Introduction
The multipotent nature of bone marrow-derived
mesenchymal stem cells (BMSCs) suggests that they may be
potentially applicable to clinical cell therapy. However, the
limited number of BMSCs, which constitute ~0.001% of the total bone
marrow cells, presents a challenge for future stem cell therapy
using these cells (1,2).
Chlorogenic acid (CGA) is a chemical monomer with
antitumor effects, which is present in various types of plants,
including Eucommia (3). CGA
also prevents obesity by inhibiting the release of glucose into the
blood (4). A previous study
demonstrated that CGA increases the phosphorylation of Akt in
response to apoptotic stimuli (5).
Shp2 is a cytoplasmic tyrosine phosphatase and is a critical
controlling factor in the interaction of the phospohinositide
3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)
signaling pathways (6,7). Shp2-mediated molecular signaling
pathways, including extracellular signal-regulated kinases (Erk)1/2
and PI3K/Akt, are associated with the regulation of stem cell
differentiation and proliferation (8,9).
The present study investigated the mechanisms
underlying CGA-induced BMSC proliferation and inhibition of
adipocyte differentiation. To the best of our knowledge, the
mechanisms underlying CGA-induced BMSC proliferation and inhibition
of adipocyte differentiation have not previously been reported. The
preent study aimed to elucidate whether, in response to treatment
with CGA, the Shp2, PI3K/Akt and cyclin D1 signaling pathways were
involved in BMSC proliferation, and the Shp2, MAPK/Erk, peroxisome
proliferator-activated receptor (PPAR)γ and CCAAT/enhancer binding
protein (C/EBP)α pathways were involved in the inhibition of
adipocyte differentiation. The results may aid the determination of
whether CGA may offer potential benefits in tissue engineering and
provide a novel theoretical basis for innovative strategies in
future clinical applications; and furthermore, may clarify the
mechanisms underyling the effects of CGA in the prevention and
treatment of obesity.
Materials and methods
Cell culture
Bone marrow aspirates were obtained from iliac crest
biopsies of two healthy donors (male, 26 years old, left calcaneus
fracture and female, 32 years old, right plateau fracture, required
open reduction and internal fixation, bone graft) during routine
surgical procedures. The bone marrow was obtained during the bone
graft procedures. The present study was approved by the Ethical
Committee of the Second Affiliated Hospital of Nanchang University
(Nanchang, China) and all the patients provided informed consent.
Mononucleated cells were isolated from the bone marrow by density
gradient centrifugation at 1,000 × g for 15 min using human
lymphocyte separation medium (density 1.077±0.0001 g/ml; Beijing
Solarbio Science & Technology, Co., Ltd., Beijing, China). The
cells were subsequently seeded and grown in Low Glucose Dulbecco’s
modified Eagle’s medium (DMEM; Hyclone Laboratories, Inc., Logan,
UT, USA), supplemented with 15% fetal bovine serum (FBS; Hyclone
Laboratories, Inc.), at 37°C in a humidified atmosphere containing
5% CO2/95% air. BMSCs were observed under a phase
contrast microscope (IX71; Olympus Corp., Guangzhou, China).
To induce adipogenesis, the BMSCs were seeded at a
density of 1×104 cell/cm2 in 24-well plates
(Corning Incorporated, Corning, NY, USA). Following a 24 h culture,
the cells were treated with adipogenic medium, consisting of growth
medium supplemented with StemPro® Adipogenesis
Differentiation kit (Gibco Life Technologies, Carlsbad, CA, USA).
After 14 days, the adipogenesis was visualized by light microscopy
(IX71; Olympus Corp.), with or without staining by Oil Red O (Bogu
Biotechnology Co., Ltd, Shanghai, China), as described previously
(10,11). Subsequently, the BMSCs were treated
with 0, 0.1, 1, 10 or 100 μM CGA (#C3878; Sigma-Aldrich, St.
Louis, MO, USA) for 15 min, and the expression levels of markers,
including Erk1/2, PPARγ and CEBPα, were assessed by reverse
transcription quantitative polymerase chain reaction (RT-qPCR) and
western blotting. Furthermore, the expression levels of the
signaling pathways, which regulate cell proliferation, including
PI3K/Akt and cyclin D1, were determined by RT-qPCR and western
blotting in the BMSCs treated with 0.1, 1, 10 or 100 μM CGA
(treated group) or 0 μM CGA (control group) for 48 h.
Western blot analysis
The cells were washed twice with phosphate-buffered
saline (PBS; Hyclone; Thermo Fisher Scientific, Rockford, IL, USA)
and then lysed on ice with radioimmunoprecipitation buffer (50 mM
Tris, 150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate and 0.1%
SDS; pH 7.4) supplemented with 10 μl/ml protease inhibitor
cocktail (cat. no. 11836170001) and PhosStop (one tablet per 10 ml;
cat no. #4906845001; Roche, Nutley, NJ, USA). The protein content
was quantified using a Bicinchoninic Acid Protein Assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA), according to the
manufacturer’s instructions. For western blotting, 30–50 μg
of the protein samples were separated by 10% SDS-PAGE
[H2O, 30% acrylamide, 1.5MTris-HCl (pH 8.8), 10% SDS,
10% ammonium persulfate and tetramethylethylenediamine] and
transferred onto polyvinylidene difluoride membranes (Ruiqi
Biotechnology Co., Ltd, Shanghai, China). The membranes were then
incubated for 8–12 h at 4–8°C with the following primary
antibodies, purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA): Rabbit polyclonal phosphorylated (p)-SHP-2
(Tyr580; cat. no. #5431), rabbit monoclonal p-p44/42 MAPK
(T202/Y204; D13.14.4E; cat. no. 4370S), rabbit monoclonal p-Akt
(ser473; cat. no. 4060S), mouse monoclonal cyclin D1 (cat. no.
2926), and Santa Cruz Biotechnology, Inc.: Rabbit polyclonal
SH-PTP2 (cat. no. sc-280), rabbit polyclonal Akt1/2/3 (H-136; cat.
no. sc-8312) and mouse monoclonal β-Actin (C4; cat. no. sc-47778).
Primary antibodies (1:1,000) and horseradish-peroxidase-conjugated
secondary antibodies (1:1,000; HRP-labeled Goat Anti-Rabbit
IgG(H+L), A0208 and HRP-labeled Goat Anti-Mouse IgG(H+L), A0216,
Beyotime, Nanjing, China) were diluted with Tris-buffered
saline-0.1% Tween® 20 (100 mmol/l Tris HCl, pH 7.5; 150
mmol/l NaCl), containing 5% bovine serum albumin (#A7906;
Sigma-Aldrich). Membranes were incubated with secondary antibodies
for 1–2 h at room temperature (12). The immunoreactive signals were then
visualized using the Enhanced Chemiluminescence Plus system
(Zhongshan Biotechnology Co., Ltd, Beijing, China).
Inhibitor studies and small interfering
(si)RNA-mediated suppression of Shp2
An ERK inhibitor (10 μM PD98059; #9900; Cell
Signaling Technology, Inc.) and PI3K inhibitor (0.1 μM
Ly294002; #S1105; Selleck, Shanghai, China) were added to the cells
(1×105 cells/ml) at 37°C, 2 h prior to the addition of
CGA. siRNA specific to Shp2, forward 5′-GAAUAUGGCGUCAUGCGUGTT-3′
and reverse 5′-CACGCAUGACGCCAUAUUCTT-3′ (13) and scrambled siRNA, forward
5′-AGUUAUAAGGCGGUCGUGCTT-3′ and reverse 5′-GCACGACCGCCUUAUAACUTT-3′
were synthesized by Invitrogen Life Technologies (Carlsbad, CA,
USA). The siRNAs were transfected into the BMSCs using
Lipofectamine® 2000 reagent (Invitrogen Life
Technologies) (14) for 6 h, and
the cells were recovered in DMEM supplemented with 10% FBS. The
cells were then harvested and reseeded at 1×105 cells/ml
in 96-well plates for subsequent bromodeoxyuridine (BrdU) and
RT-qPCR assays.
BrdU assay
The BrdU assay was performed using a Cell
Proliferation kit (cat. no. #2750; Merck Millipore, Darmstadt,
Germany), according to the manufacturer’s instructions. The
incorporation of BrdU was detected by horseradish-peroxidase
substrate (#S131125; Tiandz, Inc., Beijing, China) and the color
intensity was measured at 450 nm (ELx800; Bio-Tek Instruments,
Inc., Winooski, VT, USA).
RT-qPCR
RT-qPCR was performed, as described in our previous
study (5). The PCR conditions used
were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for
20 sec, 52°C for 30 sec and 60°C for 30 sec. The results were
analyzed using the cycle threshold (Ct) relative quantification
method (ΔΔCt), using the iQ5 Optical System Software
version 2.0 (Bio-Rad Laborotories, Inc., Hercules, CA, USA). The Ct
values were obtained using ABI 7300 software (Applied Biosysystems
Life Technologies, Foster City, CA, USA). The fold change of the
relative expression of mRNA was determined using the
2−ΔΔCt method (15).
Flow cytometry
The BMSC suspensions were washed twice with PBS. For
direct assays, 5×104 cells were incubated with 1 mg/ml
(1/1,000 dilution) fluorescein isothiocyanate-conjugated CD29, CD90
and CD105, or phycoerythrin-conjugated CD34, CD45 and CD166
(#ab81289, #ab10925 and #ab93758; Abcam, Shanghai, China) in the
dark for 15 min, and were analyzed by flow cytometric analysis
using a fluorescence-activated cell sorting (FACS)Calibur flow
cytometer (BD Biosciences, San Jose, CA, USA) with the use of
CellQuest software (TreeStar.FlowJo.v5.7; BD Biosciences, San Jose,
CA, USA).
Statistical analysis
All the data are expressed as the mean ± standard
deviation. One-way analysis of variance, followed by Student’s
t-test were used to determine statistically significant differences
between the experimental and control groups. Statistical analyses
were conducted using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference (5).
Results
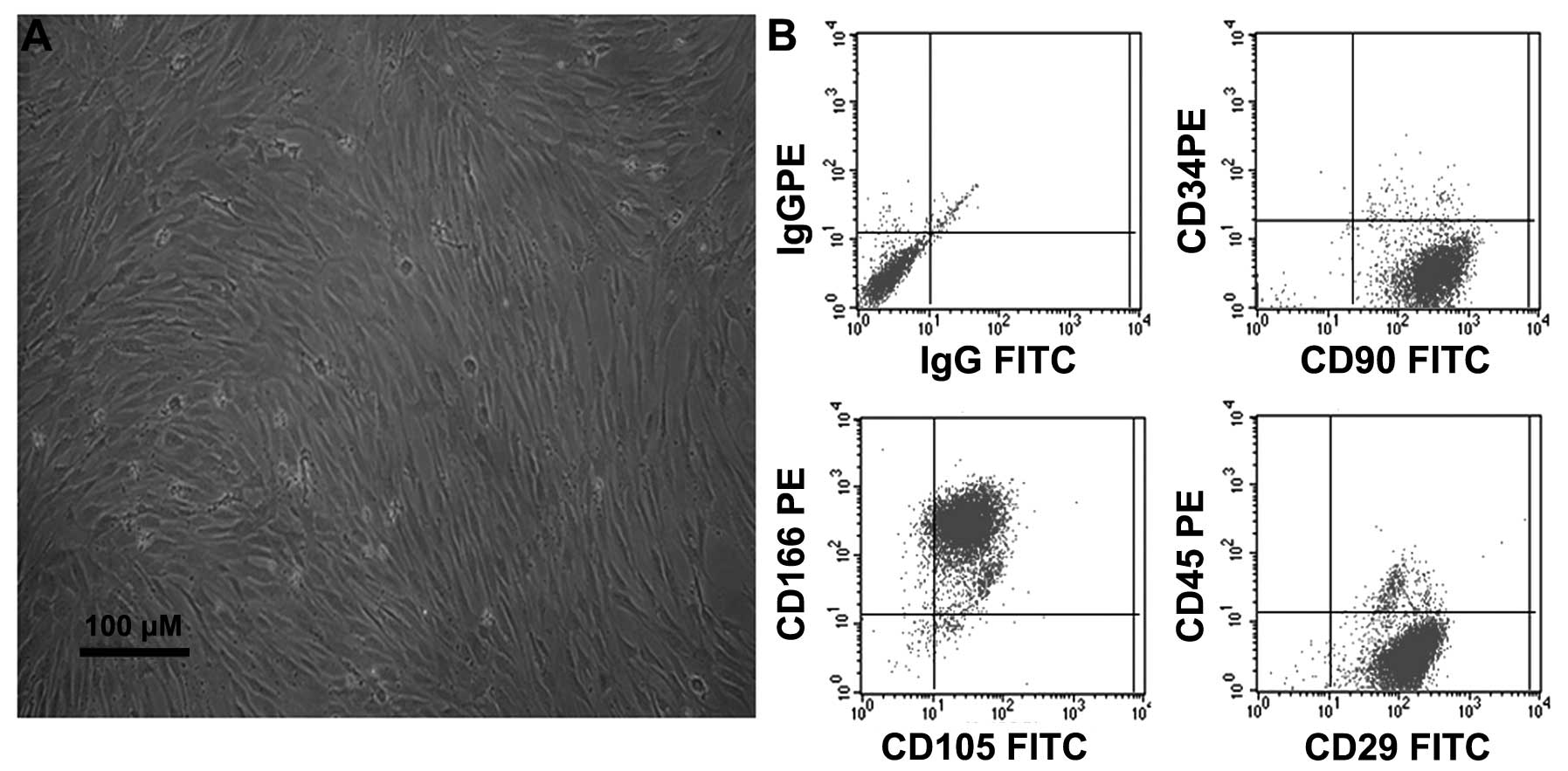
Characterization of undifferentiated
BMSCs
Following 2–3 days of culture, the non-adherent
cells were removed by replacing the low glucose DMEM, and the
attached nucleated cells were visualized. These cells displayed an
elongated fibroblast-like morphology under phase-contrast
microscopy (Fig. 1A). Furthermore,
the immunophenotypes of the expanded clonal BMSCs were analyzed by
FACS. The BMSCs expressed the CD29, CD90, CD105 and CD166 markers,
but not the CD34 or CD45 markers (Fig.
1B). This phenotype remained unchanged for >20 passages,
consistent with the results observed in a previous study by our
group (5).
Treatment with CGA induces the
proliferation of BMSCs via PI3K/Akt/cyclin D1 signaling
The effects of CGA on the proliferation of BMSCs was
investigated using a BrdU assay. Cell proliferation was
significantly enhanced following treatment with CGA at doses
between 0.1 and 10 μM, compared with the control group and
the group treated with 100 μM CGA (Fig. 2A). Cell death was observed
following treated of the cells with 100 μM CGA. To determine
the mechanism involved in the proliferation of BMSCs, the effects
of CGA on the growth of BMSCs, cultured in phenol red-free medium
containing 1% FBS, were investigated, at 48 h. Alterations in the
phosphorylation of Akt and the expression of cyclin D1 in response
to stimulation with CGA, were determined. The phosphorylation of
Akt and the protein expression levels of cyclin D1 were increased
in response to treatment with CGA at concentrations of 0.1, 1 and
10 μM (Fig. 2B); however,
the phosphorylation of Erk was not enhanced by treatment with CGA
at these concentrations (data not shown). In addition, the
inhibition of PI3K/Akt, by the specific inhibitor, LY294002,
reduced the proliferation of cells and deceased the expression
levels of cyclin D1 (Fig. 2C and
D).
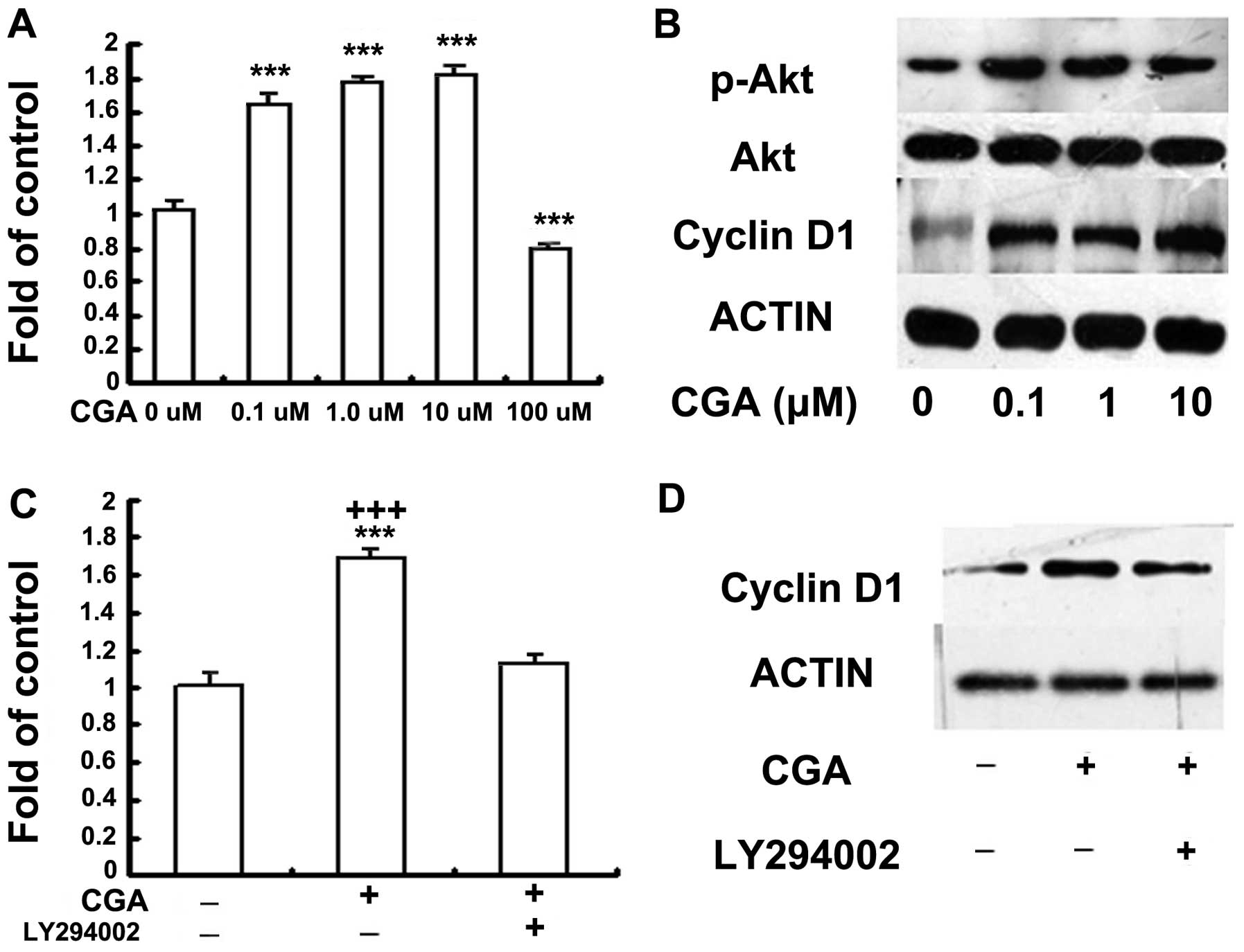 | Figure 2(A) Effects of CGA (0.1–100
μM) on the proliferation of BMSCs was investigated using a
BrdU assay. BMSCs were seeded with serum-free medium, without
phenol red, and starved for 6 h, followed by treatment with 0, 0.1,
1, 10 or 100 μM CGA for 48 h. The proliferation rate was
determined by measuring fluorescence intensity at 450 nm. BrdU
results (means ± standard error; n=3) indicate cellular BrdU
incorporation into DNA in arbitrary units. (B) BMSCs were seeded
with serum-free medium (1%) without phenol red, and starved for 6
h, followed by treatment with 0, 0.1, 1.0 or 10 μM CGA for
48 h. The protein expression levels of phosphorylated Akt and
cyclin D1 were detected by western blotting. (C) BMSCs were serum
deprived (1%) without phenol red, for 6 h and subsequently cultured
in 1 μM CGA in the presence or absence of LY294002 (20
μM) for 48 h, 2 h prior to stimulation with CGA.
Proliferation was determined by measuring fluorescence intensity at
450 nm. (D) BMSCs were serum deprived (1%) without phenol red, for
6 h and subsequently cultured in 1 μM CGA in the presence or
absence of LY294002 (20 μM) treatment for 48 h, 2 h prior to
stimulation with CGA. The protein expression levels of p-Akt and
cyclin D1 were detected by western blotting. BMSCs, bone
marrow-derived stem cells; CGA, chlorogenic acid; BrdU,
bromdeoxyuridine; p-, phosphorylated. |
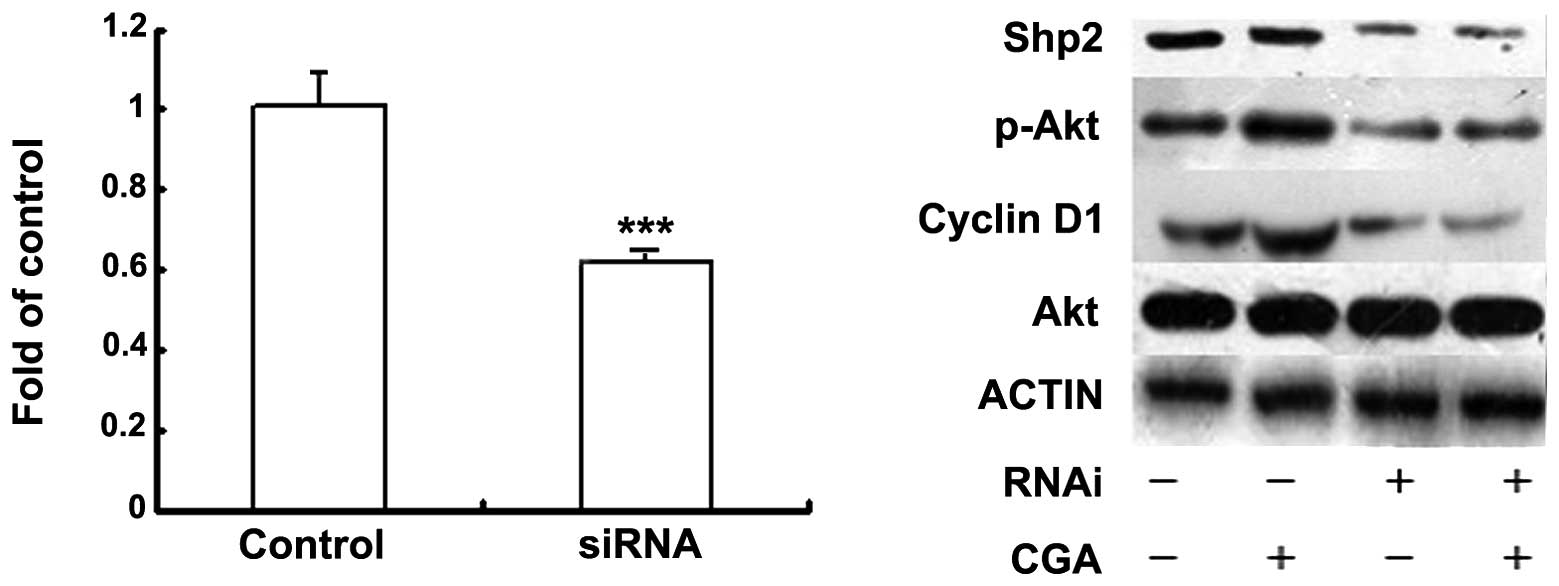
Shp2 is a regulator of CGA/PI3K/Akt and
induces BMSC proliferation
Shp2 is a major signal cytoplasmic tyrosine
phosphatase and contains two SH2 domains upstream of
PI3K/Akt/cyclin D1, which have been implicated in mitogenic
responses (16). Therefore, the
present study investigated whether Shp2 was also a regulator of
CGA-induced BMSC proliferation. The expression levels of Shp2 were
suppressed in the BMSCs, which were transfected with siRNA. The
proliferation of BMSCs was inhibited and activation of the
PI3K/Akt/cyclin D1 pathways was induced by the combination of siRNA
and Shp2 (10 nM), compared with the mock-transfected cells
(Fig. 3).
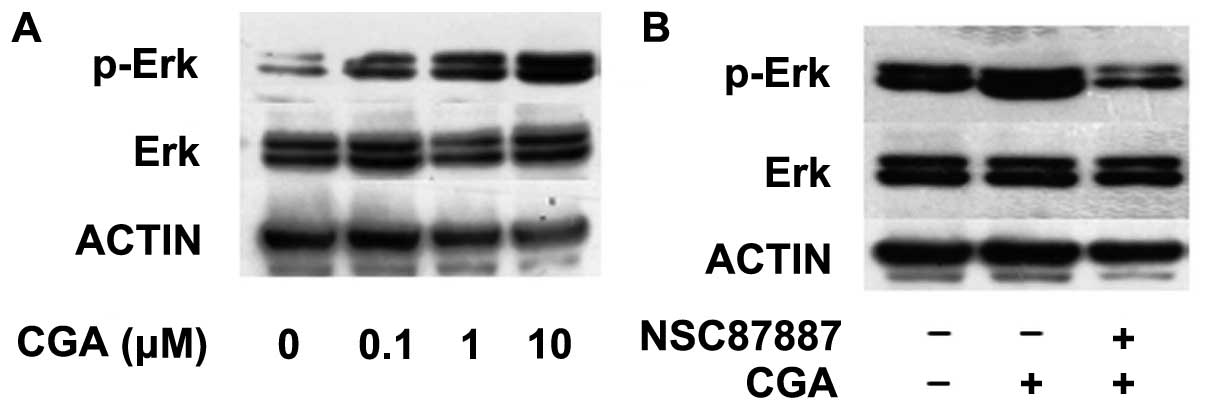
CGA-induced regulation of BMSC
differentiation occurs via Shp2/Erk signaling
The activation or inhibition of the PI3K/Akt and Erk
signaling pathways has previously been observed to regulate the
adipogenic differentiation of pre-adipocyte cell lines (17,18).
However, the role of CGA in the adipogenesis of BMSCs and the
underlying mechanisms remain to be elucidated. The present study
assessed the effect of Shp2 inhibition on the Erk and Akt signaling
pathways on BMSC adipogenic differentiation, in the absence or
presence of CGA. The phosphorylation of Erk was significantly
enhanced following treatment with CGA, at a dose between 0.1 and 10
μM, compared with the control group at 15 min (Fig. 4A); however, this effect was
inhibited by treatment with the NSC-87877 Shp2 PTPase activity
inhibitor (Fig. 4B).
Notably, treatment with NSC87887 and PD98059 had
little effect on the adipogenesis of BMSCs in differentiation
medium, however, increases in the expression levels of PPARγ and
CEBPα were detected (Fig. 5A). The
addition of LY294002 to the differentiation medium decreased the
number of lipid-containing adipocytes, and significantly inhibited
the expression levels of PPARγ and CEBPα (Fig. 5A). The activation of CGA and
inhibition of Akt resulted in reduced adipogenic differentiation of
the BMSCs (Fig. 5B). These results
suggested that other downstream pathways, rather than Akt, were
involved. As shown in Fig. 5,
NSC87887 and PD98059 inhibited the inhibition of adipogenic
differentiation of BMSCs in the presence of CGA. Therefore,
treatment with CGA inhibited the adipogenic differentiation of
BMSCs and the expression of PPARγ and CEBPα by enhancing the
phosphorylation of Erk, but not Akt.
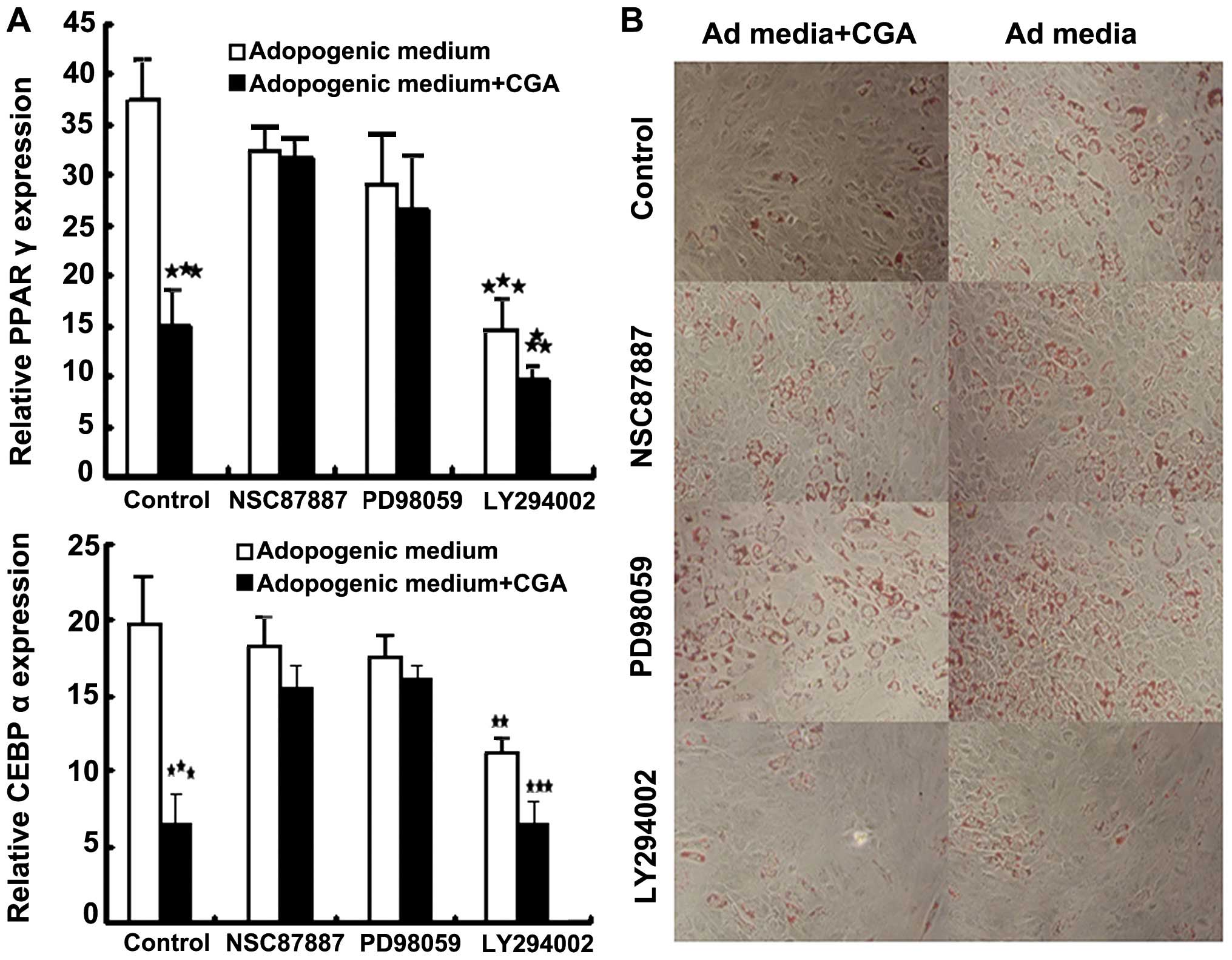 | Figure 5Bone marrow-derived stem cells were
incubated with Ad medium, with or without LY294002 (20 μM),
PD98059 (20 μM) or NSC87887 (10 μM), in the presence
or absence of 1 μM CGA for 7 days. (A) mRNA expression
levels of the adipogenic markers, PPARγ and CEBPα, were analyzed by
reverse transcription quantitative polymerase chain reaction (mean
± standard error of the mean of three experiments of duplicates).
**P<0.01 and ***P<0.001, compared with
the cells cultured in differentiation medium alone. (B) In order to
assess phenotypic changes, the cells were differentiated for a
further 7 days. Lipid accumulation in the treated and untreated
populations was visualized by Oil Red O staining (magnification,
×100). CGA, chlorogenic acid; CEBPα, CCAAT/enhancer binding
protein-α; PPARγ; peroxisome proliferator-activated receptor-γ; Ad,
adipogenic. |
Discussion
MSCs have received significant attention due to
their various applications in regenerative medicine. Understanding
the pathways controlling the fate of MSCs is likely to benefit
in vivo and in vitro tissue engineering approaches.
MSCs isolated from aged animals and humans exhibit reduced
proliferative and differentiation capacities (19). In addition, the rate of malignancy
is higher in aged stem cells and the molecular pathways underlying
tumorigenesis and proliferation of stem cells are the same
(20–22). CGA, an antioxidant and metal
chelator, has been reported to have antimicrobial (23), anti-inflammatory (24) and glycemic (25,26)
effects. Furthermore, the preventive role of CGA in human colon
cancer (27), oral tumor cell
lines (28), and murine
preadipocytes (29) has been
demonstrated.
The present study revealed that treatment with CGA
promoted the proliferation of BMSCs and inhibited the adipocyte
differentiation of BMSCs, of which Shp2 was identified as a key
regulator. Major signaling pathways, including PI3K/Akt and cyclin
D1, but not MEK/Erk, were partly involved in the growth of BMSCs,
as only inhibition of the PI3K/Akt pathway was able to reverse the
stimulatory effects of CGA on MSC proliferation. Notably, only the
activity of Akt was sustained throughout the investigation, whereas
the phosphorylation of Erk was rapid and transient. It is well
known that the duration of these pathways determines the subsequent
physiological response of the cells. Sustained Erk activation by
fibroblast growth factor-2 has been observed to induce the
proliferation of MSCs and dermal fibroblasts (30,31),
whilst the transient activation of Erk has been associated with
cell differentiation (32).
The mechanisms by which the activation of Akt or Erk
leads to cell proliferation or differentiation differ depending on
the type of cell (33,34). The present study demonstrated that
CGA had a proliferative effect on BMSCs by promoting cyclin D1. In
correlation with the cell proliferation data, the stimulation of
regulation by CGA was inhibited by Akt and Shp2 inhibitors.
Although the PI3K/Akt pathway was the principal mediator of
CGA-induced MSC proliferation, it was not involved in the
regulation of CGA-induced MSC differentiation. This was supported
by the observation that LY294002 markedly inhibited the
adipogenesis of MSCs, which was induced by differentiation medium
in the absence or presence of CGA. Furthermore, the addition of CGA
to normal medium induced the expression of the adipogenic ‘master
switch’-PPARγ (35). These results
suggested a role for CGA-induced Akt in the proliferation of MSCs,
but not in cell differentiation. Notably, although Erk was
activated by CGA signaling in the MSCs, it had a negligible effect
on proliferation and was found to be responsible for the action of
adipogenic medium on adipogenic differentiation. The ability of CGA
to inhibit adipogenesis through Erk appeared only when the cells
were induced to differentiate. In the undifferentiated cells, CGA
either upregulated or had no effect on the expression of the genes
involved in adipogenesis. Based on previous data, Akt is required
and sufficient for adipogenesis, suggesting that the upregulation
of Akt may lead to differentiation and proliferation (36,37).
However, the simultaneous activation of Erk inhibits adipogenesis
and maintains cells in an undifferentiated state. Erk signaling has
previously been implicated in adipogenesis and to have a negative
effect on adipocytes; however, stimulation was reported to be
dependent on the upstream signal (11,17,38).
These findings indicate that self-renewal of MSCs requires a
complex interaction between various signaling pathways. In the
present study, the Akt pathway promoted MSC proliferation, and Erk
signaling was required for maintaining cells in an undifferentiated
state, countering possible adipogenic differentiation through
activation of Akt. This may explain why Akt induces adipogenic
differentiation and causes spontaneous differentiation in
preadipocyte 3T3L1 cells when activated either by overexpression of
Akt or by factors, including insulin (17). However, this was not observed for
CGA with MSCs.
In conclusion, the results of the present study
demonstrated that CGA maintained the self-renewal of BMSCs by
inducing proliferation and preventing cell differentiation.
Treatment with CGA simultaneously activated two major transduction
pathways, with dual actions on proliferation and differentiation.
When the BMSCs were treated with CGA, the Shp2, PI3K/Akt and cyclin
D1 signaling pathways were found to be involved in BMSC
proliferation, whilst the Shp2, MAPK/Erk, peroxisome
proliferator-activated receptor (PPAR)γ and CCAAT/enhancer binding
protein (C/EBP)α pathways were involved in the inhibition of
adipocyte differentiation. Therefore, CGA may activate PI3K/Akt, as
the mediator of cell proliferation, and Erk, as the inhibitor of
differentiation, in MSCs. It is clear that the effects of CGA avoid
unwanted differentiation whilst MSCs undergo extensive
proliferation. These findings may contribute to the development of
novel strategies for the regulation of stem cell proliferation and
differentiation, which may be used in future clinical
applications.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81160508, 81260401 and
81160226), and the Natural Science Foundation of Jiangxi Province
(no. GZY0170).
References
|
1
|
Wong KL, Lee KB, Tai BC, Law P, Lee EH and
Hui JH: Injectable cultured bone marrow-derived mesenchymal stem
cells in varus knees with cartilage defects undergoing high tibial
osteotomy: a prospective, randomized controlled clinical trial with
2 years’ follow-up. Arthroscopy. 29:2020–2028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gopal K, Amirhamed HA and Kamarul T:
Advances of human bone marrow-derived mesenchymal stem cells in the
treatment of cartilage defects: a systematic review. Exp Biol Med
(Maywood). 239:663–669. 2014. View Article : Google Scholar
|
|
3
|
Granado-Serrano AB, Martín MA,
Izquierdo-Pulido M, et al: Molecular mechanisms of (−)-epicatechin
and chlorogenic acid on the regulation of the apoptotic and
survival/proliferation pathways in a human hepatoma cell line. J
Agric Food Chem. 55:2020–2027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wan CW, Wong CN, Pin WK, et al:
Chlorogenic acid exhibits cholesterol lowering and fatty liver
attenuating properties by up-regulating the gene expression of
PPAR-α in hypercholesterolemic rats induced with a high-cholesterol
diet. Phytother Res. 27:545–551. 2013. View
Article : Google Scholar
|
|
5
|
Li S, Bian H, Liu Z, et al: Chlorogenic
acid protects MSCs against oxidative stress by altering FOXO family
genes and activating intrinsic pathway. Eur J Pharmacol. 674:65–72.
2012. View Article : Google Scholar
|
|
6
|
Feng GS: Shp2-mediated molecular signaling
in control of embryonic stem cell self-renewal and differentiation.
Cell Res. 17:37–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishida K and Hirano T: The role of Gab
family scaffolding adapter proteins in the signal transduction of
cytokine and growth factor receptors. Cancer Sci. 94:1029–1033.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee TI, Jenner RG, Boyer LA, et al:
Control of developmental regulators by Polycomb in human embryonic
stem cells. Cell. 125:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Craddock BL, Hobbs J, Edmead CE and Welham
MJ: Phosphoinositide 3-kinase-dependent regulation of
interleukin-3-induced proliferation: involvement of
mitogen-activated protein kinases, SHP2 and Gab2. J Biol Chem.
276:24274–24283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gharibi B, Abraham AA, Ham J and Evans BA:
Contrasting effects of A1 and A2b adenosine receptors on
adipogenesis. Int JObes (Lond). 36:397–406. 2012. View Article : Google Scholar
|
|
11
|
Wu L, Cai X, Dong H, et al: Serum
regulates adipogenesis of mesenchymal stem cells via
MEK/ERK-dependent PPARgamma expression and phosphorylation. J Cell
Mol Med. 14:922–932. 2010. View Article : Google Scholar
|
|
12
|
Bai X, Ma D, Liu A, et al: Rheb activates
mTOR by antagonizing its endogenous inhibitor, FKBP38. Science.
318:977–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Y, Caday CG, Nanda A, et al:
Tyrphostin AG 1478 preferentially inhibits human glioma cells
expressing truncated rather than wild-type epidermal growth factor
receptors. Cancer Res. 56:3859–3861. 1996.PubMed/NCBI
|
|
14
|
Zhan Y, Counelis GJ and O’Rourke DM: The
protein tyrosine phosphatase SHP-2 is required for EGFRvIII
oncogenic transformation in human glioblastoma cells. Exp Cell Res.
315:2343–2357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Wu CJ, O’Rourke DM, Feng GS, Johnson GR,
Wang Q and Greene MI: The tyrosine phosphatase SHP-2 is required
for mediating phosphatidylinositol 3-kinase/Akt activation by
growth factors. Oncogene. 20:6018–6025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang HH, Huang J, Düvel K, et al: Insulin
stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS
One. 4:e61892009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Wang Y and Yamashita H: Evodiamine
inhibits adipogenesis via the EGFR-PKCalpha-ERK signaling pathway.
FEBS Lett. 583:3655–3659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roobrouck VD, Ulloa-Montoya F and
Verfaillie CM: Self-renewal and differentiation capacity of young
and aged stem cells. Exp Cell Res. 314:1937–1944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng H, Ying H, Wiedemeyer R, et al:
PLAGL2 regulates Wnt signaling to impede differentiation in neural
stem cells and gliomas. Cancer Cell. 17:497–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu HK, Wang Y, Belz T, et al: The nuclear
receptor tailless induces long-term neural stem cell expansion and
brain tumor initiation. Genes Dev. 24:683–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L and Rando TA: Manifestations and
mechanisms of stem cell aging. J Cell Biol. 193:257–266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Zhang H and Lo R: Phenolic
compounds from the leaf extract of artichoke (Cynara scolymus L.)
and their antimicrobial activities. J Agric Food Chem.
52:7272–7278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang F and Dusting GJ: Natural phenolic
compounds as cardiovascular therapeutics: potential role of their
antiinflammatory effects. Curr Vasc Pharmacol. 1:135–156. 2003.
View Article : Google Scholar
|
|
25
|
Johnston KL, Clifford MN and Morgan LM:
Coffee acutely modifies gastrointestinal hormone secretion and
glucose tolerance in humans: glycemic effects of chlorogenic acid
and caffeine. Am J Clin Nutr. 78:728–733. 2003.PubMed/NCBI
|
|
26
|
Karthikesan K, Pari L and Menon VP:
Antihyperlipidemic effect of chlorogenic acid and
tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem
Biol Interact. 188:643–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Q, Hirose Y, Yoshimi N, et al:
Further investigation of the modifying effect of various
chemopreventive agents on apoptosis and cell proliferation in human
colon cancer cells. J Cancer Res Clin Oncol. 128:539–546. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Y, Kusama K, Satoh K, et al:
Induction of cytoxicity by chlorogenic acid in human oral tumor
cell lines. Phytomedicine. 7:483–491. 2000. View Article : Google Scholar
|
|
29
|
Hsu CL, Huang SL and Yen GC: Inhibitory
effect of phenolic acids on the proliferation of 3T3-L1
preadipocytes in relation to their antioxidant activity. J Agric
Food Chem. 54:4191–4197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaragosi LE, Ailhaud G and Dani C:
Autocrine fibroblast growth factor 2 signaling is critical for
self-renewal of human multipotent adipose-derived stem cells. Stem
Cells. 24:2412–2419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Makino T, Jinnin M, Muchemwa FC, et al:
Basic fibroblast growth factor stimulates the proliferation of
human dermal fibroblasts via the ERK1/2 and JNK pathways. Br J
Dermatol. 162:717–723. 2010. View Article : Google Scholar
|
|
32
|
Choi SC, Kim SJ, Choi JH, et al:
Fibroblast growth factor-2 and -4 promote the proliferation of bone
marrow mesenchymal stem cells by the activation of the PI3K-Akt and
ERK1/2 signaling pathways. Stem Cells Dev. 17:725–736. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gharibi B1, Ghuman MS and Hughes FJ: Akt-
and Erk-mediated regulation of proliferation and differentiation
during PDGFRβ-induced MSC self-renewal. J Cell Mol Med.
16:2789–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Shen X, Wan C, Zhao Q, Zhang L,
Zhou Q and Deng L: Effects of insulin and insulin-like growth
factor 1 on osteoblast proliferation and differentiation:
differential signalling via Akt and ERK. Cell Biochem Funct.
30:297–302. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu C, Luan H, Zhang X, et al: Chlorogenic
acid protects against atherosclerosis in ApoE−/− mice and promotes
cholesterol efflux from RAW264.7 macrophages. PLoS One.
9:e954522014. View Article : Google Scholar
|
|
36
|
Fritzius T and Moelling K: Akt- and
Foxo1-interacting WD-repeat-FYVE protein promotes adipogenesis.
EMBO J. 27:1399–1410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hinoi E, Iezaki T, Fujita H, Watanabe T,
Odaka Y, Ozaki K and Yoneda Y: PI3K/Akt is involved in brown
adipogenesis mediated by growth differentiation factor-5 in
association with activation of the Smad pathway. Biochem Biophys
Res Commun. 450:255–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang M, Wang JJ, Li J, et al: Pigment
epithelium-derived factor suppresses adipogenesis via inhibition of
the MAPK/ERK pathway in 3T3-L1 preadipocytes. Am J Physiol
Endocrinol Metab. 297:E1378–E1387. 2009. View Article : Google Scholar : PubMed/NCBI
|