Introduction
The majority of malignant solid tumors, including
breast cancer, proceed to metastases, which is the most frequent
cause of cancer-associated mortality (1). Metastasis is the ability of cells to
detach from a primary tumor, invade and intravasate into the
lymphatic or blood vessels, disseminate in the lymphatic or
circulatory systems and initiate the development of new tumor cells
in distant organs (2). The
migration and invasion of a cancer cell are two crucial steps in
metastasis, as is proteolytic enzyme degradation of the
extracellular matrix (ECM) (3).
Matrix metalloproteinases (MMPs) are essential for ECM degradation
and remodeling. MMP-9 and MMP-2 are associated with elevated levels
of metastasis in several types of cancer, including breast
carcinoma, lung carcinoma and melanoma (4). Inhibition of the expression of MMP-9
by MMP-9 antisense and small interfering RNA constructs suppresses
the invasiveness and metastatic ability of tumor cells (5,6). The
formation of melanoma and lung carcinoma metastases is reduced in
MMP-9-deficient mice (7) and the
inhibition of MMP-2 and MMP-9 by potential therapeutic agents, such
as snake venom metalloproteinase inhibitor BJ46A, apigenin,
quercetin and resveratrol, reduces cancer metastasis in
vitro and in vivo (8).
Therefore, agents possessing the ability to repress the activation
of MMP-2 or MMP-9 warrant development to take advantage of their
antimetastatic properties.
The phytochemical, dibenzoylmethane (DB), is a
constituent of the root extract of licorice (Glycyrrhiza
inflata of the family Leguminosae) and has been identified as a
promising antimutagenic and anticarcinogenic compound (9–13).
DB inhibits S9-mediated mutagenicity of food-derived heterocyclic
amine mutagens (9). Furthermore,
it decreases the formation of DNA adducts following exposure to
benzo(a) pyrene, 1,6-dinitropyrene and
7,12-dimethylbenz(a)anthracene (DMBA) (10,11).
Dietary DB prevents DMBA-induced mammary carcinogenesis and
azoxymethane/dextran sulfate sodium-induced colon carcinogenesis
(12,13) and deregulation of the cell cycle by
DB has been observed in human prostate carcinoma cells (14). Treatment with DB promotes the
apoptosis of human lung carcinoma cells, epidermoid carcinoma cells
and leukemia cells (15–17). Hydroxydibenzoylmethane (HDB) and
hydroxymethyldibenzoylmethane (HMDB) are important DB analogues,
which are identical in structure to DB with the exception of a
hydroxyl group and hydroxyl and methyl groups on one of the
aromatic rings, respectively. They are more potent than DB in
inhibiting the proliferation and inducing apoptosis in COLO 205
colorectal carcinoma cells, A431 epidermoid carcinoma cells, A549
lung adenocarcinoma cells and CH27 lung squamous carcinoma cells
(15,16,18).
However, whether DB, HDB and HMDB are involved in inhibiting cancer
metastasis remains to be elucidated. The present study aimed to
examine the effects of DB, HDB and HMDB on the metastatic
properties of PMA-treated MCF-7 human breast adenocarcinoma
cells.
Materials and methods
Cell culture and reagents
The MCF-7 human breast adenocarcinoma cell line
(Bioresource Collection and Research Center, Hsinchu, Taiwan) was
routinely grown in RPMI-1640 medium (Biological Industries, Kibbutz
Beit Haemek, Israel), supplemented with 10% fetal bovine serum
(FBS; Biological Industries) at 37°C in an atmosphere of 5%
CO2/95% air under saturating humidity. DB, HDB, HMDB,
phorbol-12-myristate 13-acetate (PMA) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). LY294002
was obtained from Merck Millipore (Darmstadt, Germany). Mouse
monoclonal MMP-2, mouse monoclonal MMP-9, rabbit polyclonal
phosphorylated (phospho)-phosphatidylinositide 3-kinase (PI3K) p85
(Tyr458)/P55 (Tyr199), rabbit polyclonal phospho-protein kinase Cδ
(Thr505) and mouse monoclonal β-actin antibodies were supplied by
Thermo Fisher Scientific, Inc. (Fremont, CA, USA), Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell Signaling
Technology, Inc. (Beverly, CA, USA), respectively.
MTT cell viability assay
The MCF-7 cells (2×104) were seeded into
96-well plates for 24 h prior to treatment with 5, 10, 25, 50 and
100 μM DB, HDB or HMDB, followed by the addition of 10 ng/ml
PMA, for 24 h. Following the exposure period, the cells were
treated with MTT solution (5 μg/ml) for 4 h. The formazan
was solubilized in isopropanol (Merck Millipore) using 0.04 N HCl
(Sigma-Aldrich) and measured spectrophotometrically at 595 nm
(Synergy™ HT Multi-Detection Microplate Reader; BioTek Instruments,
Inc., Winooski, VT, USA).
Migration assay
Cell migration was assessed by a wound-healing assay
using a culture insert (19).
Briefly, the cells were seeded into the culture insert (ibidi GmbH,
Martinsried, Germany) and grown overnight to 100% confluence.
Following removal of the culture insert, a cell-free gap (500
μm) was created and the medium was replaced with 10% FBS
RPMI-1640 medium. The cells were treated with DB, HDB or HMDB at
25, 50 and 100 μM, followed by 10 ng/ml PMA and images of
cell migration from the leading edge were captured and quantified
at 12 h, using an Axio Imager A1 microscope and Axio Vision Rel.
4.7 software (Carl Zeiss, Göttingen, Germany). Each value was
observed from three randomly selected fields and the data are
expressed as the mean number of the migrating cell numbers per
field.
Invasion assay
A cellular invasion assay was performed in a
modified Boyden chamber (GenePure, Taichung, Taiwan) with 8
μm polycarbonate nucleopore filters (Neuro Probe, Inc.,
Gaithersburg, MD, USA) coated with 250 μg/ml Matrigel (BD
Biosciences, San Diego, CA, USA). RPMI-1640 medium containing 2%
FBS was added to the lower compartment of the chamber. The cells
(2.5×104) were resuspended in RPMI-1640 medium without
FBS and added to the upper compartment of the chamber in the
presence or absence of DB, HDB or HMDB at 25, 50 and 100 μM,
or 1 μM rottlerin and 10 μM LY294002, followed by 10
ng/ml PMA for 12 h. Following incubation, the cells were fixed in
methanol (Sigma-Aldrich) for 10 min, stained with Giemsa (Merck
Millipore) for 30 min and washed with H2O for 3 min. The
cells on the upper side of the filters were removed using
cotton-tipped swabs and the cells on the underside of the filters
were observed and counted under a light microscope (Axio Imager
AI).
Gelatin zymography assay
MCF-7 cells were seeded at a concentration of
2×105 cells/ml and incubated for 24 h at 37°C. Following
incubation, the culture medium was replaced with serum-free
RPMI-1640 medium. The cells were treated with 100 μM DB, 50
μM HDB or 25 μM HMDB, followed by 10 ng/ml PMA and
the supernatants were collected and subjected to gel
electrophoresis on an 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gel containing 0.1% gelatin. Following
electrophoresis, the SDS was removed from the gels by incubation
with renaturation buffer (2.7% Triton X-100; Amresco LLC, Solon,
OH, USA) for 1 h. The gels were gently agitated and incubated at
37°C for 24 h in the developing buffer containing 50 mM Tris-HCl
(pH 7.5), 0.2 M NaCl, 0.2% Brij-35 and 5 mM CaCl2 and
stained using coomassie brilliant blue (USB Corporation, Cleveland,
OH, USA) and destained with destaining solution (7% acetic acid, 5%
methanol, Sigma-Aldrich; 88% H2O) for 30 min,
respectively. The proteolytic activity of MMP-9 and MMP-2 were
observed as clear bands against the blue background of the stained
gelatin.
Reverse transcription polymerase chain
reaction (RT-PCR)
The RNA was isolated from the cells using TRIzol
reagent (MDBio, Inc., Piscataway, NJ, USA) according to the
manufacturer’s instructions. The synthesis of complementary DNA
(cDNA) was performed using the extracted total RNA (3 μg),
reverse transcriptase (200 units; Promega Corporation, Madison, WI,
USA) and dT15 primers (0.5 μg; MDBio, Inc.) and the reaction
mixture was incubated for 90 min at 37°C. For the PCR assay, 0.04
μg cDNA was added to mixture buffer containing 75 mM
Tris-HCl (pH 8.8), 20 mM
(NH4)2SO4, 0.01% Tween-20 (v/v), 1
mM MgCl2, 0.2 mM dNTPs, 0.5 μM forward and
reverse primers and 1 unit Taq DNA polymerase (MDBio, Piscataway,
NJ, USA). The PCR was performed as follows: 5 min at 95°C, 36
cycles (30 sec at 5°C; 30 sec at 55°C and 90 sec at 72°C) and 10
min at 72°C using a PC818 program temperature control system
(Astec, Fukuoka, Japan). The PCR products were analyzed on a 2%
agarose gel. The following primer pairs were used: β-actin (309 bp)
forward 5′-AGCGGGAAATCGTGCGTG-3′ and reverse
5′-CAGGGTACATGGTGGTGC-3′; MMP-2 (346 bp) forward
5′-CTTTGACGGTAAGGACGG-3′ and reverse 5′-CTGGAAGCGGAATGGAA-3′ and
MMP-9 (479 bp) forward 5′-CAACATCACCTATTGGATCC-3′ and reverse
5′-CGGGTGTAGAGTCTCTCGCT-3′.
Immunoblotting
To purify the total protein, the cells were
harvested and lysed in cold lysis buffer containing 10% (v/v)
glycerol, 1% (v/v) Triton X-100, 1 mM sodium orthovanadate, 1 mM
EGTA, 10 mM NaF, 1 mM sodium pyrophosphate, 20 mM Tris (pH 7.9),
100 μM β-glycerophosphate, 137 mM NaCl, 5 mM EDTA, 1 mM
PMSF, 10 μg/ml aprotinin and 10 μg/ml leupeptin.
Equal concentrations of protein were separated on SDS-PAGE gels and
then transferred onto polyvinylidene difluoride (PVDF) membranes
(Pall Corporation, Ann Arbor, MI, USA). Following blotting, the
PVDF membranes were incubated with anti-phospho-PKCδ (Thr505),
anti-phospho-PI3K p85 (Tyr458)/anti-phospho-p55 (Tyr199) or
anti-β-actin antibodies (dilutions 1:2,500) for 6 h at 4°C.
Following washing with washing solution [50 mM tris-HCl (pH 7.5),
150mM NaCl, 0.1% Tween-20 (v/v)], the secondary antibody labeled
with horseradish-peroxidase was added for 1 h at 4°C. The targeted
proteins were visualized using enhanced chemiluminescence (Western
Lightning Plus ECL; PerkinElmer, Inc., Waltham, MA, USA).
Statistical Analysis
Data are expressed as the mean ± standard deviation.
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used for statistical analysis. Statistical comparisons
were made by a one-way analysis of variance, followed by Dunnett’s
multiple-comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
DB, HDB and HMDB reduce PMA-induced MCF-7
cancer cell migration and invasion
To determine whether DB and its analogues were
involved in inhibiting the PMA-induced migration of the MCF-7
cells, the cells were treated with PMA and/or DB, HDB or HMDB and
migration was assessed using a wound-healing assay. Treatments with
DB, HDB and HMDB significantly inhibited PMA-induced cell migration
(Fig. 1). Treatment with 25, 50
and 100 μM DB reduced the migration of the cancer cells by
21, 60 and 84%, respectively, compared with those treated with PMA
only. PMA-induced MCF-7 cell migration was reduced by 44, 79 and
90% following exposure to 25, 50 and 100 μM HDB,
respectively, which was more markedly inhibited to 67, 79 and 92%
following treatment with 25, 50 and 100 μM HMDB,
respectively, compared with those treated with PMA only. To undergo
invasion, cancer cells promote the degradation of the extracellular
matrix in order to cross it (3).
Therefore, the effect of DB, HDB or HMDB on PMA-induced MCF-7
cancer cell invasion was determined using an invasion chamber
assay. The MCF-7 cells were seeded onto Matrigel-coated filters in
the presence of DB, HDB or HMDB at 25, 50 and 100 μM and the
number of cells, which invaded through the Matrigel were counted
following incubation for 12 h. Treatments with DB, HDB and HMDB
significantly inhibited PMA-induced cell invasion (Fig. 2). Treatment with 25, 50 and 100
μM DB reduced the cancer cell invasion by 1, 32 and 68%,
respectively, compared with the PMA-only-treated cells. The
PMA-induced MCF-7 cell invasion was reduced by 33, 81 and 90%
following exposure to 25, 50 and 100 μM HDB, respectively,
and was significantly inhibited to 57, 75 and 98% following
treatment with 25, 50 and 100 μM of HMDB, respectively,
compared with the PMA-only group. On the basis of these results, it
was concluded that DB, HDB and HMDB inhibited the migration and
invasion of the MCF-7 cells.
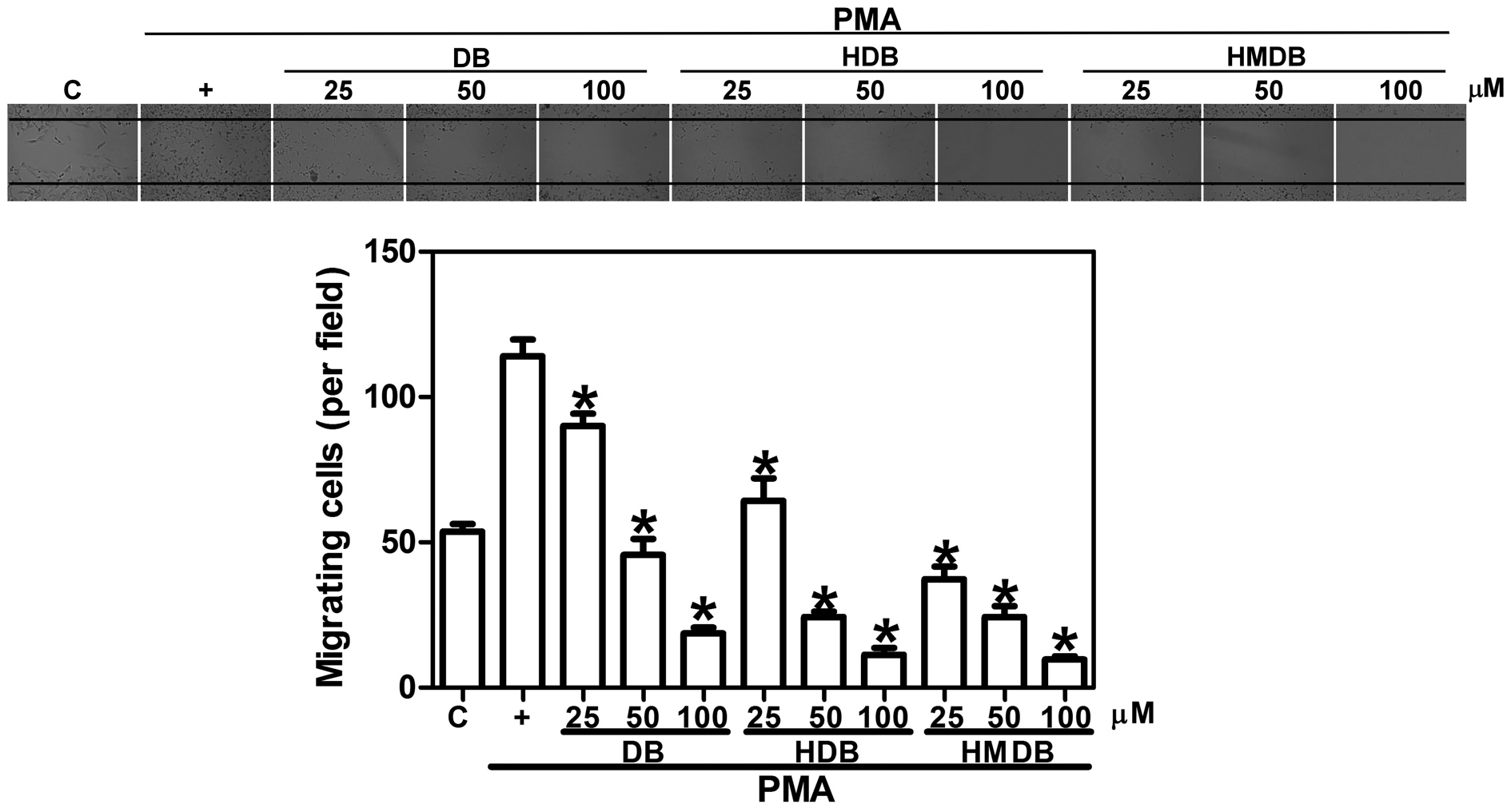 | Figure 1Effects of DB and its analogues, HDB
and HMDB, on MCF-7 breast cancer cell migration. The MCF-7 cells
were treated with various concentrations of DB, HDB and HMDB,
followed by 10 ng/ml PMA for 12 h. Images of the migrating cells
were captured using phase contrast microscopy (magnification,
x100). The quantification data are expressed as the mean ± standard
deviation of three independent experiments. *P<0.05,
compared with the PMA only group. DB, dibenzoylmethane; HDB,
hydroxydibenzoylmethane; HMDB, hydroxymethyldibenzoylmethane; PMA,
phorbol-12-myristate 13-acetate; C, control. |
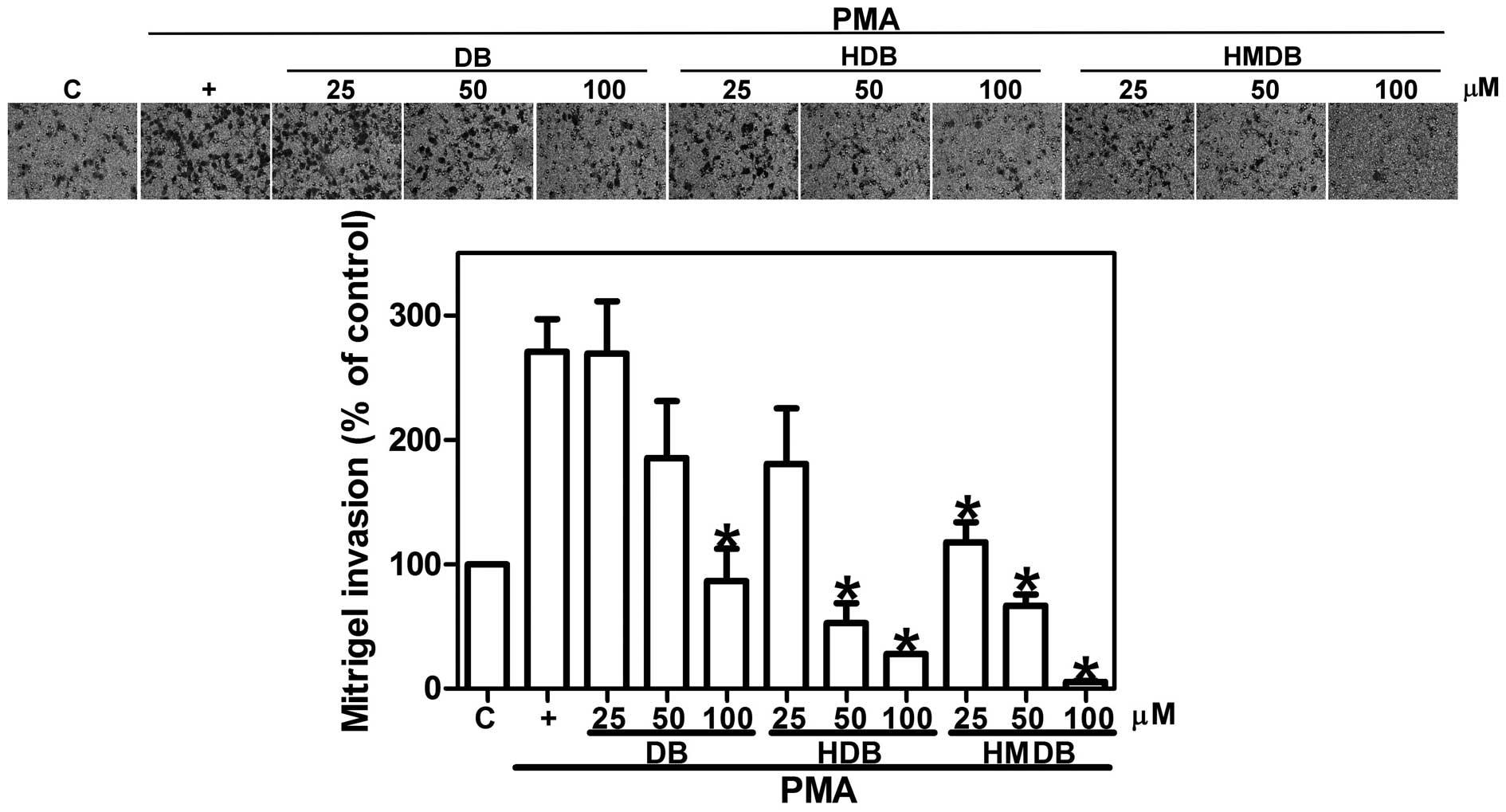 | Figure 2Effects of DB, HDB and HMDB on MCF-7
breast cancer cell invasion. The MCF-7 cells were treated with
various concentrations of DB, HDB and HMDB, followed by 10 ng/ml
PMA for 12 h. The invasive ability was assessed using a
Matrigel-coated in vitro invasion assay, as described
(magnification, x200). The quantification data are expressed as the
mean ± standard deviation of three independent experiments.
*P<0.05, compared with the PMA only group. DB,
dibenzoylmethane; HDB, hydroxydibenzoylmethane; HMDB,
hydroxymethyldibenzoylmethane; PMA, phorbol-12-myristate
13-acetate; C, control. |
Effects of DB, HDB and HMDB on the
viability of the PMA-treated MCF-7 cells
To determine the cytotoxicity of DB and its
analogues, HDB and HMDB, on the PMA-mediated MCF-7 cancer cells,
the cells were treated with different concentrations (5, 10, 25, 50
and 100 μM) of DB, HDB or HMDB followed by the addition of
PMA for 24 h. The cellular viability was then assessed using an MTT
assay. The proportional viability (%) of the cells was determined
by comparing each treated group with the PMA-only group, the
viability of which was assumed to be 100% (Fig. 3). Treatment of the MCF-7 cells with
DB, up to a maximal concentration of 100 μM, caused no
significant change in cell viability. The cells had significantly
lower viability following treatment with 100 μM HDB and 50
μM HMDB. Therefore, the antimetastatic effects of DB, HDB
and HMDB were not associated with cell viability.
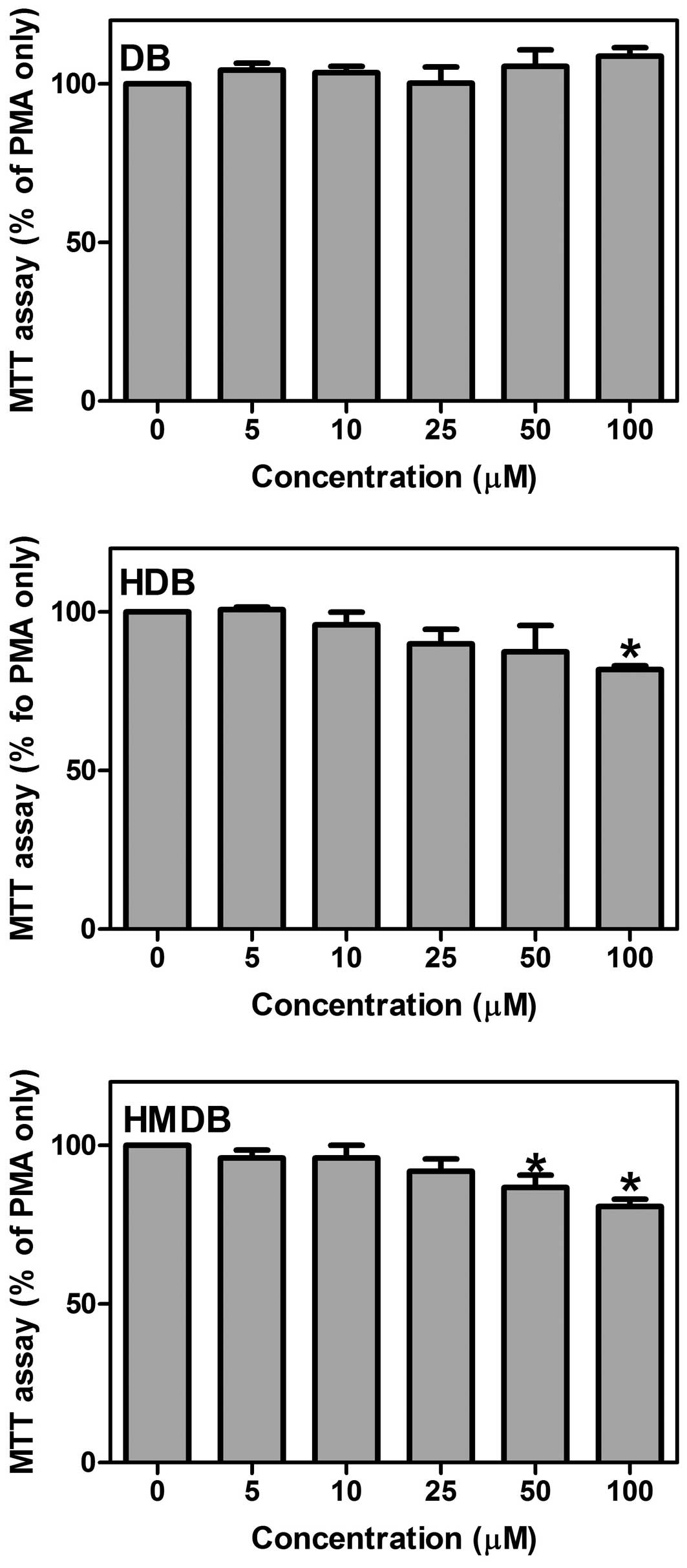 | Figure 3Effects of DB, HDB and HMDB on MCF-7
breast cancer cell viability following PMA treatment. The MCF-7
cells were treated with various concentrations of DB, HDB and HMDB,
followed by the addition of 10 ng/ml PMA for 24 h. The cell
viability was assessed using an MTT assay. The quantification data
are expressed as the mean ± standard deviation of three independent
experiments. *P<0.05, compared with the PMA only
group. DB, dibenzoylmethane; HDB, hydroxydibenzoylmethane; HMDB,
hydroxymethyldibenzoylmethane; PMA, phorbol-12-myristate
13-acetate; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
DB and its analogues inhibit the
PMA-induced expression of MMP-9
The MMPs are a family of extracellular matrix
degrading enzymes, which are associated with cancer cell
metastasis. The type IV collagenases and gelatinases, MMP-9 and
MMP-2, have been linked with high metastatic potential in breast
carcinoma (20). Therefore, the
effects of DB, HDB and HMDB on the enzyme activities and the gene
expression levels of MMP-2 and MMP-9 were examined. The MCF-7 cells
were treated with various concentrations of DB, HDB or HMDB
followed by treatment with PMA in serum-free medium. The enzyme
activities of the secreted MMP-2 and MMP-9 were assayed by gelatin
zymography. As shown in Fig. 4A,
DB, HDB and HMDB inhibited the enzyme activity of MMP-9, but not
MMP-2, in a time-dependent manner. To determine whether DB, HDB and
HMDB can inhibit the enzymatic activity of the secreted MMP-9
through the direct inhibition of these enzymes, various
concentrations of DB, HDB and HMDB were incubated with conditioned
medium derived from PMA-treated MCF-7 cells. The enzymatic
activities of MMP-9 and MMP-2 were assessed using gelatin
zymography. As shown in Fig. 4B,
MMP-9 and MMP-2 activity was detected in the conditioned media, and
no significant differences were observed between the groups with or
without treatment. Subsequently, to determine whether the
inhibition of the enzymatic activities of MMP-9 and MMP-2 by DB,
HDB or HMDB was due to a decreased level of mRNA transcription and
protein synthesis, immunoblotting and RT-PCR were performed to
examine the protein and mRNA expression levels of MMP-9 and MMP-2.
As shown in Fig. 4C, DB, HDB and
HMDB reduced the protein expression of the PMA-induced MMP-9,
however no changes in the protein or mRNA expression of MMP-2 were
observed. MMP-9 inhibitor I, a cell-permeable and reversible
inhibitor of MMP-9, deceased the PMA-induced MCF-7 cell invasion
(Fig. 4D). These results
demonstrated that DB, HDB and HMDB disrupted the mRNA transcription
and protein synthesis of MMP-9 in PMA-mediated metastasis.
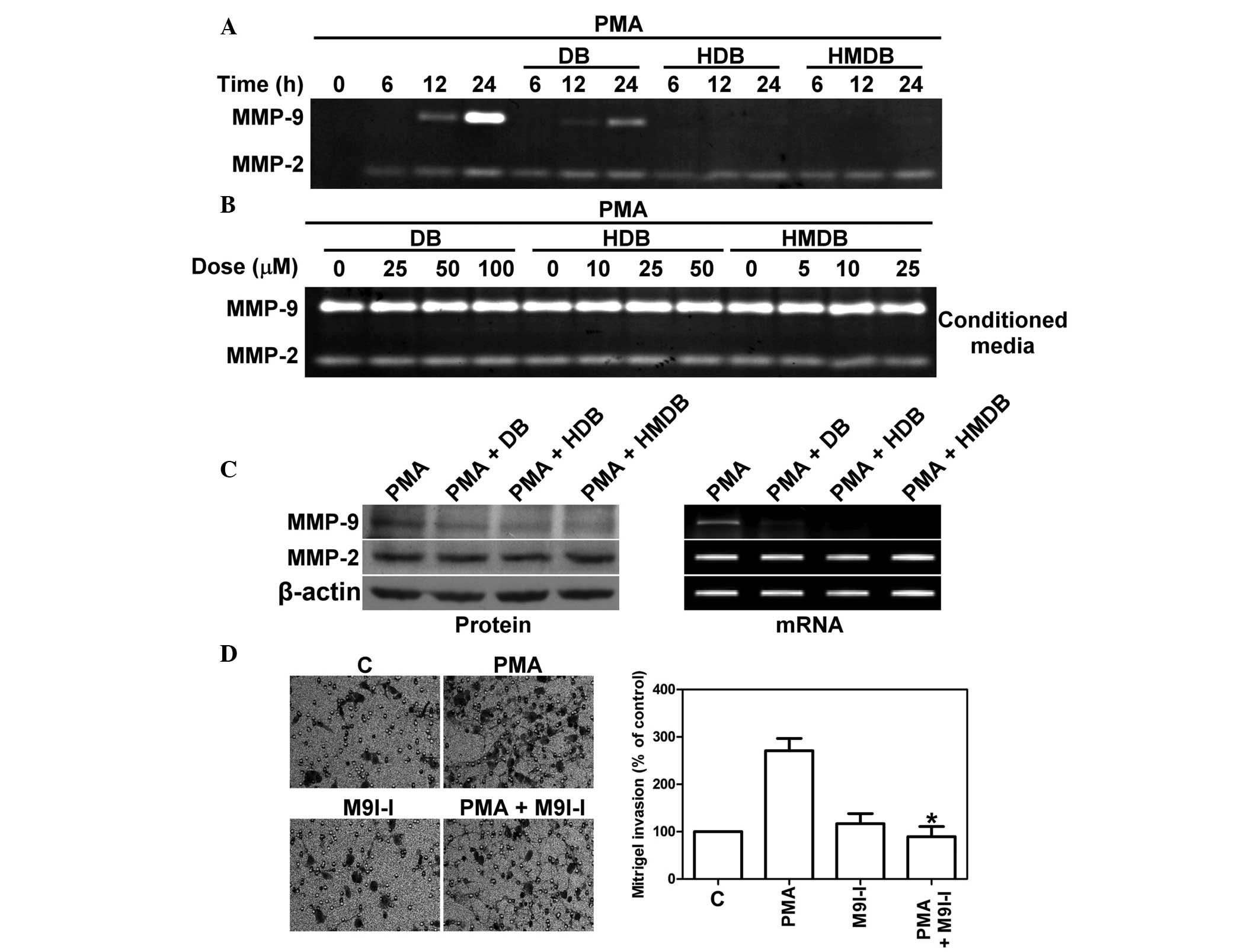 | Figure 4DB, HDB and HMDB inhibit the
PMA-induced expression of MMP-9 in MCF-7 cells. (A) MCF-7 cells
were treated with 100 μM DB, 50 μM HDB and 25
μM HMDB, followed by the addition of 10 ng/ml PMA for 6, 12
and 24 h. The activity of the secreted MMP-9 and MMP-2 proteins was
analyzed by gelatin zymography. (B) MMP-9 and MMP-2, derived from
PMA-treated conditioned medium following stimulation for 24 h, was
incubated with various concentrations of DB, HDB and HMDB for 30
min. The enzyme activities of MMP-2 and MMP-9 were analyzed using
gelatin zymography. The light areas represent zones of lysis in
gelatin gel were quantified by laser scanning densitometry. (C)
MCF-7 cells were treated with DB, HDB and HMDB followed by the
addition of 10 ng/ml PMA for 24 h. The protein and mRNA expression
levels of MMP-9 and MMP-2 were analyzed using immunoblotting and
reverse transcription polymerase chain reaction. (D) MCF-7 cells
were treated with or without M9I-I and with PMA and then invasive
ability was assessed using a Matrigel invasion assay
(magnification, x200). The data are expressed as the mean ±
standard deviation *P<0.05, compared with the PMA
only group. DB, dibenzoylmethane; HDB, hydroxydibenzoylmethane;
HMDB, hydroxymethyldibenzoylmethane; PMA, phorbol-12-myristate
13-acetate; M9I-I, MMP-9 inhibitor I; C, control. |
PMA-induced activation of PKCδ and PI3K
is reduced by DB, HDB and HMDB
The PKCδ and PI3K signaling pathways are important
in cancer invasion (21,22). The present study aimed to determine
the involvement of the activation of PKCδ and PI3K in the
PMA-induced expression of MMP-9. The MCF-7 cells were treated with
rottlerin (Rot), a PKCδ inhibitor or LY294002, a PI3K inhibitor,
for 24 h, followed by the addition of PMA. As shown in Fig. 5A, Rot and LY effectively reduced
the PMA-induced enzymatic activity and the mRNA expression of MMP-9
in a dose-dependent manner, as measured by gelatin zymography and
RT-PCR, respectively. In addition, it was observed that the MCF-7
cells treated with 1 μM Rot and 10 μM LY reduced
PMA-induced MCF-7 cell invasion (Fig.
5B). The activity of PKCδ was increased by the phosphorylation
of Thr 505 and the activity of PI3K kinase was increased by the
phosphorylation of Tyr458 in PI3K-p85 and of Tyr199 in PI3K-p55.
Therefore, the phosphorylation levels of PKCδ-Thr505, PI3K
p85-Tyr458 and p55-Tyr199 were assessed by immunoblotting. As shown
in Fig. 5C, the PMA-stimulated
MCF-7 cells exhibited increased phosphorylation levels of PKCδ and
PI3K. The increases in phosphorylated PKCδ and PI3K by
PMA-stimulation were suppressed by treatment for 0.5, 1 and 3 h
with 100 μM DB, 50 μM HDB and 25 μM HMDB. The
order of potency of these compounds on the inhibition of PKCδ and
PI3K phosphorylation were in the order HMDB>HDB>DB.
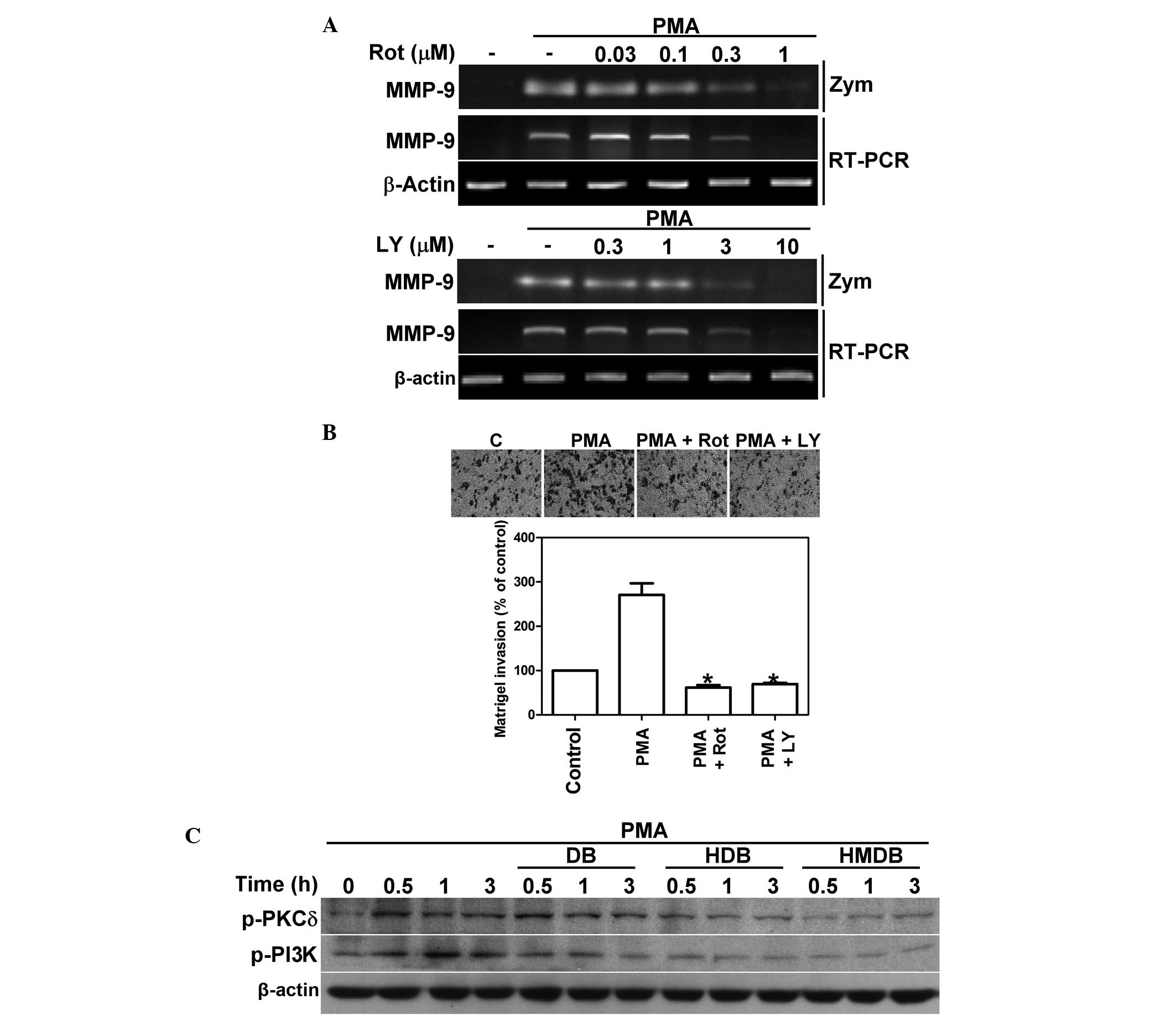 | Figure 5Effects of DB, HDB and HMDB on the
PMA-induced activation of PKCδ and PI3K. (A) MCF-7 cells were
treated with various concentrations of Rot or LY and the addition
of PMA for 24 h. The activity and mRNA expression of MMP-9 were
assessed using gelatin zymography and reverse transcription
polymerase chain reaction, respectively. (B) MCF-7 cells were
treated with Rot or LY followed by PMA and the invasive ability was
assessed using a Matrigel invasion assay (magnification, x200). The
data are expressed as the mean ± standard deviation.
*P<0.05 compared with the PMA only group. (C) MCF-7
cells were treated with 100 μM DB, 50 μM HDB and 25
μM HMDB followed by the addition of PMA for 0.5, 1 and 3 h.
The expression levels of p-PKCδ and p-PI3K were analyzed by
immunoblotting. DB, dibenzoylmethane; HDB, hydroxydibenzoylmethane;
HMDB, hydroxymethyldibenzoylmethane; PMA, phorbol-12-myristate
13-acetate; PKCδ, protein kinase C δ; PI3K, phosphatidylinositide
3-kinase; Rot, rottlerin; LY, LY294002; p-, phosphorylated; MMP,
metalloproteinase, Zym, zymography; C, control. |
Discussion
The cause of mortality in the majority of patients
with breast cancer is not from the primary cancer growth, but due
to spread of the cancer cells to other parts of the body (23). Synthesized chemotherapeutic
compounds demonstrate efficacy in treating metastatic breast
cancer, however, certain chemotherapeutic agents possess toxic side
effects (24). Therefore, the
identification of naturally-derived antimetastatic, non-toxic,
compounds is of particular interest. Natural products containing
phenolic compounds have been widely reported to have the ability to
prevent cancer metastasis (25).
Flavonoids are a large subclass of phenolic compounds, prevalent in
food and herbal nutraceuticals (26). DB, a β-diketone analogue of
curcumin, belongs to the flavonoid family. Skin cancer frequently
occurs at sun-exposed sites of the body, and sunscreen derivatives
from DB have been used for protection against ultraviolet radiation
(27). DB modulates the phase
I/phase II detoxification enzymatic systems (10,28)
and with its analogues, HDB and HMDB, induces apoptosis in various
types of cancer (15–18). However, their antimetastatic
effects remain to be elucidated. The present study found that DB,
HDB and HMDB significantly inhibited PMA-mediated MCF-7 cell
migration and invasion (Figs. 1
and 2) and was the first, to the
best of our knowledge, to demonstrate that DB, HDB and HMDB
inhibiti cancer cell migration and invasion.
MMPs are a family of Zn2+- and
Ca2+-dependent endopeptidases, comprising four
subclasses (collagenases, gelatinases, stromelysins and
membrane-type MMPs) based on their substrate (4). Increased levels of MMP-9 and MMP-2
are functionally associated with metastasis in several types of
malignant tumor, including breast cancer (4,20).
The present study indicated that DB, HDB and HMDB inhibited the
expression of MMP-9, but not MMP-2, following PMA treatment
(Fig 4). MMP-2 is generally
constitutively expressed in tissues and is maximally present in
malignant neoplasms (29). By
contrast, the expression of MMP-9 can be induced by various growth
factors and inflammatory mediators during pathological states and
by agents, including PMA (19,30).
PMA is a tumor promoter and an inflammatory stimulator, which
increases the invasiveness of cancer cells, including breast
cancer, by activating MMP-9 (19).
DB is an effective inhibitor of PMA-induced mouse skin inflammation
and tumor promotion (31). In the
present study, the potency of inhibition by DB and its structural
analogs on the expression of MMP-9 and their effects on the
invasive potential of the PMA-treated MCF-7 cells was in the order
HMDB>HDB>DB. Higher numbers of OH groups are present in
flavonoid structures with increased anti-MMP activation (32,33).
Compared with DB and HDB, HMDB has more marked anticancer effects
in solid cancer cell lines (15).
PMA is a diacylglycerol mimic, which directly binds
to and activates classical (α, β and γ) and novel (δ, ε, θ and η)
PKC isozymes (34). The activation
of PKC involves the phosphorylation of PKC isoforms, causing cell
proliferation, apoptosis, and cancer metastasis. The
phosphorylation of PKCδ is involved in promoting cell invasion
(35). PKCδ is an important
signaling molecule for the expression of MMP-9 in cancer cell
lines, including MCF-7 human breast carcinoma cells, CaSki cells
and HP-75 pituitary adenoma cells (32,36,37).
The present study found that the DB analogues inhibited the
phosphorylation of PKCδ in the MCF-7 cells following PMA treatment
(Fig. 5C). PKCδ activates
Ha-Ras-mediated signal transduction pathways (38) and human telomerase reverse
transcriptase (hTERT) is phosphorylated by PKCδ (39). DB attenuates the expression of
oncogenes Ha-Ras and hTERT oncogenes (40). The activation of MMP-9 is
downstream of Ha-Ras (41) and
knockdown of hTERT reduces the expression of MMP-9 in human
glioblastoma cell lines (42).
Inhibiting the activity of PKCδ by Rot enhances p38 mitogen
activated protein kinase (MAPK) activation (43). HMDB activates p38 MAPK signaling
(44), which suppresses the
expression of MMP-9 in human stomach cancer (45). Nutraceuticals derived from spices,
including curcumin, capsaicin and diosgenin, target the PI3K
signaling pathway to regulate cancer metastasis (25) and activation of this pathway in
breast cancer is associated with a poor prognosis (46). In the present study, DB, HDB and
HMDB prevented the PMA-induced phosphorylation of PI3K in the MCF-7
cells (Fig. 5C). PI3Ks are
heterodimeric serine/threonine kinases consisting of p110 catalytic
and regulatory subunits and tyrosine phosphorylation of the p85
regulatory subunit reduces its inhibitory activity on PI3K
(47). The PI3K inhibitor,
LY294002, significantly diminishes the expression of phosphorylated
PI3K-p85 (Tyr458)/p55 (Tyr199) (48). The results of the present and
previous studies have revealed that the inhibition of PI3K activity
can suppress the expression of MMP-9 and cancer cell invasion
(21). These findings suggest that
DB analogues inhibits PMA-induced metastasis by interfering with
the PI3K/PKCδ-mediated expression of MMP-9.
In conclusion, the present study demonstrated that
DB, HDB and HMDB exerted antimetastatic effects in PMA-treated
MCF-7 breast cancer cells. The mechanism underlying this was the
inhibiton of the activity and expression of MMP-9 via the PKCδ and
PI3K signaling pathways. Therefore, DB, HDB and HDMB may be potent
chemopreventive agents in therapeutic strategies for metastatic
cancers in the future.
Acknowledgments
This study was supported by grants from the National
Science Council of Taiwan (NSC 98-2320-B-324-001 and NSC
102-2320-B-324-002)
References
|
1
|
Weigelt B, Peterse JL and van’t Veer LJ:
Breast cancer metastasis: markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320.
2012.PubMed/NCBI
|
|
3
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kondraganti S, Mohanam S, Chintala SK, et
al: Selective suppression of matrix metalloproteinase-9 in human
glioblastoma cells by antisense gene transfer impairs glioblastoma
cell invasion. Cancer Res. 60:6851–6855. 2000.
|
|
6
|
Lakka SS, Gondi CS, Yanamandra N, et al:
Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma
cell line via RNA interference reduces tumor cell invasion, tumor
growth and angiogenesis. Oncogene. 23:4681–4689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh T, Tanioka M, Matsuda H, et al:
Experimental metastasis is suppressed in MMP-9-deficient mice. Clin
Exp Metastasis. 17:177–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji MK, Shi Y, Xu JW, et al: Recombinant
snake venom metalloproteinase inhibitor BJ46A inhibits invasion and
metastasis of B16F10 and MHCC97H cells through reductions of matrix
metalloproteinases 2 and 9 activities. Anticancer Drugs.
24:461–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shishu, Singla AK and Kaur IP: Inhibitory
effect of dibenzoylmethane on mutagenicity of food-derived
heterocyclic amine mutagens. Phytomedicine. 10:575–582. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CC, Lu YP, Lou YR, et al: Inhibition
by dietary dibenzoylmethane of mammary gland proliferation,
formation of DMBA-DNA adducts in mammary glands, and mammary
tumorigenesis in Sencar mice. Cancer Lett. 168:125–132. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singletary K and MacDonald C: Inhibition
of benzo[a]pyrene- and 1,6-dinitropyrene-DNA adduct formation in
human mammary epithelial cells bydibenzoylmethane and sulforaphane.
Cancer Lett. 155:47–54. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung KL, Khor TO, Huang MT and Kong AN:
Differential in vivo mechanism of chemoprevention of tumor
formation in azoxymethane/dextran sodium sulfate mice by PEITC and
DBM. Carcinogenesis. 31:880–885. 2010. View Article : Google Scholar :
|
|
13
|
Singletary K, MacDonald C, Iovinelli M,
Fisher C and Wallig M: Effect of the beta-diketones
diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary
DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene.
Carcinogenesis. 19:1039–1043. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson KM, DeLeon M, Verret CR and Harris
WB: Dibenzoylmethane induces cell cycle deregulation in human
prostate cancer cells. Cancer Lett. 178:161–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan MH, Sin YH, Lai CS, et al: Induction
of apoptosis by 1-(2-hydr
oxy-5-methylphenyl)-3-phenyl-1,3-propanedione through reactive
oxygen species production, GADD153 expression, and caspases
activation in human epidermoid carcinoma cells. J Agric Food Chem.
53:9039–9049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng CJ, Yang YT, Ho CT and Yen GC:
Mechanisms of apoptotic effects induced by resveratrol,
dibenzoylmethane, and their analogues on human lung carcinoma
cells. J Agric Food Chem. 57:5235–5243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu CL, Liao YF, Hung YC, Lu KH, Hung HC
and Liu GY: Ornithine decarboxylase prevents
dibenzoylmethane-induced apoptosis through repressing reactive
oxygen species generation. J Biochem Mol Toxicol. 25:312–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan MH, Huang MC, Wang YJ, Lin JK and Lin
CH: Induction of apoptosis by hydroxydibenzoylmethane through
coordinative modulation of cyclin D3, Bcl-X(L), and Bax, release of
cytochrome c, and sequential activation of caspases in human
colorectal carcinoma cells. J Agric Food Chem. 51:3977–3984. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao YF, Rao YK and Tzeng YM: Aqueous
extract of Anisomeles indica and its purified compound exerts
anti-metastatic activity through inhibition of
NF-kappaB/AP-1-dependent MMP-9 activation in human breast cancer
MCF-7 cells. Food Chem Toxicol. 50:2930–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: Role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar
|
|
21
|
Park SK, Hwang YS, Park KK, Park HJ, Seo
JY and Chung WY: Kalopanaxsaponin A inhibits PMA-induced invasion
by reducing matrix metalloproteinase-9 via PI3K/Akt- and
PKCdelta-mediated signaling in MCF-7 human breast cancer cells.
Carcinogenesis. 30:1225–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu HS, Lin TH and Tang CH: Bradykinin
enhances cell migration in human prostate cancer cells through B2
receptor/PKCdelta/c-Src dependent signaling pathway. Prostate.
73:89–100. 2013. View Article : Google Scholar
|
|
23
|
Pockaj BA, Wasif N, Dueck AC, et al:
Metastasectomy and surgical resection of the primary tumor in
patients with stage IV breast cancer: time for a second look? Ann
Surg Oncol. 17:2419–2426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson KA and Brown PH: Drug development
for cancer chemoprevention: focus on molecular targets. Semin
Oncol. 37:345–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar
|
|
26
|
Kandaswami C, Lee LT, Lee PP, et al: The
antitumor activities of flavonoids. In Vivo. 19:895–909.
2005.PubMed/NCBI
|
|
27
|
Nogueira MA, Magalhaes EG, Magalhaes AF,
et al: A novel sunscreen agent having antimelanoma activity.
Farmaco. 58:1163–1169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dinkova-Kostova AT and Talalay P: Relation
of structure of curcumin analogs to their potencies as inducers of
Phase 2 detoxification enzymes. Carcinogenesis. 20:911–914. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brummer O, Athar S, Riethdorf L, Loning T
and Herbst H: Matrix-metalloproteinases 1, 2, and 3 and their
tissue inhibitors 1 and 2 in benign and malignant breast lesions:
an in situ hybridization study. Virchows Arch. 435:566–573. 1999.
View Article : Google Scholar
|
|
30
|
Mishra A, Paul S and Swarnakar S:
Downregulation of matrix metalloproteinase-9 by melatonin during
prevention of alcohol-induced liver injury in mice. Biochimie.
93:854–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang MT, Lou YR, Xie JG, et al: Effect of
dietary curcumin and dibenzoylmethane on formation of
7,12-dimethylbenz[a] anthracene-induced mammary tumors and
lymphomas/leukemias in Sencar mice. Carcinogenesis. 19:1697–1700.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CW, Hou WC, Shen SC, et al: Quercetin
inhibition of tumor invasion via suppressing PKC
delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in
breast carcinoma cells. Carcinogenesis. 29:1807–1815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sim GS, Lee BC, Cho HS, et al: Structure
activity relationship of antioxidative property of flavonoids and
inhibitory effect on matrix metalloproteinase activity in
UVA-irradiated human dermal fibroblast. Arch Pharm Res. 30:290–298.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu WS and Heckman CA: The sevenfold way
of PKC regulation. Cell Signal. 10:529–542. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuoka H, Tsubaki M, Yamazoe Y, et al:
Tamoxifen inhibits tumor cell invasion and metastasis in mouse
melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt
pathways. Exp Cell Res. 315:2022–2032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hussaini IM, Trotter C, Zhao Y, et al:
Matrix metalloproteinase-9 is differentially expressed in
nonfunctioning invasive and noninvasive pituitary adenomas and
increases invasion in human pituitary adenoma cell line. Am J
Pathol. 170:356–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woo JH, Lim JH, Kim YH, et al: Resveratrol
inhibits phorbol myristate acetate-induced matrix
metalloproteinase-9 expression by inhibiting JNK and PKC delta
signal transduction. Oncogene. 23:1845–1853. 2004. View Article : Google Scholar
|
|
38
|
Vuong H, Patterson T, Shapiro P, et al:
Phorbol ester-induced expression of airway squamous cell
differentiation marker, SPRR1B, is regulated by protein kinase
Cdelta/Ras/MEKK1/MKK1-dependent/AP-1 signal transduction pathway. J
Biol Chem. 275:32250–32259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang JT, Lu YC, Chen YJ, et al: hTERT
phosphorylation by PKC is essential for telomerase holoprotein
integrity and enzyme activity in head neck cancer cells. Br J
Cancer. 94:870–878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin CC, Tsai YL, Huang MT, et al:
Inhibition of estradiol-induced mammary proliferation by
dibenzoylmethane through the E2-ER-ERE-dependent pathway.
Carcinogenesis. 27:131–136. 2006. View Article : Google Scholar
|
|
41
|
Lee KW, Kim MS, Kang NJ, et al: H-Ras
selectively up-regulates MMP-9 and COX-2 through activation of
ERK1/2 and NF-kappaB: an implication for invasive phenotype in rat
liver epithelial cells. Int J Cancer. 119:1767–1775. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
George J, Banik NL and Ray SK: Knockdown
of hTERT and concurrent treatment with interferon-gamma inhibited
proliferation and invasion of human glioblastoma cell lines. Int J
Biochem Cell Biol. 42:1164–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park EJ, Lim JH, Nam SI, Park JW and Kwon
TK: Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through
reactive oxygen species (ROS) dependent and PKC delta-independent
pathway in human colon cancer HT29 cells. Biochimie. 92:110–115.
2010. View Article : Google Scholar
|
|
44
|
Pan YC, Li CF, Ko CY, et al: CEBPD
reverses RB/E2F1-mediated gene repression and participates in
HMDB-induced apoptosis of cancer cells. Clin Cancer Res.
16:5770–5780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee KH and Kim JR: Kiss-1 suppresses MMP-9
expression by activating p38 MAP kinase in human stomach cancer.
Oncol Res. 18:107–116. 2009. View Article : Google Scholar
|
|
46
|
Adamo B, Deal AM, Burrows E, et al:
Phosphatidylinositol 3-kinase pathway activation in breast cancer
brain metastases. Breast Cancer Res. 13:R1252011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cuevas BD, Lu Y, Mao M, et al: Tyrosine
phosphorylation of p85 relieves its inhibitory activity on
phosphatidylinositol 3-kinase. J Biol Chem. 276:27455–27461. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu C, Su T, Li F, et al: PI3K/Akt
signaling transduction pathway is involved in rat vascular smooth
muscle cell proliferation induced by apelin-13. Acta Biochim
Biophys Sin (Shanghai). 42:396–402. 2010. View Article : Google Scholar
|



















