Introduction
The vasculature is predominantly comprised of
vascular smooth muscle cells (VSMCs), and these cells are involved
in the maintenance of vessel tone and blood pressure (1,2).
Abnormal proliferation of VSMCs is key in the pathogenesis of
cardiovascular diseases, such as coronary heart disease,
hypertension and atherosclerosis (3,4).
Several growth factors and cytokines have been reported to be
capable of stimulating the migration and proliferation of VSMCs,
for example leptin, which is vital in restenosis (5–7). A
previous study indicated that tumor necrosis factor-α (TNF-α) is a
major risk factor in atherosclerosis and coronary heart disease
(8). TNF-α is a pleiotropic
inflammatory cytokine that has been reported to serve a
pathophysiological role in vascular atherosclerosis (9,10).
In addition, TNF-α has been demonstrated to regulate the
proliferation, apoptosis, migration and differentiation of VSMCs,
which are critical in the pathogenesis of cardiovascular disease
(8,11,12).
However, the precise mechanism underlying TNF-α-induced
proliferation of VSMCs remains to be fully elucidated.
MicroRNAs (miRNAs) are a class of small
(22-nucleotide) noncoding RNA molecules that function as endogenous
silencers of various target genes (13–15).
The majority of mature miRNAs suppress gene expression either by
facilitating the cleavage of their target messenger RNAs (mRNAs) or
by inhibiting mRNA translation upon imperfect base pairing to the
3′-untranslated region (3′-UTR) of the mRNA (16,17).
It has been reported that miRNAs are highly conserved among species
and are crucial in various physiological and pathological
processes, including age-associated diseases, developmental
abnormalities, autoimmune diseases and various types of cancer
(18–21). The role of miRNAs in the
cardiovascular system has been previously investigated (22,23);
however, the function of miRNAs in the proliferation of VSMCs
induced by TNF-α remains to be fully investigated.
In the current study, it was hypothesized that the
expression of miR-25 was inhibited in TNF-α-stimulated VSMCs and
that overexpression of miR-25 in the VSMCs inhibited cell
proliferation by targeting cyclin-dependent kinase 6 (CDK6). Thus,
the current study aimed to elucidate whether miR-25 was impaired in
the pathogenesis of atherosclerosis and coronary heart disease, and
may be a potential therapeutic target in atherosclerosis and
coronary heart disease.
Materials and methods
Ethical statement
All experiments were approved by the Clinical
Research Ethics Committee of The Fourth Affiliated Hospital, Harbin
Medical University (Harbin, China).
Vectors and cell culture
The pcDNA-CDK6 vector was purchased from
Sigma-Aldrich (Oakville, ON, Canada). The CDK6 3′UTR sequence with
the binding site for miR-25 was cloned into the pMIR-REPORT
luciferase construct (Ambion Life Technologies, Carlsbad, CA, USA).
The mutant construct of CDK6 3′UTR was generated using the
QuikChange Site-Directed Mutagenesis kit (Agilent Technologies,
Inc., Santa Clara, CA, USA). The reagents for cell culture (fetal
bovine serum, penicillin and streptomycin) were purchased from
Gibco Life Technologies (Carlsbad, CA, USA). Human VSMCs were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in the medium 231 supplemented with smooth muscle
cell growth supplement (Gibco Life Technologies) at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Cell transfection
The miR-25 mimics and the control were synthesized
by Shanghai GenePharma Co., Ltd. (Shanghai, China) and transfected
into the cells to a final oligonucleotide concentration of 20
nmol/l. All cell transfections were introduced using DharmaFECT 1
reagent (GE Healthcare Biosciences, Pittsburgh, PA, USA) according
to the manufacturer’s instructions.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and the miRNA was
reverse transcribed into cDNA (miRNA reverse kit, Promega Corp.,
Madison, WI, USA). The reaction mixture contained 1 μg
purified total RNA, 5X M-MLV buffer (Invitrogen Life Technologies),
200 U/μl (M-MLV; Invitrogen Life Technologies), 1.0
μl dithiothreitol (Invitrogen Life Technologies), 1.0
μl of 10 μmol/l stem-loop RT primer (Invitrogen Life
Technologies), 0.5 μl of 40 U/μl RNase inhibitor
(Invitrogen Life Technologies) and 1.0 μl of 10 mmol/l
deoxyribonucleotide triphosphate (Invitrogen Life Technologies).
Relative transcript levels of mRNA were determined by RT-qPCR using
the ABI 7300 Real-time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The RT-qPCR reaction was
composed of 1X SYBR green fluorescent dye (Takara Biotechnology
Co., Ltd., Dalian, China), 1 μl of 10 μM forward
primers, 1 μl of 10 μM reverse primers, 1X qPCR mix
(Invitrogen Life Technologies) and 1 μl cDNA. Reactions were
run with the following thermal cycling parameters: 95°C for 5 min
followed by 35 cycles of 95°C for 5 sec and 60°C for 30 sec;
melting curve program (60–95°C) with a heating rate of 0.1°C/sec.
The relative gene expression was assessed by the ΔΔCt method. GAPDH
or U6 was used as an internal control (Table I).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Sequence
(5′-3′) |
|---|
| MicroRNA reverse
transcription primer |
| MicroRNA-25 |
GTCGTATCCAGTGCGTGTCGTGGAGTCG
GCAATTGCACTGGATACGACTCAGAC |
| U6 snRNA |
AAAATATGGAACGCTTCACGAATTTG |
| Real-time
polymerase chain reaction primer sequence |
| MicroRNA-25 | F:
CATTGCACTTGTCTCGGTCTG
R: ATTGCGTGTCGTGGAGTCG |
| U6 snRNA | F:
CTCGCTTCGGCAGCACATATACT
R: ACGCTTCACGAATTTGCGTGTC |
| GAPDH | F:
AATGGGCAGCCGTTAGGAAA
R: TGAAGGGGTCATTGATGGCA |
| Ki-67 | F:
TCCTTTGGTGGGCACCTAAGACCTG
R: TGATGGTTGAGGTCGTTCCTTGATG |
| CDK6 | F:
GGACTTTCTTCATTCACACCG
R: GACCACTGAGGTTAGGCCA |
|
Cell proliferation assays
Cells were seeded in 96-well plates at a density of
1,000 cells/well with 100 μl complete culture medium. The
cells were then cultured for an additional 24, 48 or 72 h. The
supernatant was removed and 100 μl medium 231 containing 10
μl Cell Counting kit 8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well for a
2-h incubation at 37°C. The culture plates were then agitated for
10 min and the optical density values were read at a wavelength of
450 nm using a Thermo Fisher Scientific microplate reader (Beijing,
China).
Dual luciferase assays
Cells were co-transfected with 0.4 μg miR-25
or negative controls and the reporter construct 0.2 μg pGL-3
control vector (Promega Corp.). Cells were plated at a density of
5×105 cells/well in 24-well plates 24 h prior to
transfection. The cells were harvested 24 h post-transfection and
assayed using the Dual-Luciferase Reporter Assay system (Promega
Corp.) according to manufacturer’s instructions. Firefly luciferase
values were normalized to Renilla and the ratio of
Firefly/Renilla luciferase values was reported.
Western blotting
Total cellular protein extraction and western
blotting procedures were conducted using standard methods (14). CDK-6 and GAPDH proteins were
incubated with rat polyclonal anti-human CDK-6 (1:1,000; Abcam,
Cambridge, UK) and mouse polyclonal anti-human GAPDH (1:5,000:
ProteinTech Group, Inc., Chicago, IL, USA) primary antibodies,
respectively. The membranes were subsequently incubated with
horseradish peroxidase-labeled rabbit anti-mouse and goat
anti-rabbit antibodies (ZSGB-BIO, Beijing, China). the signal was
detected using an enhanced chemiluminescence kit (EMD Millipore,
Billerica, MA, USA).
Statistical analysis
Each experiment was repeated a minimum of three
times. Statistical analysis was performed using SPSS, version 15.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. Either the analysis of variance or Student’s
t-test was completed in order to analyze the data and P<0.05
(two-tailed) was considered to indicate a statistically significant
difference.
Results
Expression of miR-25 is reduced in VSMCs
induced by TNF-α
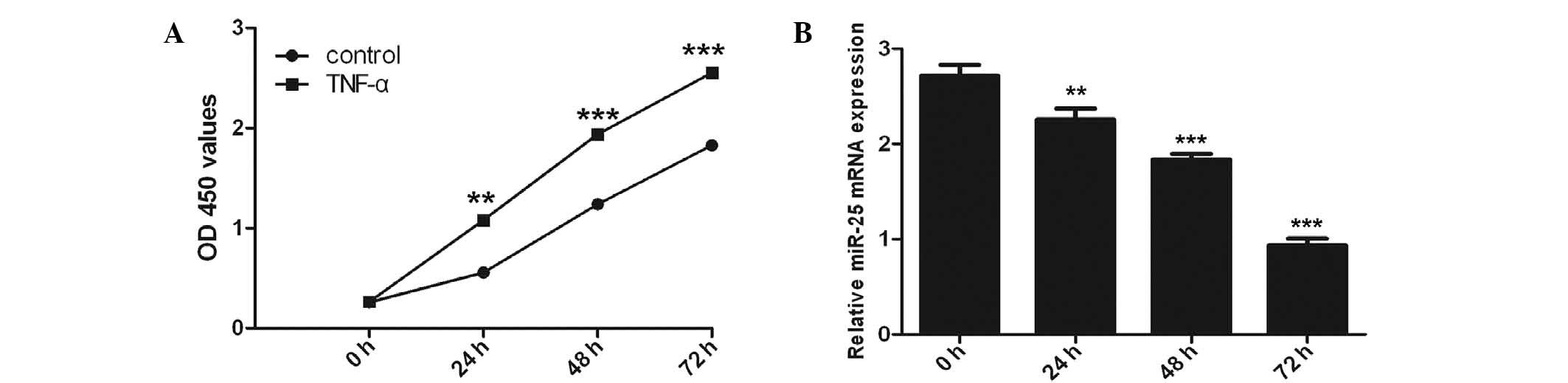
A significant time-dependent induction of cell
proliferation by TNF-α (100 ng/ml) was observed with the maximal
response at 72 h (P<0.001) using the CCK-8 proliferation assay
(Fig. 1A). Inhibition of miR-25
expression was observed in VSMCs following TNF-α stimulation with
RT-qPCR analysis (Fig. 1B).
Overexpression of miR-25 inhibits VSMC
proliferation
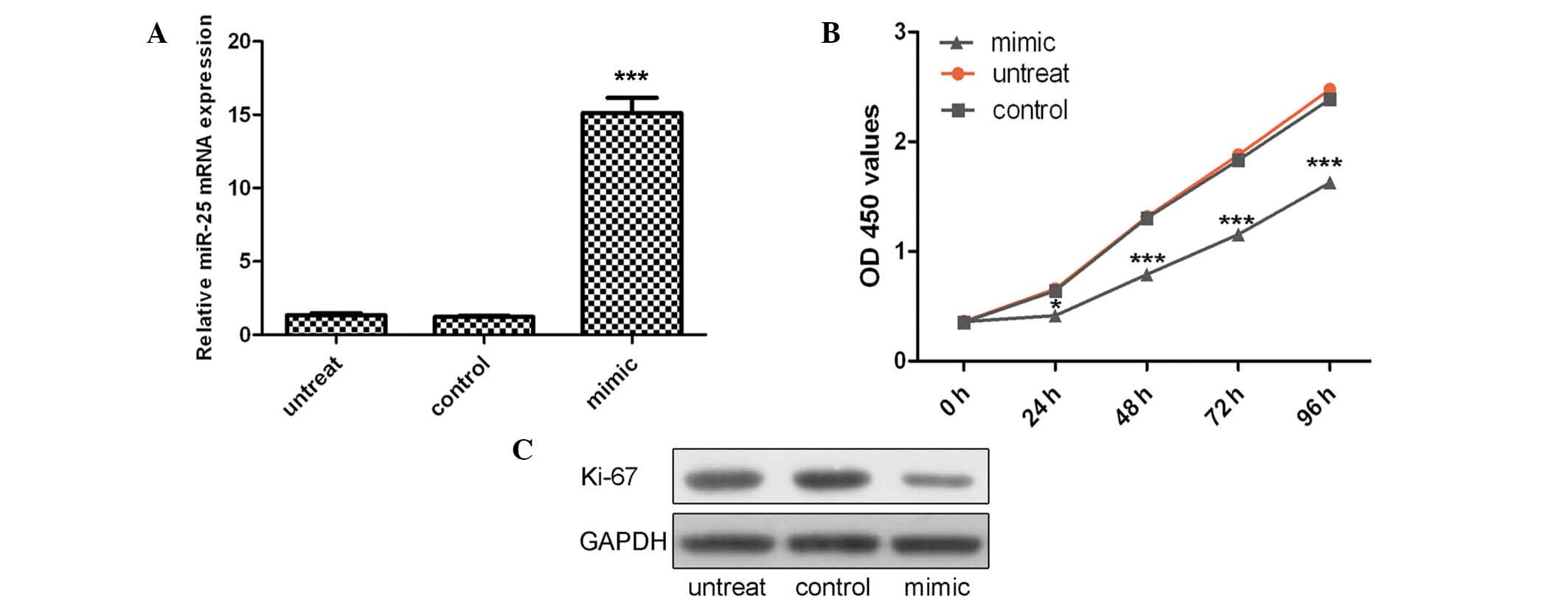
VSMCs were transfected with miR-25 mimics or control
oligo, which were demonstrated to have high transfection efficiency
(Fig. 2A; P<0.001). The CCK-8
proliferation assay indicated that cell proliferation was
significantly decreased in miR-25 mimic-transfected VSMCs compared
with the control oligo-transfected cells or untreated cells
(Fig. 2B). This proliferative
effect of miR-25 was further confirmed by Ki-67 expression. As
demonstrated in Fig. 2C, a
significant decrease in the protein expression of Ki-67 was
observed in the group transfected with miR-25 mimics as compared
with the control group or untreated group.
miR-25 directly regulates the CDK6 gene
in VSMCs
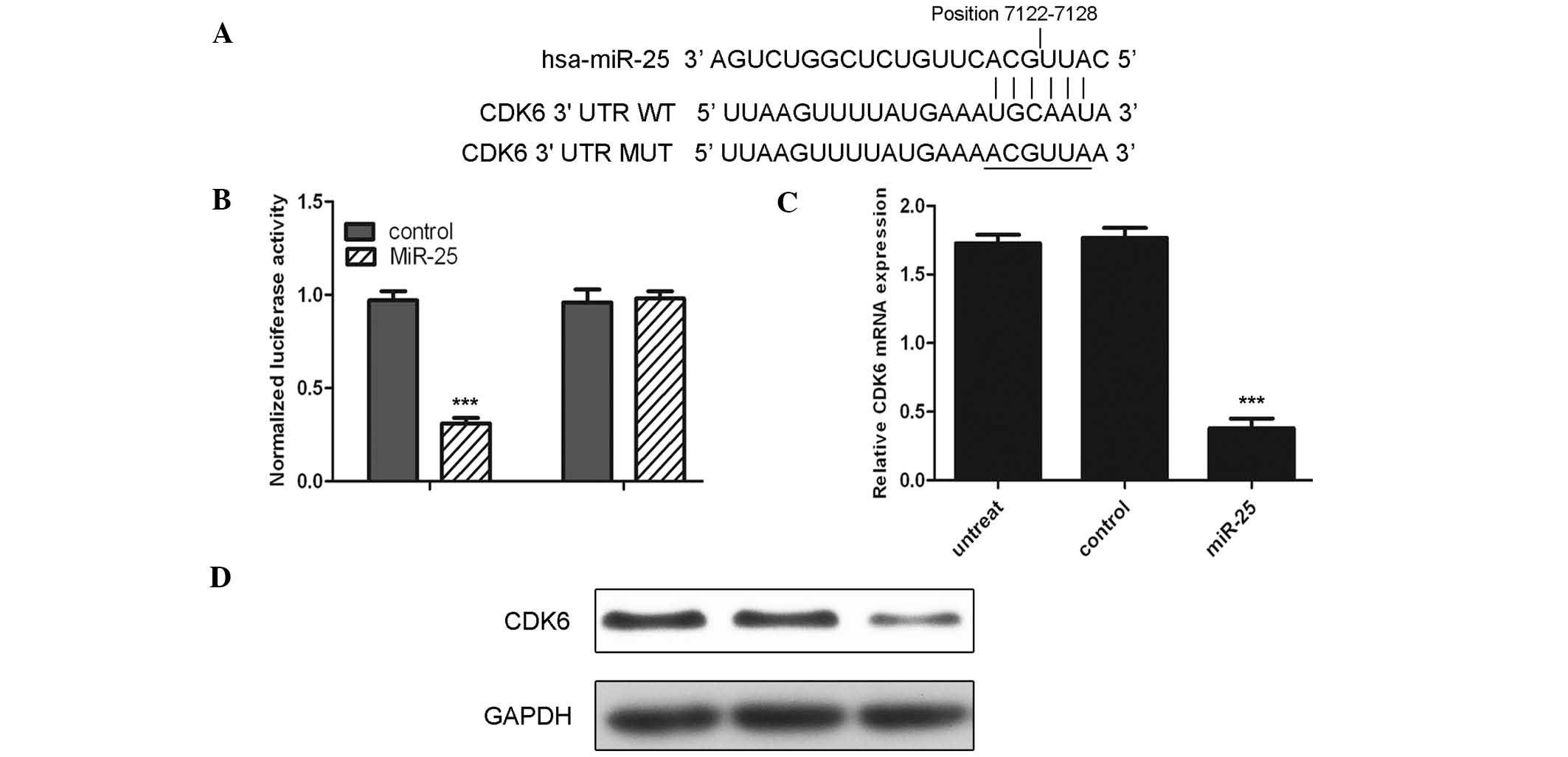
The current study suggested CDK6 as a potential
target of miR-25 (Fig. 3A). To
confirm whether CDK6 is a target, luciferase reporter gene assays
were conducted. The miR-25 mimic rather than control significantly
suppressed the luciferase activity of the reporter gene containing
wild-type 3′-UTR of CDK6 (P<0.001); however, did not affect the
activity of the gene containing the mutant 3′-UTR (Fig. 3B). Furthermore, the mRNA and
protein levels of CDK6 were detected following transfection of
cells with the miR-25 mimic. Notably, mRNA and protein levels of
CDK6 were substantially reduced upon transfection with the miR-25
mimic (Fig. 3C and D;
P<0.001).
CDK6 is involved in miR-25-mediated VSMC
proliferation
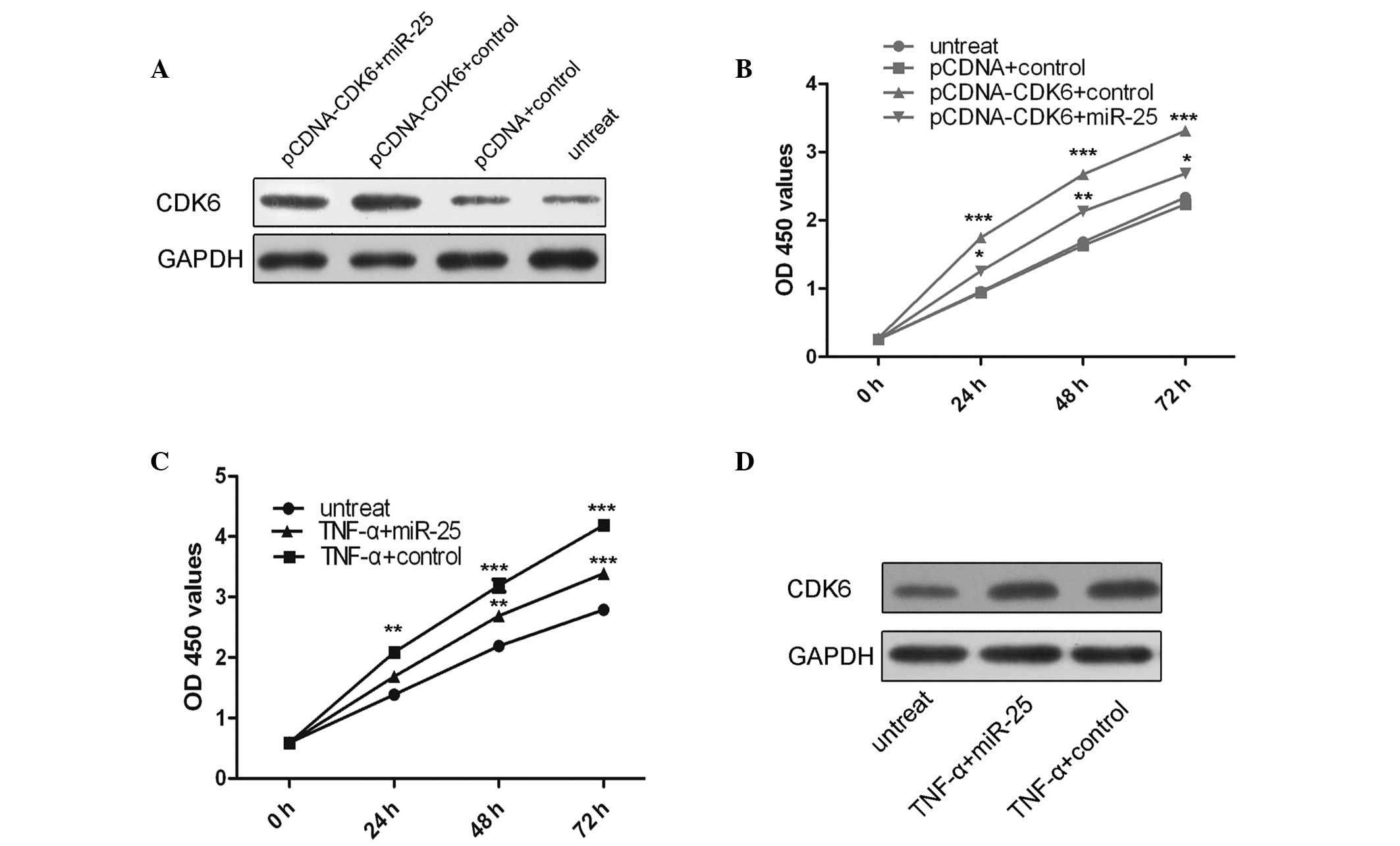
To explore whether miR-25 mediated tumor suppression
in VSMCs via directly targeting CDK6, a ‘rescue’ methodology was
used. The expression of CDK6 was restored in cells that had been
previously treated with miR-25 mimics. In agreement with the
restored expression of CDK6 protein (Fig. 4A), an increase in cell
proliferation was observed upon transfection with the CDK6 plasmid
(Fig. 4B). Furthermore, miR-25
mimics were able to inhibit cell proliferation and the promotion of
cell proliferation following TNF-α treatment was significantly
attenuated by the re-introduction of miR-25 (Fig. 4C). In addition, TNF-α was able to
enhance CDK6 protein expression and miR-25 attenuated this effect
(Fig. 4D).
Discussion
The abnormal proliferation of VSMCs is critical in
the pathogenesis of atherosclerosis, coronary heart disease and
restenosis (24,25). The presence of chronic or mild
inflammation in the arteries is a risk factor for cardiovascular
disease (26,27) and TNF-α is a potent inflammatory
cytokine. TNF-α has been reported to possess the ability to induce
vascular damage and promote the pathogenesis of atherosclerosis
(8,28). In addition, TNF-α is known to
regulate the proliferation and migration of VSMCs, which results in
calcium deposition and an increase in arterial stiffness (12,29,30).
However, the precise mechanisms of TNF-α in the regulation of
abnormal VSMC proliferation remains to be fully elucidated. The
data of the current study suggested that TNF-α inhibits miR-25
expression, overexpression of miR-25 suppresses the proliferation
of VSMCs and overexpression of CDK6 impairs the miR-25-induced
inhibition of proliferation of VSMCs. These observations suggest
that TNF-α may function to promote abnormal proliferation of the
VSMCs via the inhibition of miR-25. Thus, miR-25 may be a potential
therapeutic target in the treatment of vascular disease.
Previous studies have demonstrated that miR-25 is
able to regulate various developmental and cellular processes, and
is implicated in a number of human diseases (31–33).
miR-25 is known to be important in several types of cancer,
including hepatocellular carcinoma, and colon, gastric and lung
cancer (34–36). miR-25 has been indicated to be
involved in various cellular processes, including cell
proliferation, apoptosis and the release of cytokines (37–40).
Esposito et al (36)
demonstrated that miR-25 was reduced in anaplastic thyroid
carcinomas, and ectopic expression of miR-25 suppressed the
proliferation and colony formation of anaplastic thyroid carcinoma
cells via the induction of G2/M-phase cell cycle arrest
(36). However, the precise
function of miR-25 in VSMCs remains unknown. In the present study,
TNF-α was observed to inhibit the expression of miR-25.
Furthermore, overexpression of miR-25 was suggested to inhibit VSMC
proliferation. Thus, it is possible that TNF-α induces VSMC
proliferation partly via the inhibition of miR-25 expression.
Various previous studies have suggested that cell
proliferation may be caused by dysregulation of cell
cycle-associated proteins, including cyclins, CDKs and CDK
inhibitors (41,42). CDK6 is a member of the family of
serine-threonine kinases, which predominantly mediate the
regulation of cell cycle progression (43). The CDK6 gene has been observed to
often be amplified or overexpressed in various types of human
cancer, including gastric cancer, Ewing’s sarcoma and lymphoid
malignancies (44–46). A previous study demonstrated that
acetylbritannilactone (ABL) treatment inhibited platelet-derived
growth factor-induced synthesis and proliferation of DNA in
cultured VSMCs. The ABL-mediated inhibition of cell growth was
associated with G1 phase arrest. This was correlated
with a reduction in expression levels of cyclins D1, A and E, and
CDK2, 4 and 6, in addition to an increase in the expression of the
CDK inhibitory protein p21cip1 and enhanced binding of p21cip1 to
CDKs (47). The current study
demonstrated that CDK8 is the target of miR-25 in VSMCs, as
transfection of miR-25 resulted in a substantial reduction of
luciferase activity by the luciferase expression constructs that
carry the target CDK6 fragment compared with the mutant constructs
that lack this site. Furthermore, ectopic expression levels of
miR-25 significantly downregulated the transcription of the CDK6
gene and the expression of CDK6 protein. Thus, decreased expression
of miR-25 in tumor cells is suggested to contribute to the
increasing expression levels of CDK6 at the post-transcriptional
level and in atherosclerotic progression.
In conclusion, the present study suggests that TNF-α
inhibits the expression of miR-25 and overexpression of miR-25
inhibits the proliferation of VSMC via targeting CDK6. These
results aid in the understanding of the pro-atherogenic mechanisms
of TNF-α. miR-25 is suggested to serve an important role in the
proliferation of VSMCs and atherosclerosis induced by TNF-α.
However, in order to further elucidate the precise mechanisms of
the TNF-α-mediated regulation of VSMC proliferation, further
investigation is required.
References
|
1
|
Zeidan A, Purdham DM, Rajapurohitam V,
Javadov S, Chakrabarti S and Karmazyn M: Leptin induces vascular
smooth muscle cell hypertrophy through angiotensin II- and
endothelin-1-dependent mechanisms and mediates stretch-induced
hypertrophy. J Pharmacol Exp Ther. 315:1075–1084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen NX, Kiattisunthorn K, O’Neill KD, et
al: Decreased microRNA is involved in the vascular remodeling
abnormalities in chronic kidney disease (CKD). PloS One.
8:e645582013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis-Dusenbery BN, Wu C and Hata A:
Micromanaging vascular smooth muscle cell differentiation and
phenotypic modulation. Arterioscler Thromb Vasc Biol. 31:2370–2377.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kothapalli D, Castagnino P, Rader DJ,
Phillips MC, Lund-Katz S and Assoian RK: Apolipoprotein E-mediated
cell cycle arrest linked to p27 and the Cox2-dependent repression
of miR221/222. Atherosclerosis. 227:65–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bohlen F, Kratzsch J, Mueller M, et al:
Leptin inhibits cell growth of human vascular smooth muscle cells.
Vascul Pharmacol. 46:67–71. 2007. View Article : Google Scholar
|
|
6
|
Bodary PF, Shen Y, Ohman M, et al: Leptin
regulates neointima formation after arterial injury through
mechanisms independent of blood pressure and the leptin
receptor/STAT3 signaling pathways involved in energy balance.
Arterioscler Thromb Vasc Biol. 27:70–76. 2007. View Article : Google Scholar
|
|
7
|
Beltowski J: Leptin and atherosclerosis.
Atherosclerosis. 189:47–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis R, Pillai S, Lawrence N, Sebti S and
Chellappan SP: TNF-α-mediated proliferation of vascular smooth
muscle cells involves Raf-1-mediated inactivation of Rb and
transcription of E2F1-regulated genes. Cell Cycle. 11:109–118.
2012. View Article : Google Scholar :
|
|
9
|
Gao X, Belmadani S, Picchi A, et al: Tumor
necrosis factor-alpha induces endothelial dysfunction in Lepr(db)
mice. Circulation. 115:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajesh M, Mukhopadhyay P, Haskó G, Huffman
JW, Mackie K and Pacher P: CB2 cannabinoid receptor agonists
attenuate TNF-alpha-induced human vascular smooth muscle cell
proliferation and migration. Br J Pharmacol. 153:347–357. 2008.
View Article : Google Scholar
|
|
11
|
Lee SJ, Kim WJ and Moon SK: TNF-alpha
regulates vascular smooth muscle cell responses in genetic
hypertension. Int Immunopharmacol. 9:837–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rho MC, Ah Lee K, Mi Kim S, et al:
Sensitization of vascular smooth muscle cell to TNF-alpha-mediated
death in the presence of palmitate. Toxicol Appl Pharmacol.
220:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song B, Wang Y, Xi Y, et al: Mechanism of
chemoresistance mediated by miR-140 in human osteosarcoma and colon
cancer cells. Oncogene. 28:4065–4074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu X, Li Z, Shen J, et al: MicroRNA-10b
promotes nucleus pulposus cell proliferation through RhoC-Akt
pathway by targeting HOXD10 in intervetebral disc degeneration.
PloS One. 8:e830802013. View Article : Google Scholar :
|
|
15
|
Hutcheson R, Terry R, Chaplin J, et al:
MicroRNA-145 restores contractile vascular smooth muscle phenotype
and coronary collateral growth in the metabolic syndrome.
Arterioscler Thromb Vasc Biol. 33:727–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martello G, Rosato A, Ferrari F, et al: A
MicroRNA targeting dicer for metastasis control. Cell.
141:1195–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Majid S, Dar AA, Saini S, et al:
MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in
bladder cancer. PloS One. 8:e676862013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu WK, Lee CW, Cho CH, et al: MicroRNA
dysregulation in gastric cancer: a new player enters the game.
Oncogene. 29:5761–5771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brenner B, Hoshen MB, Purim O, et al:
MicroRNAs as a potential prognostic factor in gastric cancer. World
J Gastroenterol. 17:3976–3985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cullen BR: MicroRNAs as mediators of viral
evasion of the immune system. Nat Immunol. 14:205–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rotllan N and Fernández-Hernando C:
MicroRNA regulation of cholesterol metabolism. Cholesterol.
2012:8478492012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen KC and Juo SH: MicroRNAs in
atherosclerosis. Kaohsiung J Med Sci. 28:631–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen LJ, Lim SH, Yeh YT, Lien SC and Chiu
JJ: Roles of microRNAs in atherosclerosis and restenosis. J Biomed
Sci. 19:792012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Chen D, Cao L, et al: MiR-490-3p
modulates the proliferation of vascular smooth muscle cells induced
by ox-LDL through targeting PAPP-A. Cardiovasc Res. 100:272–279.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu ML, Wang JF, Wang GK, et al: Vascular
smooth muscle cell proliferation is influenced by let-7d microRNA
and its interaction with KRAS. Circ J. 75:703–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S and Kang H: miR-15b induced by
platelet-derived growth factor signaling is required for vascular
smooth muscle cell proliferation. BMB Rep. 46:550–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Liu Y, Yi B, et al: MicroRNA-638 is
highly expressed in human vascular smooth muscle cells and inhibits
PDGF-BB-induced cell proliferation and migration through targeting
orphan nuclear receptor NOR1. Cardiovasc Res. 99:185–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gómez-Hernández A, Escribano Ó, Perdomo L,
et al: Implication of insulin receptor A isoform and IRA/IGF-IR
hybrid receptors in the aortic vascular smooth muscle cell
proliferation: role of TNF-α and IGF-II. Endocrinology.
154:2352–2364. 2013. View Article : Google Scholar
|
|
29
|
Jiang F, Jiang R, Zhu X, Zhang X and Zhan
Z: Genipin inhibits TNF-α-induced vascular smooth muscle cell
proliferation and migration via induction of HO-1. PloS One.
8:e748262013. View Article : Google Scholar
|
|
30
|
Kim HH and Kim K: Enhancement of
TNF-alpha-mediated cell death in vascular smooth muscle cells
through cytochrome c-independent pathway by the proteasome
inhibitor. FEBS Lett. 535:190–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Zou C, Han Z, et al: MicroRNA-25
functions as a potential tumor suppressor in colon cancer by
targeting Smad7. Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Razumilava N, Bronk SF, Smoot RL, et al:
miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death
receptor-4 and promotes apoptosis resistance in cholangiocarcinoma.
Hepatology. 55:465–475. 2012. View Article : Google Scholar :
|
|
33
|
Lu D, Davis MP, Abreu-Goodger C, et al:
MiR-25 regulates Wwp2 and Fbxw7 and promotes reprogramming of mouse
fibroblast cells to iPSCs. PloS One. 7:e409382012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su ZX, Zhao J, Rong ZH, Geng WM, Wu YG and
Qin CK: Upregulation of microRNA-25 associates with prognosis in
hepatocellular carcinoma. Diagn Pathol. 9:472014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu FX, Su YL, Zhang H, Kong JY, Yu H and
Qian BY: Prognostic implications for high expression of MiR-25 in
lung adenocarcinomas of female non-smokers. Asian Pac J Cancer
Prev. 15:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Esposito F, Tornincasa M, Pallante P, et
al: Down-regulation of the miR-25 and miR-30d contributes to the
development of anaplastic thyroid carcinoma targeting the polycomb
protein EZH2. J Clin Endocrinol Metab. 97:E710–E718. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Yang C, Wang X, Zhang J, Zhang R and
Liu R: The expression of miR-25 is increased in colorectal cancer
and is associated with patient prognosis. Med Oncol. 31:7812014.
View Article : Google Scholar
|
|
38
|
Wahlquist C, Jeong D, Rojas-Muñoz A, et
al: Inhibition of miR-25 improves cardiac contractility in the
failing heart. Nature. 508:531–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Setyowati Karolina D, Sepramaniam S, Tan
HZ, Armugam A and Jeyaseelan K: miR-25 and miR-92a regulate insulin
I biosynthesis in rats. RNA Biol. 10:1365–1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: MiR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.
|
|
41
|
Grossel MJ and Hinds PW: Beyond the cell
cycle: a new role for Cdk6 in differentiation. J Cell Biochem.
97:485–493. 2006. View Article : Google Scholar
|
|
42
|
Pauls E, Ruiz A, Badia R, et al: Cell
cycle control and HIV-1 susceptibility are linked by CDK6-dependent
CDK2 phosphorylation of SAMHD1 in myeloid and lymphoid cells. J
Immunol. 193:1988–1997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kohrt D, Crary J, Zimmer M, et al: CDK6
binds and promotes the degradation of the EYA2 protein. Cell Cycle.
13:62–71. 2014. View Article : Google Scholar :
|
|
44
|
Wu J, Qian J, Li C, et al: miR-129
regulates cell proliferation by downregulating Cdk6 expression.
Cell Cycle. 9:1809–1818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dauphinot L, De Oliveira C, Melot T, et
al: Analysis of the expression of cell cycle regulators in Ewing
cell lines: EWS-FLI-1 modulates p57KIP2and c-Myc expression.
Oncogene. 20:3258–3265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kollmann K and Sexl V: CDK6 and p16INK4A
in lymphoid malignancies. Oncotarget. 4:1858–1859. 2013.PubMed/NCBI
|
|
47
|
Liu B, Han M, Sun RH, Wang JJ, Liu YP and
Wen JK: Acetylbritannilactone induces G1 arrest and apoptosis in
vascular smooth muscle cells. Int J Cardiol. 149:30–38. 2011.
View Article : Google Scholar
|