Introduction
Schisandra chinensis is a member of the
family Schisandraceae and the genus Schisandra. This
climbing plant is widely distributed in the Russian Far East,
China, Japan and Korea, and is often used to treat asthenia, cough,
asthma and exhaustion (1,2). In addition, it is widely used to
treat skin diseases, such as, atopic dermatitis, photo-aging and
hair loss (3–6). Recently, S. chinensis has been
reported to aid liver regeneration, inhibit hepatocarcinogenesis,
exhibit antioxidant activity, restore blood sugar balance, reduce
blood pressure, and to exhibit anti-inflammatory, wound healing and
antitumor effects (2,7,8).
Contact dermatitis (CD) is one of the most common
occupational skin diseases (9).
Furthermore, its timely and accurate diagnosis is important for
achieving satisfactory outcomes (9). Primary treatment for CD requires
avoidance of contact with the offending agent, which is often
difficult in cases of occupational CD (10). As a result, treatment for CD tends
to consist of the repeated administration of immune-modulatory and
anti-inflammatory agents, such as corticosteroids. However,
although they are highly effective when used to treat allergic and
inflammatory diseases, the dosages and treatment schedules of
corticosteroids are frequently restricted due to serious potential
side effects (10), which minimize
their use, particularly when continuous application is required.
Conversely, traditional medicines have been used in Korea as
alternatives and to complement corticosteroids as they are safe and
inexpensive (11).
However, the use of S. chinensis to treat CD
with or without corticosteroids has not been fully investigated.
Therefore, in the present study, the anti-inflammatory effects of
S. chinensis was evaluated using a mouse model of CD. In
particular, the effects of S. chinensis on ear thickness,
ear weight, histopathological changes in ear tissue, and cytokine
levels in inflamed tissues were assessed in vivo.
Materials and methods
Chemicals and reagents
1-Fluoro-2,4-dinitrofluorobenzene (DNFB), dimethyl
sulfoxide (DMSO) and dexamethasone (DEX/PLGA) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Protein extraction kits were
obtained from Intron Bio (Daejeon, Korea) and cytometric bead array
kits were acquired from BD Biosciences (Franklin Lakes, NJ,
USA).
Preparation of the methanol extract of S.
chinensis (MESC)
Ripe S. chinensis fruits were purchased from
Hwalim Medicinal Herbs (Pusan, Korea). The extraction was performed
using a standard procedure (8).
Briefly, 50 g ripe S. chinensis fruits were immersed in
1,000 ml methanol, sonicated for 30 min, and agitated for 24 h. The
extract was then filtered through Whatman filter paper (no. 20; VWR
International, LLC, Arlington Heights, IL, USA), evaporated under
reduced pressure using a vacuum evaporator (Eyela, Tokyo, Japan),
and lyophilized using a freeze dryer (Labconco, Kansas City, MO,
USA). Finally, 15.9 g lyophilized powder (MESC) was obtained
(yield, 31.8%). A sample of MESC (voucher no. MH2010-010) was
deposited at the Division of Pharmacology, School of Korean
Medicine, Pusan National University (Yansan, Korea).
Animals
Male 6 week-old BALB/c mice were purchased from
Samtaco (Incheon, Korea) and housed under specific pathogen-free
conditions with a 12 h light/dark cycle and free access to standard
rodent food and water. The animals were sacrificed by cervical
dislocation. All animal experiments were approved by the Pusan
National University Animal Care and Use Committee and conducted
according to institutional guidelines (PNU-2011-000406).
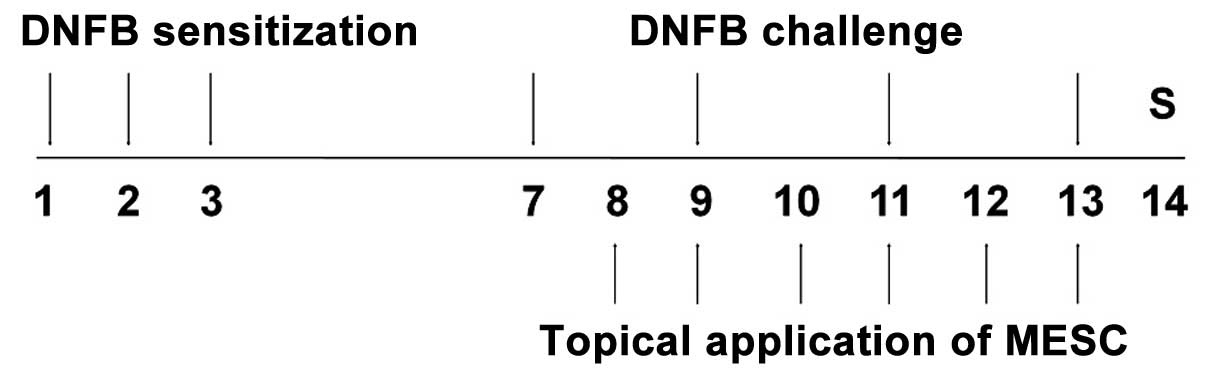
Induction of CD and experimental
design
CD induction was performed using DNFB. Briefly, mice
were sensitized by applying 50 µl DNFB (0.1%, v/v) in
acetone and olive oil (AOO, 4:1) onto shaved backs for three
consecutive days. Four days after sensitization, each mouse was
challenged by applying 30 µl DNFB (0.2%, v/v) in AOO onto
the dorsum of both ears on alternate days. For topical
applications, MESC and DEX were dissolved in ethanol, filtered
using a 0.45-µm syringe filter and finally diluted in AOO.
MESC in solution (30, 100 or 300 µg/ear) was applied onto
the dorsum of both ears daily for six consecutive days. Naïve
animals (NOR) were neither sensitized nor challenged with DNFB
(n=6). Control animals (CTL) were sensitized and challenged with
DNFB in AOO, and then vehicle was applied (ethanol and AOO) (n=9).
MESC treated animals were sensitized and challenged with DNFB, and
then administered with 30, 100 or 300 µg/ear MESC (n=9). DEX
treated animals were sensitized and challenged with DNFB and then
75 µg/ear DEX in ethanol and AOO (n=6) was applied as a
positive control. The experimental design is summarized in Fig. 1.
Measurement of ear thickness and
weight
Mice were anesthetized with 30 mg/kg Zoletil
(Virbac, Carros, France) and the thickness of both ears was
measured using vernier calipers (Mitutoyo, Carros, Japan). The
weights of ear samples (5 mm in diameter) were measured at the same
time.
Hematoxylin-eosin (H&E) staining
After measuring ear thickness and weight, ear
tissues were resected and embedded in paraffin. Sections were then
stained with H&E to observe immune cell infiltration and
spongiosis. Stained tissues were observed under a light microscope
(×100; SZX7; Olympus, Tokyo, Japan).
Measurement of epithelial thickness
H&E stained slides were thoroughly examined
under the light microscope equipped with a digital camera (Coolpix
P600; Nikon, Tokyo, Japan), and five images per slide were captured
at ×200. To measure epithelium thickness, vertical distances
between basal lamina and outermost stratum granulosum were
measured. Five random measurements were made per slide using Motic
Images Plus 2.0 (Motic Instrument Inc., Causeway Bay, Hong
Kong).
Evaluation of immune cell
infiltration
To evaluate immune cell infiltration, numbers of
immune cells in connective tissue were counted in five photographs
per slide (×200). Macrophages, polymorphonuclear leucocytes (PMNL),
lymphocytes, eosinophils, plasma cells and giant cells were viewed
as immune cells.
Measurement of cytokine production
Resected ear tissues were lysed and homogenized with
protein extraction solution (Intron Bio) using a bullet blender
(Next Advance, Averill Park, NY, USA) to obtain tissue lysates. The
levels of tumor necrosis factor (TNF)-α, interferon (IFN)-γ,
interleukin-6 (IL-6) and monocyte chemotactic protein-1 (MCP-1)
were then measured in 50 µg lysate using a cytometric bead
array kit (BD Biosciences, San Jose, CA, USA).
Statistical analysis
The Mann-Whitney test was used for all statistical
comparisons, and Prism 5 version 5.01 (GraphPad Software Inc., La
Jolla, CA, USA) was used for all analyses. Results are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
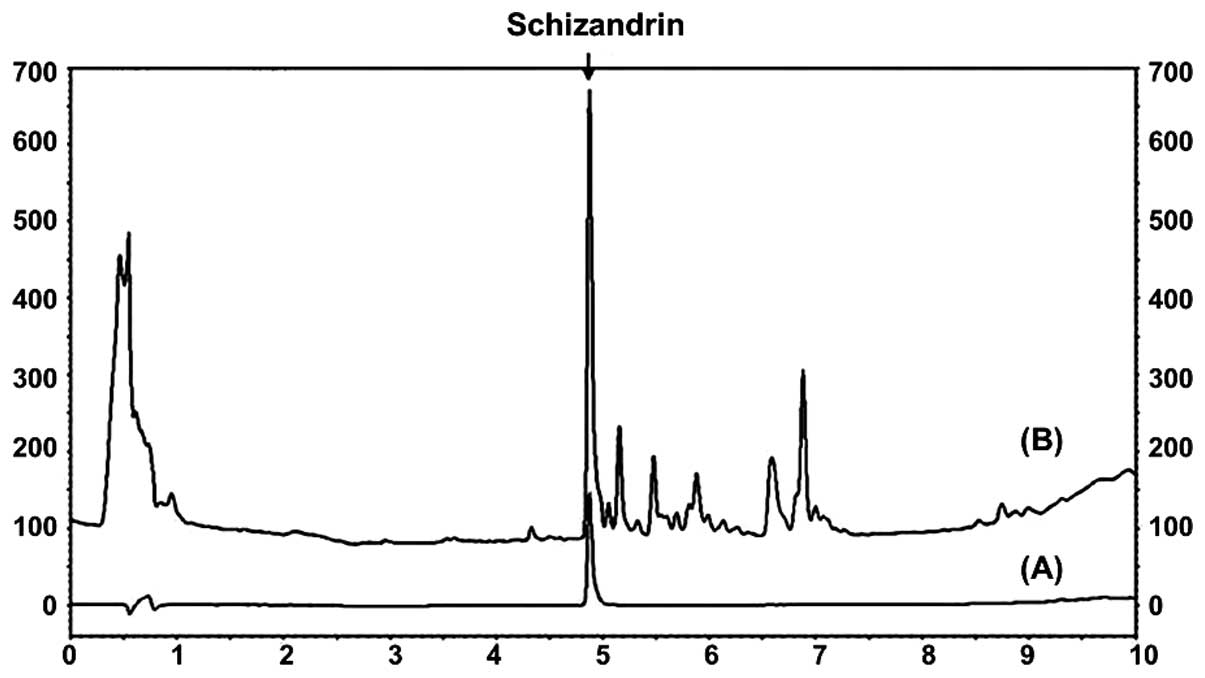
Identification of schizandrin in
MESC
The schizandrin peak was detected at a retention
time of 4.870 min (Fig. 2).
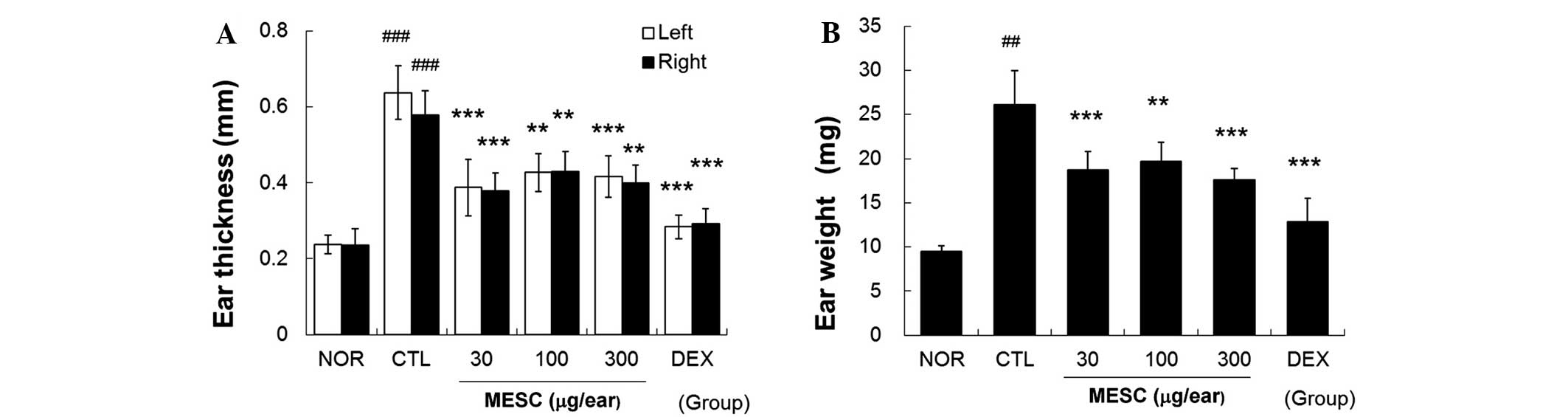
MESC inhibits increases in ear swelling
in CD mice
Repeated application of DNFB increased ear thickness
and weight. Marked increases in the thickness and weight of ear
tissues were observed in the CTL group. Topical treatment with MESC
effectively inhibited increases in thickness and weight, although
inhibitory effects were greater in the DEX group than in the MESC
group (Fig. 3).
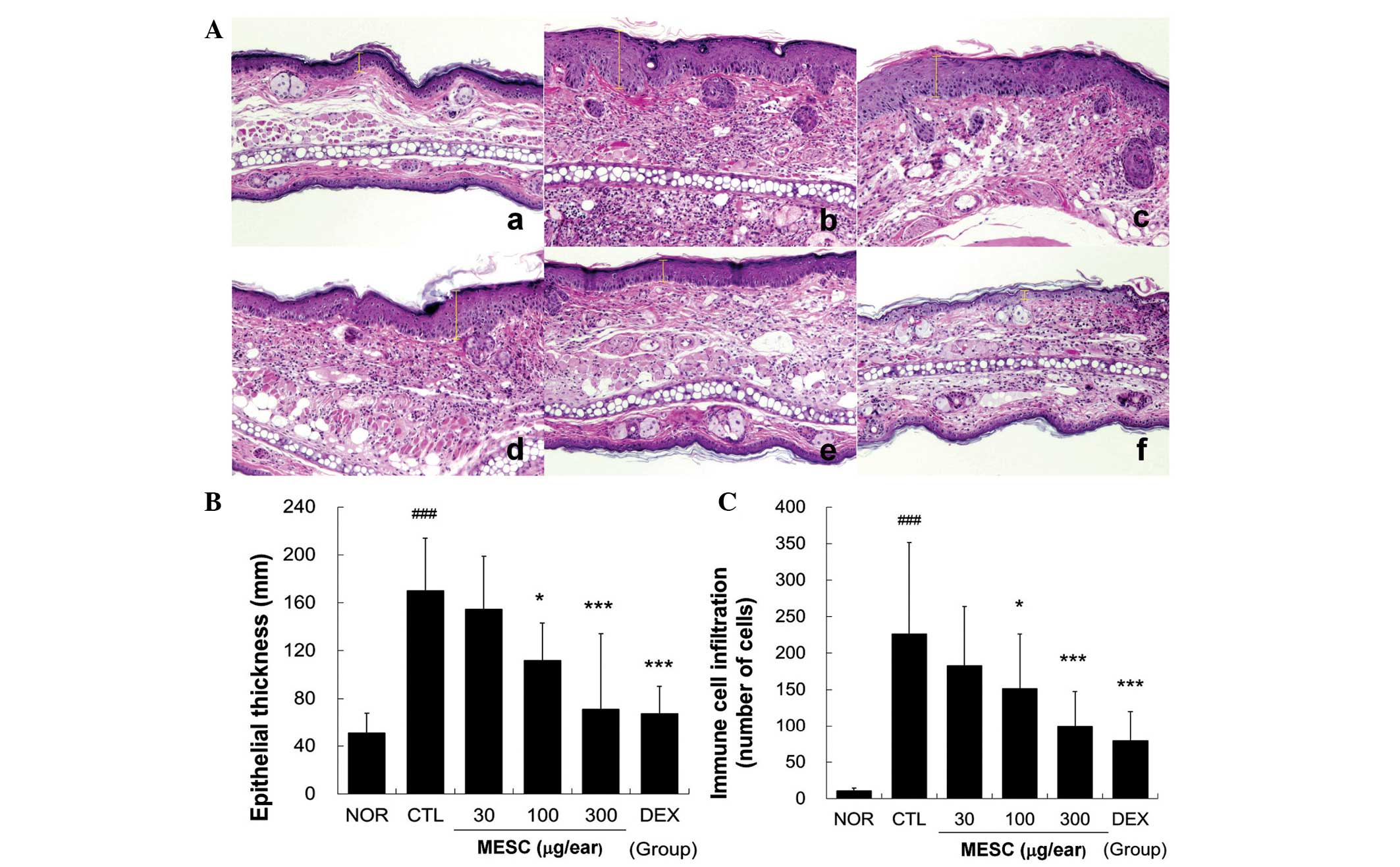
MESC prevents hyperplasia, spongiosis and
immune cell infiltration
Repeated application of DNFB induced hyperplasia and
significant edema and spongiosis, which are the hallmarks of skin
inflammation. Marked immune cell infiltration was observed in the
CTL group, whereas little hyperplasia, edema or spongiosis was
observed in the MESC group. Treatment with 30 µg/ear MESC
had a marginal effect on hyperplasia and immune cell infiltration.
However, treatment with DEX more effectively prevented spongiosis,
hyperplasia and immune cell infiltration (Fig. 4).
MESC reduces the levels of TNF-α, IFN-γ,
IL-6 and MCP-1 in the ear tissues of CD mice
Marked increases in TNF-α, IFN-γ, IL-6 and MCP-1
levels were observed in the CTL group, but these increases in
TNF-α, IFN-γ, IL-6 and MCP-1 levels were effectively reduced by
topical MESC. Treatment with MESC at <100 µg/ear did not
affect IL-6 levels (Fig. 5).
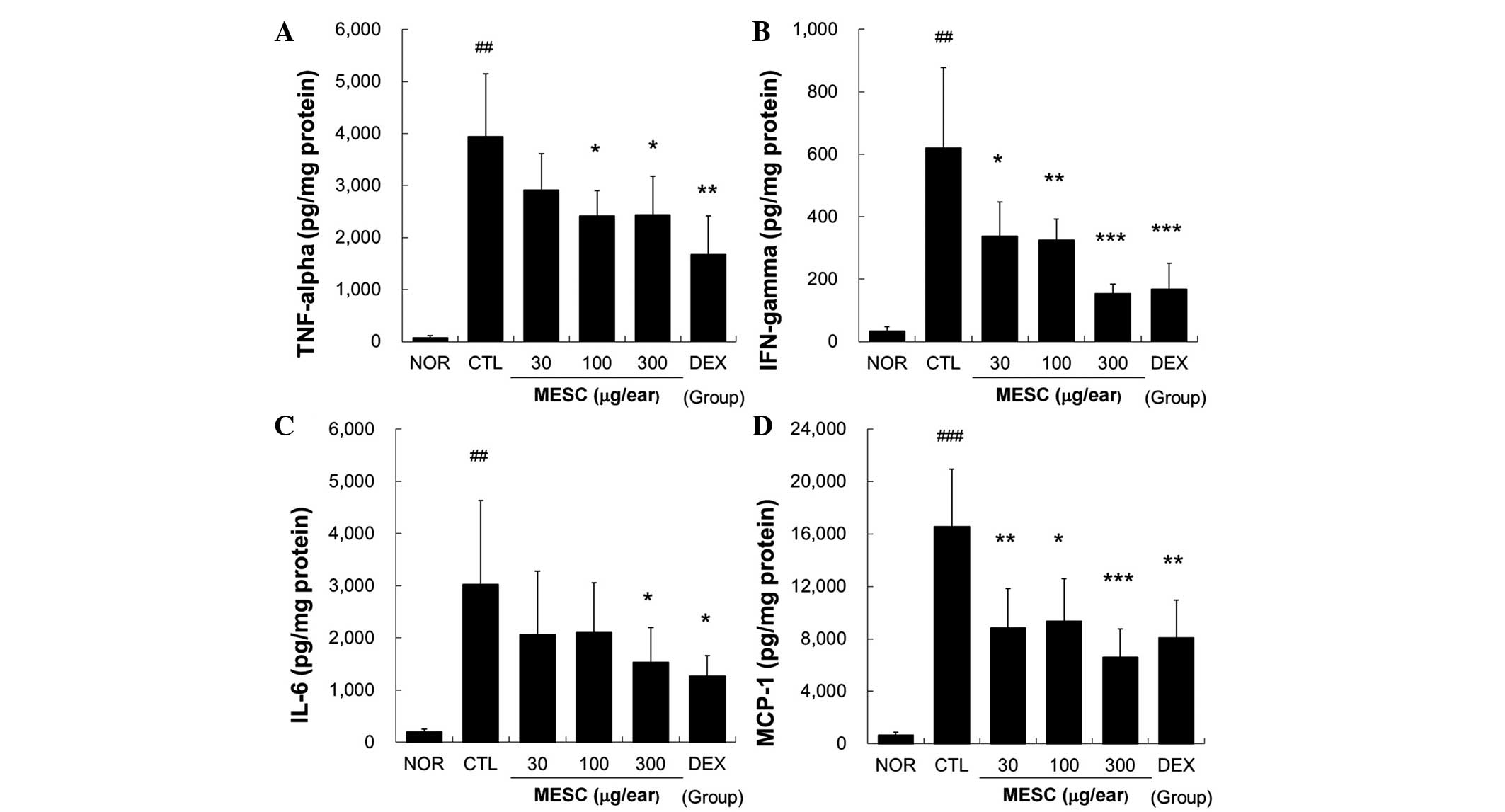 | Figure 5Effects of MESC on cytokine production
in mice with CD. Levels of TNF-α, IFN-γ, IL-6 and MCP-1 in ear
tissues were measured using the cytometric bead array method. A
total of 50 µg tissue lysate was used to measure cytokine
levels. (A) TNF-α; (B) IFN-γ; (C) IL-6 and (D) MCP-1. All values
are presented as the mean ± standard deviation.
##P<0.01 and ###P<0.001 vs. NOR;
*P<0.05, **P<0.01 and
***P<0.001 vs. CTL. NOR, treatment naïve mice; CTL,
non-treated CD mice; DEX, 75 µg/ear DEX. CD, contact
dermatitis; TNF, tumor necrosis factor; IFN, interferon; IL,
interleukin; MCP, monocyte chemoattractant protein; MESC, methanol
extract of S. chinensis; DEX, dexamethasone. |
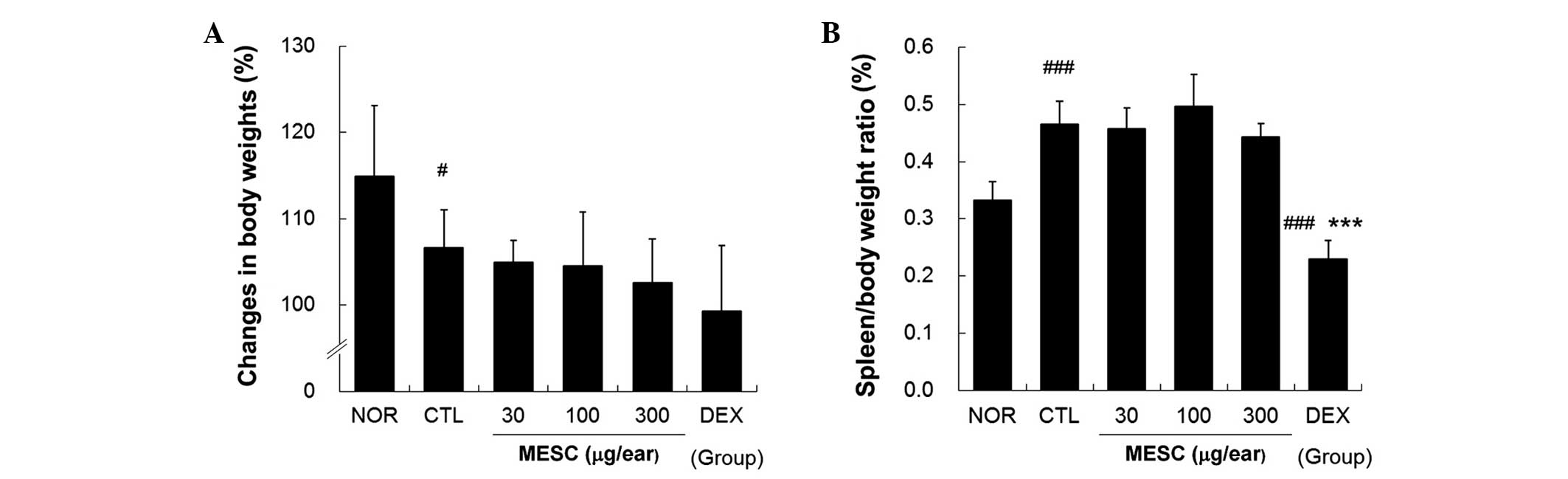
MESC does not affect spleen/body weight
ratio in CD mice
Body weight gains in the CTL group were lower than
in the NOR group (Fig. 6A),
whereas no body weight gains were observed in the MESC or DEX
groups (Fig. 6A). The effects of
MESC on spleen enlargement were estimated by determining
spleen/body weight ratios, which were significantly smaller in the
DEX group than in the CTL group, but almost the same in the MESC
and CTL groups (Fig. 6B).
Discussion
Inflammation is mediated by a variety of soluble
factors, including a group of secreted polypeptides termed
cytokines (12). Several cytokines
are important in mediating acute or chronic inflammatory reactions,
such as IFN-γ, IL-6, MCP-1 and TNF-α (13). The cytokines known to mediate
chronic inflammatory processes can be divided into those
participating in humoral inflammation, such as IL-6, and those
contributing to cellular inflammation, such as IFN-γ and TNF-α
(13). In the present study, MESC
effectively reduced immune cell infiltration into affected tissues
and reduced pro-inflammatory cytokine production. These results
suggest that MESC acts as an anti-inflammatory agent and that the
major mechanism underlying its effects involves suppression of
pro-inflammatory cytokine production.
S. chinensis Turcz. fruit is a well-known
traditional Korean herbal medicine and is used as an antitussive
and sedative agent, as well as to improve liver function in
patients with viral hepatitis (14). Furthermore, it has been shown to
have anti-oxidative, antimicrobial and nitrite scavenging effects
(15). Yasukawa et al
(16) concluded that S.
chinensis (gomesin A) exhibits anti-inflammatory activity, and
found that it inhibited tumor promotion by
12-O-tetradecanoylphorbol-13-acetate in a mouse skin model of
carcinogenesis. Huyke et al (17) suggested that S. chinensis
may be useful for the prevention and treatment of
hyperproliferative and inflammatory skin diseases, whereas Kang
et al (18) reported that
S. chinensis inhibited the secretion of pro-inflammatory
cytokines by mast cells. These cells contain potent inflammatory
mediators, such as histamine and multifunctional cytokines, which
can promote the production of pro-inflammatory cytokines, adhesion
molecules and growth factors in resident cells (19). In the present study, MESC reduced
TNF-α, IFN-γ, IL-6 and MCP-1 levels, showing that its
anti-inflammatory effects are attributable to cytokine
regulation.
In the present study, DEX reduced body weight gain
and spleen/body weight ratios. Spleen/body weight ratios in the DEX
group were significantly lower than in the NOR group. By contrast,
MESC did not affect body weight gains or spleen/body weight ratios.
In a previous study by Nagao et al (20), it was reported that
corticosteroid-induced weight loss was the result of adverse
reactions. In addition, the spleen mass reduction observed in the
DEX group could have reflected general immune suppression, which is
one of the major side effects of corticosteroids.
This study reports the anti-inflammatory effects of
MESC on CD in vivo. MESC effectively prevented ear swelling,
hyperplasia, spongiosis and the infiltration of immune cells into
inflamed tissues. In addition, levels of TNF-α, IFN-γ and IL-6 in
affected tissues were dose-dependently reduced by MESC. These
results suggest that MESC can effectively prevent inflammatory
reactions, and that it should be considered as a complementary or
alternative treatment for patients with inflammatory skin
diseases.
Acknowledgments
This study was supported by the National Research
Foundation of Korea funded by the Korean government (grant no.
2014R1A5A2009936).
References
|
1
|
Huang T, Shen P and Shen Y: Preparative
separation and purification of deoxyschisandrin and
gamma-schisandrin from Schisandra chinensis (Turcz) Baill by
high-speed counter-current chromatography. J Chromatogr A.
1066:239–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panossian A and Wi k man G: Pha r macology
of Schisandra chinensis Bail: an overview of Russian research and
uses in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang YH and Shin HM: Inhibitory effects of
Schizandra chinensis extract on atopic dermatitis in NC/Nga mice.
Immunopharmacol Immunotoxicol. 34:292–298. 2012. View Article : Google Scholar
|
|
4
|
Chiu PY, Lam PY, Yan CW and Ko KM:
Schisandrin B protects against solar irradiation-induced oxidative
injury in BJ human fibroblasts. Fitoterapia. 82:682–691. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lam PY, Yan CW, Chiu PY, Leung HY and Ko
KM: Schisandrin B protects against solar irradiation-induced
oxidative stress in rat skin tissue. Fitoterapia. 82:393–400. 2011.
View Article : Google Scholar
|
|
6
|
Kang JI, Kim SC, Hyun JH, Kang JH, Park
DB, Lee YJ, Yoo ES and Kang HK: Promotion effect of Schisandra
nigra on the growth of hair. Eur J Dermatol. 19:119–125.
2009.PubMed/NCBI
|
|
7
|
Liu C, Zhang S and Wu H: Non-thermal
extraction of effective ingredients from Schisandra chinensis Baill
and the antioxidant activity of its extract. Nat Prod Res.
23:1390–1401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY,
Hong YN, Kang SS, Kim HP and Kim YS: Anti-inflammatory effects of
schisandrin isolated from the fruit of Schisandra chinensis Baill.
Eur J Pharmacol. 591:293–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diepgen TL: Occupational skin diseases. J
Dtsch Dermatol Ges. 10:297–313. 2012.PubMed/NCBI
|
|
10
|
Cohen DE and Heidary N: Treatment of
irritant and allergic contact dermatitis. Dermatol Ther.
17:334–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HH, Lee JS, Yun H, Hwang G and Chong
MS: Effect of Schisandrae chinensis fructus on keratinocyte damage
by UV irradiation. Korean J Oriental Physiology and Pathology.
26:330–337. 2012.
|
|
12
|
Oskeritzian CA: Mast cell plasticity and
sphingosine-1-phosphate in immunity, inflammation and cancer. Mol
Immunol. 63:104–112. 2015. View Article : Google Scholar
|
|
13
|
Sebastiani S, Albanesi C, De PO, Puddu P,
Cavani A and Girolomoni G: The role of chemokines in allergic
contact dermatitis. Arch Dermatol Res. 293:552–559. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ou M: China-English manual of common-used
prescriptions in traditional Chinese medicine. Guangdong Science
and Technology Press; Guangzhou, China: pp. 69–70. 1992
|
|
15
|
Jung GT, Ju IO, Choi JS and Hong JS: The
antioxidative, antimicrobial and nitrite scavenging effects of
Schisandra chinensis RUPRECHT (Omija) seed. Korean J Food Sci
Technol. 32:928–935. 2000.
|
|
16
|
Yasukawa K, Ikeya Y, Mitsuhashi H, Iwasaki
M, Aburada M, Nakagawa S, Takeuchi M and Takido M: Gomisin A
inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in
two-stage carcinogenesis in mouse skin. Oncology. 49:68–71. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huyke C, Engel K, Simon-Haarhaus B, Quirin
KW and Schempp CM: Composition and biological activity of different
extracts from Schisandra sphenanthera and Schisandra chinensis.
Planta Med. 73:1116–1126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang OH, Chae HS, Choi JH, Choi HJ, Park
PS, Cho SH, Lee GH, So HY, Choo YK, Kweon OH and Kwon DY: Effects
of the Schisandra fructus water extract on cytokine release from a
human mast cell line. J Med Food. 9:480–486. 2006. View Article : Google Scholar
|
|
19
|
Kanda N and Watanabe S: Histamine enhances
the production of granulocyte-macrophage colony-stimulating factor
via protein kinase Calpha and extracellular signal-regulated kinase
in human keratinocytes. J Invest Dermatol. 122:863–872. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagao K, Akabane H, Masuda T, Komai M,
Tanaka H and Nagai H: Effect of MX-68 on airway inflammation and
hyper-responsiveness in mice and guinea-pigs. J Pharm Pharmacol.
56:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|