was subjected to reverse transcription (RT) by PCR
immediately subsequent to extraction. The 20-μl RT-PCR
reaction system (ReverTra Ace-α-Reverse Transcription kit; Toboyo
Co., Ltd., Osaka, Japan) contained 2 μl 10X buffer, 1
μl 2.5 mM deoxynucleotide triphosphate, 3 μl mixed
downstream primer, 0.5 μl reverse transcriptase Moloney
murine leukemia virus and 13.5 μl RNA template. The RT-PCR
program comprised 25°C for 15 min followed by 50°C for 50 min. A
total of 2 μl RT product (cDNA) was utilized for qPCR. The
20-μl reaction systems for the qPCR detection of miR-1,
miR-133 and U6 contained 10 μl SYBR Premix Taq II (2X;
Takara Bio, Inc., Shiga, Japan), 0.2 μl 30 pmol/μl
upstream primer, 0.2 μl 30 pmol/μl downstream primer,
7.6 μl dH2O and 2 μl cDNA. The fluorescent
qPCR program comprised 95°C for 5 min, followed by 40 cycles of
94–80°C (94°C for 10 sec, 55°C for 20 sec, 72°C for 10 sec and 80°C
for 35 sec) using an ABI 7500 fluorescent quantitative PCR machine
in plate read mode (Applied Biosystems Life Technologies, Foster
City, CA, USA), which identifies genotypes, detects gene locus
mutations and analyzes single nucleotide polymorpyhsms. The program
ended with the preparation of a melting curve at 60–95°C.
Statistical analysis
The standard curves of miR-1 and miR-133 were used
to calculate the absolute quantities of miR-1 and miR-133. The
levels of circulating miR-1 and miR-133 in the plasma are expressed
as the mean ± standard deviation. Measurement data were analyzed by
the independent two-sample t-test with P<0.05 considered
statistically significant. The sensitivity of miR-1 and miR-133 to
detect arrhythmia was tested with receiver operating characteristic
(ROC) curves. All data were processed using SPSS 16.0 (SPSS Inc.,
Chicago, IL, USA) statistical software.
Results
Baseline data
This study included 40 pediatric patients: 24 with
arrhythmia and 16 healthy controls (24 males and 16 females) with
average ages of 6.6±3.9 and 9.8±1.8 years in the arrhythmia and
control groups, respectively. In the arrhythmia group, there were
seven cases of ventricular tachycardia and 17 cases of
supraventricular tachycardia (SVT). Baseline data of the pediatric
patients are shown in Table I.
 | Table IBaseline data of the pediatric
patients. |
Table I
Baseline data of the pediatric
patients.
| Arrhythmic | Non-arrhythmic |
|---|
| Number | 24 | 16 |
| Gender (n male/n
female) | 15/9 | 9/7 |
| Age (years) | 6.6±3.9 | 9.8±1.8 |
Circulating miRNA levels in the plasma of
pediatric patients
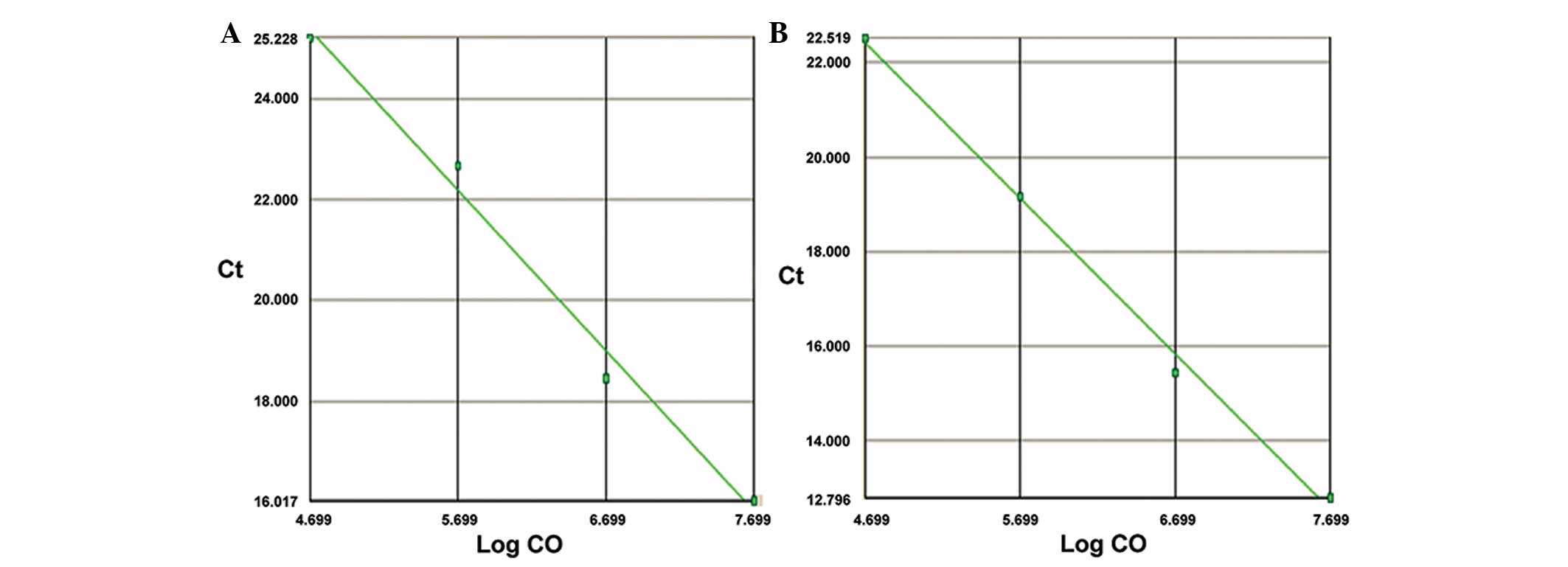
The standard curves prepared by double dilution of
miR-1 and miR-133 standards (y, cycle threshold value;
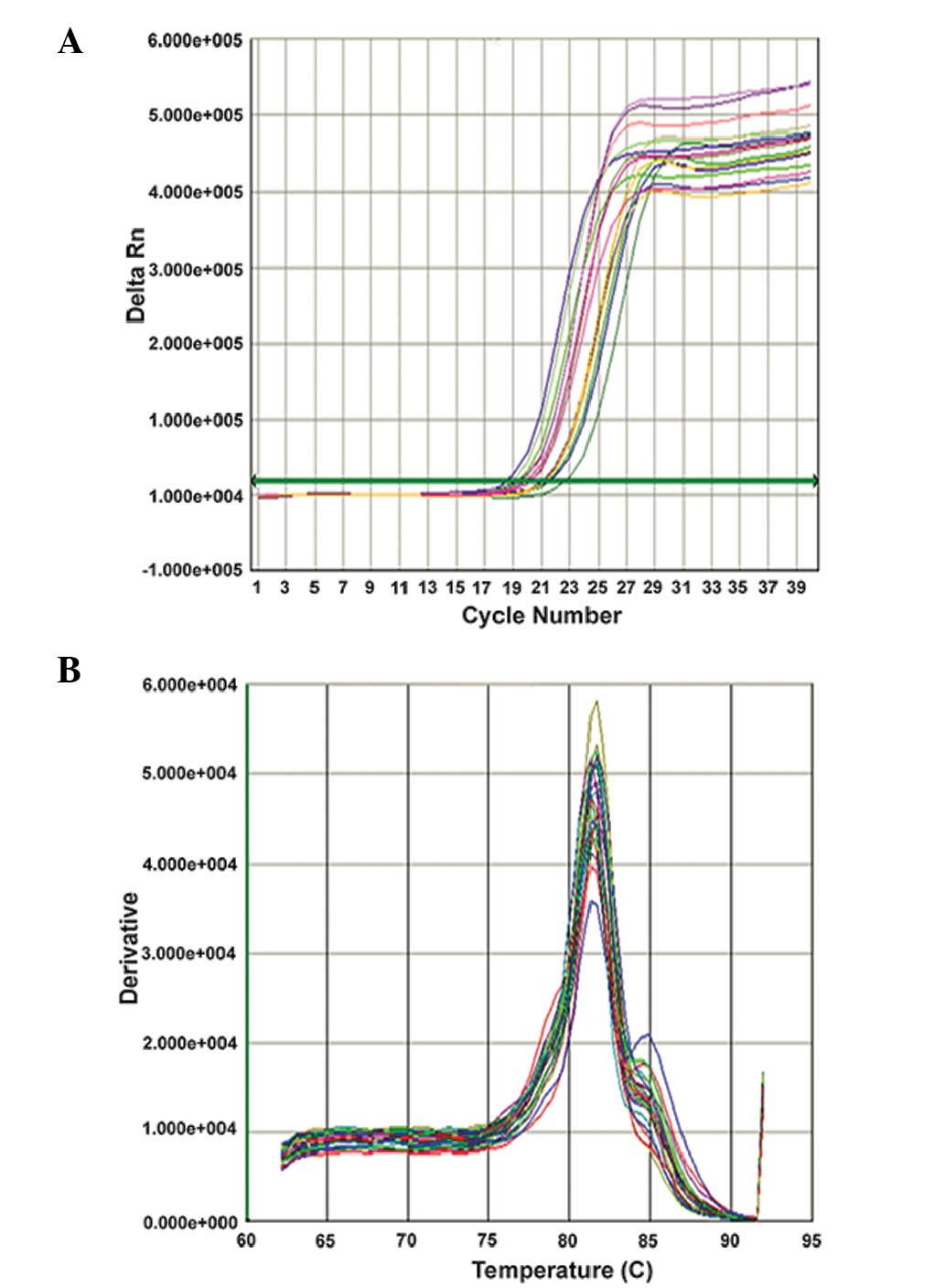
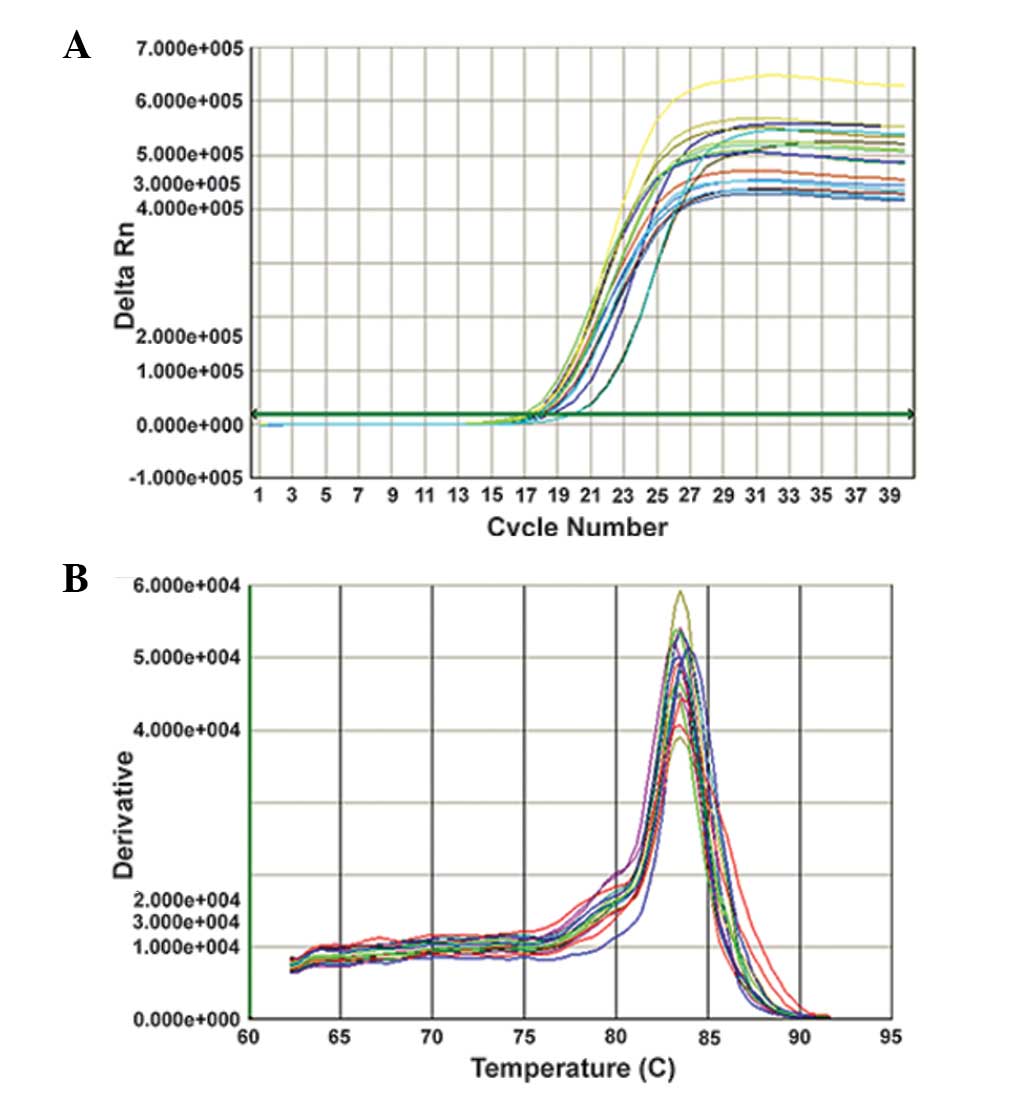
x, Log inital copies; CO) are shown in Fig. 1. The amplification and melting
curves for the qPCR are shown in Figs.
2 and 3.
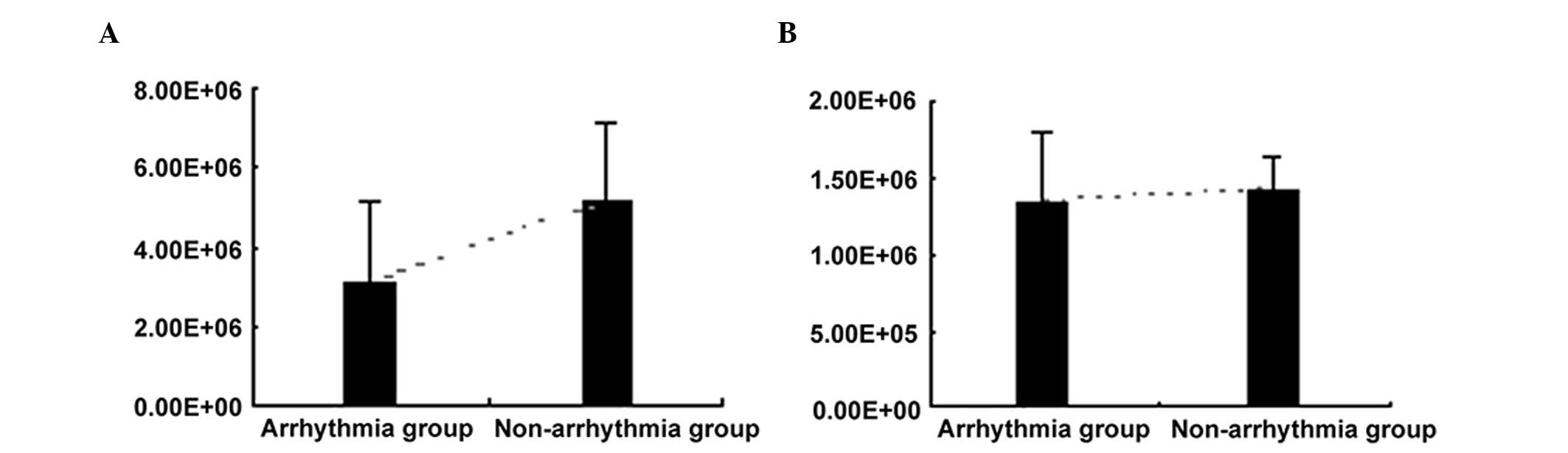
The miR-1 levels in the plasma of pediatric patients
showed a significant difference between the arrhythmia and
non-arrhythmia groups (3.09×106±2.11×106 vs.
5.16×106±1.99×106 copies/ml, P=0.004),
whereas no statistically significant differences were observed in
the miR-133 levels between the two groups
(1.34×106±4.74×105 vs. 1.
43×106±2.03×105 copies/ml, P=0.456) (Table II). The above results indicate
that miR-1 levels were decreased in the plasma of the patients with
arrhythmia whereas miR-133 levels exhibited no significant
variation (Fig. 4).
 | Table IImiR-1 and miR-133 levels in the
plasma of pediatric patients. |
Table II
miR-1 and miR-133 levels in the
plasma of pediatric patients.
| miR type | Arrhythmic
(copies/ml) | Non-arrhythmic
(copies/ml) | P-value |
|---|
| miR-1 |
3.09×106±2.11×106 |
5.16×106±1.99×106 | 0.004a |
| miR-133 |
1.34×106±4.74×105 |
1.43×106±2.03×105 | 0.456 |
miRNA levels in the plasma of pediatric
patients of different genders
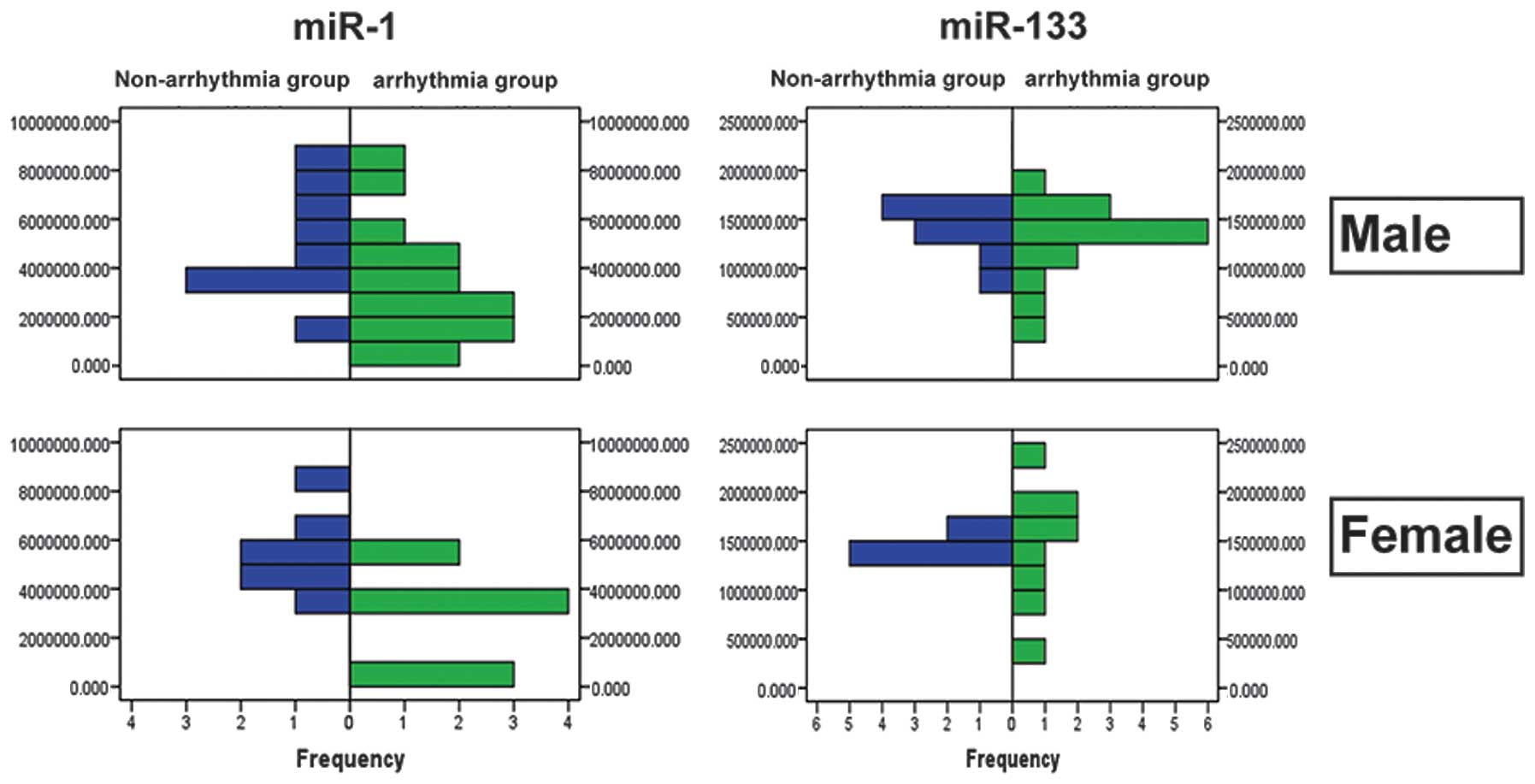
The levels of miR-1 and miR-133 in the plasma of
pediatric patients with arrhythmia showed no statistically
significant differences between the males and females (miR-1,
3.86×106± 2. 41×10 6 vs. 4. 01×10
6±2.15×106 copies/ml, P=0.842; miR-133,
1.33×106±3.47×105 vs.
1.45×106±4.44×105 copies/ml, P=0.351)
(Table III). The frequency
distribution of miR-1 and miR-133 in the plasma of male and female
pediatric patients with arrhythmia and controls is shown in
Fig. 5.
 | Table IIImiR-1 and miR-133 levels in the
plasma of male and female pediatric patients. |
Table III
miR-1 and miR-133 levels in the
plasma of male and female pediatric patients.
| miR type | Male
(copies/ml) | Female
(copies/ml) | P-value |
|---|
| miR-1 |
3.86×106±2.41×106 |
4.01×106±2.15×106 | 0.842 |
| miR-133 |
1.33×106±3.47×105 |
1.45×106±4.44×105 | 0.351 |
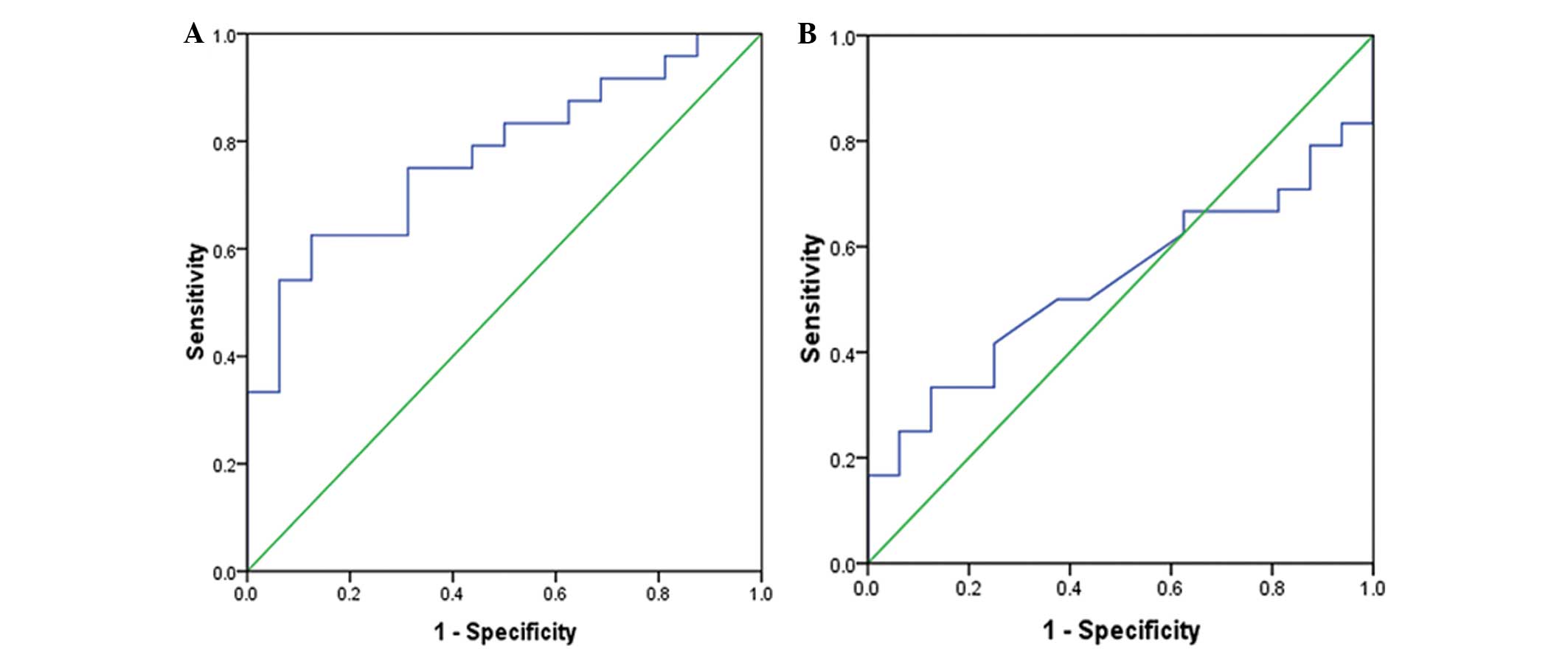
Sensitivity and specificity of 1/miRNA
for the evaluation of arrhythmia (ROC curve)
ROC curves were prepared with 1/miR-1 and 1/miR-133.
Arrhythmia was defined as the positive event (Fig. 6). The results showed that the
diagnosis of tachycardia with 1/miR-1 had statistical significance
(P=0.004; area under the ROC curve, 0.773; 95% confidence interval
of the area, 0.630–0.917), whereas the diagnosis of tachycardia
with 1/miR-133 had no statistical significance (P=0.73) (Table IV).
 | Table IVSensitivity and specificity of
1/miRNA for the evaluation of arrhythmia. |
Table IV
Sensitivity and specificity of
1/miRNA for the evaluation of arrhythmia.
| 1/miR type | Area under ROC
curve | P-value | 95% CI |
|---|
| 1/miR-1 | 0.773 | 0.004a | 0.630–0.917 |
| 1/miR-133 | 0.533 | 0.73 | 0.534–0.711 |
Subgroup analysis of miRNA levels in the
plasma of patients with arrhythmia
Subgroup analysis was performed on the pediatric
patients with arrhythmia for the comparison of plasma miR-1 and
miR-133 levels between the SVT and VT groups (Table V). Statistically significant
differences were observed in the miR-1 and miR-133 levels in the
plasma between the SVT and VT groups (miR-1,
2.41×106±1.62×106 vs.
4.76×106±2.36×106 copies/ml, P=0.004;
miR-133, 1.22×106±5.08×105 vs.
1.64×106±1.69×105 copies/ml, P=0.046). In
addition, statistically significant differences in plasma miR-1
levels were observed between the patients with SVT and the control
group (2.41×106±1.62×106 vs.
5.16×106±1.99×106 copies/ml, P<0.001)
(Table VI), whereas the plasma
miR-133 levels showed no significant difference between these two
groups. The expression levels of miR-133 in the plasma were,
however, significantly different between the VT and normal control
groups (1.64×106±1.69×105 vs.
1.43×106±2.03×105 copies/ml, P=0.024)
(Table VII), whereas no
significant difference was observed in the plasma miR-1 levels
between these two groups. Together, these results indicate that
circulating miR-1 levels in the plasma of pediatric patients with
SVT were downregulated, whereas miR-133 levels in the plasma of
pediatric patients with VT were upregulated.
 | Table VComparison of miR-1 and miR-133
between the supraventricular and ventricular tachycardia
groups. |
Table V
Comparison of miR-1 and miR-133
between the supraventricular and ventricular tachycardia
groups.
| miR type | Supraventricular
tachycardia (copies/ml) | Ventricular
tachycardia (copies/ml) | P-value |
|---|
| miR-1 |
2.41×106±1.62×106 |
4.76×106±2.36×106 | 0.004a |
| miR-133 |
1.22×106±5.08×105 |
1.64×106±1.69×105 | 0.046a |
 | Table VIComparison of miR-1 and miR-133
between the supraventricular tachycardia and normal control
groups. |
Table VI
Comparison of miR-1 and miR-133
between the supraventricular tachycardia and normal control
groups.
| miR type | Supraventricular
tachycardia (copies/ml) | Normal control
(copies/ml) | P-value |
|---|
| miR-1 |
2.41×106±1.62×106 |
5.16×106±1.99×106 | <0.001a |
| miR-133 |
1.22×106±5.08×105 |
1.43×106±2.03×105 | 0.143 |
 | Table VIIComparison of miR-1 and miR-133
between the ventricular tachycardia and normal control groups. |
Table VII
Comparison of miR-1 and miR-133
between the ventricular tachycardia and normal control groups.
| miR type | Ventricular
tachycardia (copies/ml) | Normal control
(copies/ml) | P-value |
|---|
| miR-1 |
4.76×106±2.36×106 |
5.16×106±1.99×106 | 0.68 |
| miR-133 | 1.64×106
±1.69×105 |
1.43×106±2.03×105 | 0.024a |
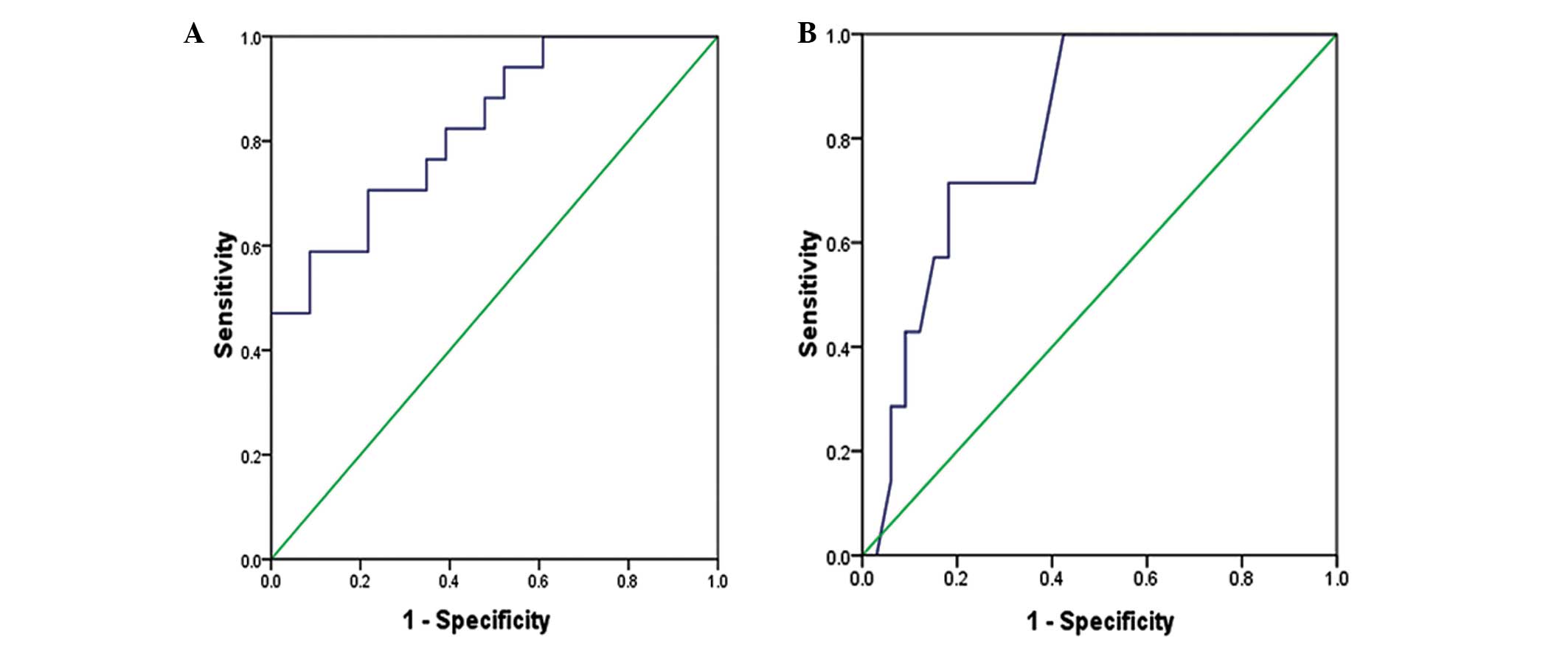
Sensitivity of ROC curves for the
evaluation of SVT and VT
According to the ROC curves, miR-1 had enhanced
sensitivity and specificity for the evaluation of SVT (P<0.001;
area under the ROC curve, 0.826; 95% confidence interval of the
area, 0.699–0.953) (Fig. 7A),
whereas miR-133 had better sensitivity and specificity for the
evaluation of VT (P=0.01; area under the ROC curve, 0.814; 95%
confidence interval of the area, 0.671–0.957) (Fig. 7B).
Discussion
miRNAs regulate cell proliferation and
differentiation by mRNA-specific base pairing and their expression
exhibits cell or tissue specificity (14–16).
Studies have demonstrated that numerous miRNAs are involved in the
reconstruction of ion channels by regulating gene expression in
cardiomyocytes during the process of arrhythmia (17–19).
At present, studies on the association between miRNA and arrhythmia
are primarily focusing on pathological and physiological processes
such as myocardial ischemia and cardiac hypertrophy (20–23).
The interactions of miRNAs with ion channel-encoding genes and
calmodulin regulate cardiac contractility, rhythm and excitement,
thereby inducing arrhythmia (24,25).
However, less research has been undertaken into arrhythmia in
pediatric patients without organ disease, and, to the best of our
knowledge, no reports are available on the specific expression of
circulating miRNA in pediatric patients with persistent
tachycardia.
In the present study, miR-1 and miR-133 levels in
the plasma of pediatric patients with tachycardia and healthy
controls were quantitatively detected by a qPCR. The results showed
that circulating miR-1 levels in the plasma were lower in the
patients with arrhythmia than those in the healthy controls, whilst
no significant differences were observed in the miR-133 expression
levels. Of note, the results of the subgroup analysis revealed that
there were significant differences in circulating miR-1 and miR-133
levels in the plasma of pediatric patients between the SVT and VT
groups. Additionally, circulating plasma miR-1 levels were
decreased in patients with SVT, whereas plasma miR-133 levels were
significantly increased in patients with VT, as compared with those
in the normal controls. Together, these results demonstrate that
different circulating miRNAs in the plasma of pediatric patients
with SVT and VT may cause the reconstruction of various ion
channels, thereby inducing arrhythmias of different types.
Myocardial ischemia-induced arrhythmia is a major
cardiovascular pathology associated with miR-1 and miR-133. It has
been reported that miR-1 expression is significantly increased in
patients with myocardial ischemia and infarction and is causative
of arrhythmias. A possible mechanism for this is that the
ischemia-related abnormally high expression of miR-1 leads to a
reduction in gap junction α-1 protein/Connexin43 expression and a
delay in cardiac conduction, as well as to a reduction in potassium
inwardly-rectifying channel, subfamily J, member 2 expression, a
decline in inward rectifier current density and resting potential
rise, ultimately leading to the occurrence of ischemic arrhythmia
(26–29). Zhang et al (30) found in an animal model of
myocardial ischemia that miR-1 overexpression in the ventricular
heart muscle caused atrioventricular block; the underlying
mechanism was associated with a reduction in Connexin43 expression
and a decline in L-type Ca2+ currents. In the present
study, none of the pediatric patients included had organic heart
disease. The finding of down-regulated miR-1 expression in the
plasma of pediatric patients contrasted with upregulated miR-1
expression following myocardial ischemia, indicating that pediatric
SVT involves a different pathogenesis. Connexin43 is a protein
component of gap junctions, involved in regulating synchronized
contraction of the heart. The expression level of Connexin43 is
relatively low in the sinoatrial and atrioventricular nodes,
whereas its expression in the surrounding tissues is high (31). Therefore, data from the present
study suggest that the pathogenesis of SVT in pediatric patients
without heart disease is associated with the presence of
considerable Connexin43 expression adjacent to the atrioventricular
nodes and in the atrioventricular accessory pathways. The
upregulation of Connexin43 expression may inhibit the expression of
miR-1, leading to increases in the myocardial conduction velocity.
A major limitation of the present study lies in the difficulty of
obtaining myocardial tissues from pediatric patients to
quantitatively determine Connexin43 expression in the myocardial
tissue.
miR-133 is another miRNA with specific expression in
myocardial tissue that is known to affect the autorhythmicity of
heart tissue through pacemaker channels [potassium/sodium
hyperpolarization-activated cyclic nucleotide-gated ion channel 2
(HCN2) and HCN4] (32,33) or potassium ion channels [potassium
voltage-gated channel, KQT-like subfamily, member 1 (KCNQ1) and
potassium voltage-gated channel, Isk-related family, member 1
(KCNE1)] (34,35). In miR-133a transgenic mice,
miR-133a inhibits the rapid delayed rectifier potassium current
encoded by the congenital long QT2 syndrome gene and the slow
delayed rectifier potassium current encoded by the KCNQ1 and KCNE1
genes. Damage to the repolarizing current channels leads to the
extension of action potential duration or prolongation of the QT
interval (36). In the present
study, miR-133 levels were increased in pediatric patients with VT;
this finding may be associated with damage to the repolarizing
current channels, although further investigations are required to
validate this hypothesis.
This study investigated whether the levels of
circulating miR-1 and miR-133 in the plasma of pediatric patients
differed between male and female patients; the results showed no
significant differences in miR-1 and miR-133 levels between the
genders (P>0.05). However, Stauffer et al (37) found that gender was associated with
significantly different Connexin43 and miR-1 expression, with miR-1
expression in the plasma lower in female than in male patients
(37). This inconsistency between
the studies may be due to the small sample size and different
ethnicities and ages of patients included. Studies with a larger
sample size are required, as well as comparisons between the levels
of circulating miRNAs in the plasma of pediatric and adult
patients.
Since differences in the levels of specific miRNAs
have been identified in the plasma of pediatric patients with
arrhythmias, miRNA interference technology may be used as a
suitable treatment of refractory arrhythmias. To rectify the
downregulation of miR-1 expression in pediatric patients with SVT,
we speculate that synthetic miR-1 may be introduced into cells by
exogenous, double-stranded miRNA or miRNA mimic technologies. To
target the upregulation of miR-133 expression in pediatric patients
with VT, miRNA antisense oligonucleotide technology and miRNA
barrier oligonucleotide technology can be used to inhibit the
expression of miR-133, further controlling the occurrence of
arrhythmia. However, these and other relevant technologies are
still in the early stages of testing.
The identification of specific miRNAs has provided a
novel perspective for studying the pathogenesis of arrhythmia.
Accordingly, new ideas have been considered for the future
development of novel, secure and effective drugs for treating
arrhythmia. The expression of specific circulating miRNA in plasma
has great medical significance for early arrhythmia prevention. In
the near future, gene-targeted therapy and the prevention of
arrhythmia in pediatric patients are likely to have great
benefit.
References
|
1
|
Balaguer Gargallo M, Jordán García I,
Caritg Bosch J, et al: Supraventricular tachycardia in infants and
children. An Pediatr (Barc). 67:133–138. 2007.In Spanish.
View Article : Google Scholar
|
|
2
|
Calabrò MP1, Cerrito M, Luzza F, et al:
Supraventricular tachycardia in infants: epidemiology and clinical
management. Curr Pharm Des. 14:723–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weindling SN, Saul JP and Walsh EP:
Efficacy and risks of medical therapy for supraventricular
tachycardia in neonates and infants. Am Heart J. 131:66–72. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kugler JD, Danford DA, Deal BJ, et al:
Radiofrequency catheter ablation for tachyarrhythmias in children
and adolescents. N Engl J Med. 330:1481–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwartz P, Crotti L and Insolia R:
Arrhythmogenic disorders of genetic origin: long QT syndrome: from
genetics to management. Circ Arrhythm Electrophysiol. 5:868–873.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu W and Horie M: Phenotypical
manifestations of mutations in genes encoding subunits of cardiac
potassium channels. Circ Res. 109:97–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miranda KC, Huynh T, Tay Y, et al: A
pattern-based method for the identification of MicroRNA binding
sites and their corresponding heteroduplexes. Cell. 126:1203–1217.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
da Costa Martins PA, Leptidis S, Salic K
and De Windt LJ: MicroRNA regulation in cardiovascular disease.
Curr Drug Targets. 11:900–906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeda S and Pu WT: Expression and function
of microRNAs in heart disease. Curr Drug Targets. 11:913–925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jazbutyte V and Thum T: MicroRNA-21: from
cancer to cardiovascular disease. Curr Drug Targets. 11:926–935.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lagos-Quintana M, Rauhut R, Yalcin A, et
al: Identification of tissue-specific microRNAs from mouse. Curr
Biol. 12:735–739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marsit CJ, Eddy K and Kelsey KT: MicroRNA
responses to cellular stress. Cancer Res. 66:10843–10848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Etheridge A, Lee I, Hood L, et al:
Extracellular microRNA: a new source of biomarkers. Mutat Res.
717:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Rooij E, Sutherland LB, Qi X,
Richardson JA, Hill J and Olson EN: Control of stress-dependent
cardiac growth and gene expression by a microRNA. Science.
316:575–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Rooij E, Quiat D, Johnson BA, et al: A
family of microRNAs encoded by myosin genes governs myosin
expression and muscle performance. Dev Cell. 17:662–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo X, Zhang H, Xiao J and Wang Z:
Regulation of human cardiac ion channel genes by microRNAs:
theoretical perspective and pathophysiological implications. Cell
Physiol Biochem. 25:571–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye Y, Hu Z, Lin Y, et al: Downregulation
of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist
protects against myocardial ischaemia-reperfusion injury.
Cardiovasc Res. 87:535–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin Y, Yu Y, Dong H, et al: MicroRNA 21
inhibits left ventricular remodeling in the early phase of rat
model with ischemia-reperfusion injury by suppressing cell
apoptosis. Int J Med Sci. 9:413–423. 2012. View Article : Google Scholar
|
|
22
|
Zhang X, Wang X, Zhu H, et al: Synergistic
effects of the GATA-4-mediated miR-144/451 cluster in protection
against simulated ischemia/reperfusion-induced cardiomyocyte death.
J Mol Cell Cardiol. 49:841–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ganesan J, Ramanujam D, Sassi Y, et al:
MiR-378 controls cardiac hypertrophy by combined repression of
mitogen-activated protein kinase pathway factors. Circulation.
127:2097–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo X, Zhang H, Xiao J, et al: Regulation
of human cardiac ion channel genes by microRNAs: theoretical
perspective and pathophysiological implications. Cell Physiol
Biochem. 25:571–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terentyev D, Belevych AE, Terentyeva R, et
al: miR-1 over-expression enhances Ca(2+) release and promotes
cardiac arrhythmogenesis by targeting PP2A regulatory subunit
B56alpha and causing CaMKII dependent hyperphosphorylation of RyR2.
Circ Res. 104:514–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ai J, Zhang R, Li Y, et al: Circulating
microRNA-1 as a potential novel biomarker for acute myocardial
infarction. Biochem Biophys Res Commun. 391:73–77. 2010. View Article : Google Scholar
|
|
27
|
Wang GK, Zhu JQ, Zhang JT, et al:
Circulating microRNA: a novel potential biomarker for early
diagnosis of acute myocardial infarction in humans. Eur Heart J.
31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D’Alessandra Y, Pompilio G and Capogrossi
MC: MicroRNAs and myocardial infarction. Curr Opin Cardiol.
27:228–235. 2012. View Article : Google Scholar
|
|
29
|
Iekushi K, Seeger F, Assmus B, Zeiher AM
and Dimmeler S: Regulation of cardiac microRNAs by bone marrow
mononuclear cell therapy in myocardial infarction. Circulation.
125:1765–1773. S1–S7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Sun L, Zhang Y, et al:
Overexpression of microRNA-1causes atrioventricular block in
rodents. Int J Biol Sci. 9:455–462. 2013. View Article : Google Scholar
|
|
31
|
Boyett MR, Inada S, Yoo S, et al:
Connexins in the sinoatrial and atrioventricular nodes. Adv
Cardiol. 42:175–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo X, Lin H, Pan Z, et al:
Down-regulation of miR-1/m iR-133 contributes to re-expression of
pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol
Chem. 283:20045–20052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao J, Yang B, Lin H, Lu Y, Luo X and
Wang Z: Novel approaches for gene-specific interference via
manipulating actions of microRNAs: examination on the pacemaker
channel genes HCN2 and HCN4. J Cell Physiol. 212:285–292. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Yang CM, Xi Y, et al: MicroRNA-1/133
targeted dysfunction of potassium channels KCNE1 and KCNQ1 in human
cardiac progenitor cells with simulated hyperglycemia. Int J
Cardiol. 167:1076–1078. 2013. View Article : Google Scholar
|
|
35
|
Matkovich SJ, Wang W, Tu Y, et al:
MicroRNA-133a protects against myocardial fibrosis and modulates
electrical repolarization without affecting hypertrophy in
pressure-overloaded adult hearts. Circ Res. 106:166–175. 2010.
View Article : Google Scholar :
|
|
36
|
Xiao J, Luo X, Lin H, et al: MicroRNA
miR-133 represses HERG K+ channel expression
contributing to QT prolongation in diabetic hearts. J Biol Chem.
282:12363–12367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stauffer BL, Sobus RD and Sucharov CC: Sex
differences in cardiomyocyte connexin43 expression. J Cardiovasc
Pharmacol. 58:32–39. 2011. View Article : Google Scholar : PubMed/NCBI
|