Introduction
Obesity is an increasing public health problem
worldwide, which is correlated with an increased risk of certain
types of cancer, including breast, colon and prostate cancer
(1). The underlying mechanisms
regarding the increased cancer risk as a result of obesity are
currently unclear. Obese individuals have an increased risk of
developing cancer, as compared with normal weight individuals
(2). In patients with cancer,
oxidative stress may take place either at the onset of disease or
as a function of disease progression (3). Adipose tissue is an endocrine organ
that produces biologically active substances, including leptin,
ghrelin, vaspin, visfatin, resistin and adiponectin (4). Recent studies have suggested that
adipose-derived hormones and cytokines, termed adipokines, such as
leptin, adiponectin and inflammatory markers, may be associated
with mechanisms linked to tumorigenesis (2).
Ghrelin has been identified as a hormone that is a
natural ligand of the orphan growth hormone secretagogue (GHS)
receptor type 1a (GHS-R1a) (5).
Ghrelin is a unique acylated 28-amino acid peptide, which is
produced and secreted by the X/A-like cells of the oxyntic glands
of the stomach. Ghrelin stimulates growth hormone (GH) secretion,
gastric motility, food intake, gastric acid secretion, modulation
of pancreatic exocrine and endocrine functions, and modulation of
the proliferation of neoplastic cells, as well as exhibiting
effects on the immune system (6–8). Two
major molecular forms of ghrelin are found in the stomach and
plasma: Acylated and deacylated ghrelin (9). Ghrelin is commonly expressed in
various organs. The highest levels of ghrelin are found in the
small intestine, pancreatic islet cells, gallbladder, liver, spleen
and immune cells (10). Recent
evidence has demonstrated that ghrelin has dual proliferative
effects, according to the type of cell or tissue, via mechanisms
that are dependent on or independent of GHS-R1a (11). In addition, ghrelin has possible
antioxidant and anti-inflammatory effects (7,12).
Ghrelin exhibits a strong gastroprotective role, at least in part
due to its anti-inflammatory actions (13). The marked gastroprotective effects
of ghrelin are supposedly derived from its antioxidant properties
(7). An in vitro study of
human polymorphonuclear cells incubated with ghrelin demonstrated
that ghrelin was able to inhibit the generation of reactive oxygen
species (ROS), as measured by chemiluminescence. The mechanism by
which ghrelin inhibits ROS may be through the blockade of enzymes
required for their production (14).
Leptin (OB protein) is a 167-amino acid peptide
hormone that regulates food intake and energy balance. Leptin is a
protein product of the ob gene, which is mainly expressed in
adipose tissue, with receptors located in the central nervous
system and in peripheral tissue (15). ObRb is the main isoform of the
leptin receptor, which is expressed by colonocytes and has been
shown to be preserved in human colonic adenomas and carcinomas
(16). Leptin promotes body weight
loss by acting on the brain, in order to decrease food intake and
increase sympathetic nervous system activity (17). The antioxidant enzymes catalase
(CAT) and glutathione peroxidase (GSH-Px) are known to be lower in
ob/ob mice, and leptin treatment is able to correct these
alterations (18). Recent studies
have suggested that leptin may be associated with mechanisms
underlying tumorigenesis and cancer progression (19–21).
Melatonin (N-acetyl-5-methoxytryptamine) is a pineal
hormone with structural similarities to 5-hydroxytryptamine.
Melatonin is able to act via MT1, MT2 and MT3 membrane receptors,
and the retinoid nuclear receptor RZR/ROR (22). Melatonin is capable of reducing
free radical damage by acting directly as a free radical scavenger,
and indirectly, by stimulating the activities of antioxidant
enzymes (23). In addition,
melatonin protects against lipid peroxidation and decreases the
synthesis of malondialdehyde (MDA), which is an end-product of
lipid peroxidation in non-cancerous cells (24,25).
Numerous studies have demonstrated that melatonin has important
oncostatic properties (26–29).
It has been demonstrated that melatonin inhibits cell proliferation
in various cancer cell lines, including human B-lymphoma cells,
HL-60 human myeloid leukemia cells and human neuroblastoma cancer
cells (27). Fan et al
(27) suggested that chemotherapy
combined with melatonin may increase the therapeutic effects of
anticancer drugs. In addition, Osseni et al (28) previously demonstrated that
melatonin can act as an antioxidant and pro-oxidant on a human
liver cell line, depending on the concentration and the duration of
incubation. Normal human cells produce minimal amounts of ROS,
which are reduced by antioxidant enzymes and low molecular weight
radical scavengers; however, the blood levels of ROS are
significantly higher in patients with cancer, as compared with
healthy donors (3).
The present study aimed to determine the effects of
the adipokines ghrelin and leptin, and the melatonin, on
intracellular ROS levels and the activity of selected antioxidant
enzymes: Superoxide dismutase (SOD), CAT and glutathione peroxidase
(GSH-Px). In addition, the effects of these adipokines and
melatonin were determined on the viability of HCT 116 human
colorectal carcinoma cells in vitro.
Materials and methods
Cell culture
The HCT 116 human colorectal carcinoma cell line was
obtained from the Silesian University of Technology, (Gilwice,
Poland) as a gift from Dr M. Skonieczna. The cells were plated at a
density of 5×105 cells per dish (10 cm2) and
were cultured in McCoy’s 5A modified medium (Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS;
BioWhittaker™, Lonza, Verviers, Belgium), 100 U/ml penicillin and
0.1 mg/ml streptomycin (BioWhittaker™), at 37°C in an atmosphere
containing 95% air and 5% CO2. The HCT 116 cell line was
free of mycoplasma, pathogenic viruses and bacteria.
BacT/ALERT®3D automated microbial detection system was
used to assess microbial contamination of the culture medium
(bio-Mérieux, Marcy l’Etoile, France). Mycoplasma species
identification were made using ELISA assay with commercial
diagnostic Mycoplasma detection kit (Roche, Mannheim, Germany),
according to the manufacturer’s instructions. The kit contains
species-specific polyclonal antibodies to Acholeplasma
laidlawii, Mycoplasma arginini, M. hyorhinis and
M. orale. The cell cultures were maintained for no longer
than 4 weeks after recovery from the frozen stock.
Experimental protocol
Cell cultures with or without ghrelin
(10−8 M), leptin (10−6 M) and melatonin
(10−6 M) (all Sigma-Aldrich) were incubated for 24 h.
The substances were applied separately in ROS and antioxidant
enzyme assays. Melatonin was dissolved in a minimum amount of
ethanol (95%), and then diluted with the aforementioned medium, in
order to reach the final concentration of 10−6 M. An
equivalent quantity of ethanol (95%) was added to the control
cells, and all remaining studied groups treated solely with ghrelin
or leptin. Leptin and ghrelin were dissolved in growth medium to
reach the final concentrations of 10−6 M and
10−8 M, respectively. Incubation media were not changed
during this time. Following the incubation period, the cell
supernatants were removed, centrifuged and maintained at −80°C
until further use.
Intracellular ROS detection
Intracellular ROS levels were detected in 2′,
7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA)-loaded cells (Molecular Probe, Leiden,
Netherlands) using a fluorescent measurement system (Astroscan
Cytofluor 2300/2350, Millipore, Billerica, MA, USA). HCT-116 cells
(2×106), untreated or treated with ghrelin, leptin or
melatonin for 24 h, were plated in Corning six-well plates
(Sigma-Aldrich) and were pre-incubated with 5 µM
H2DCF-DA for 1 h at 37°C. The plates were then
centrifuged at 250 × g for 10 min, and the fluorescence of the
control and treated cells was measured using a Cytofluor reader
(excitation at 504 nm, emission at 526 nm). The background of
deacetylated, oxidized DCF (2′, 7′-dichlorofluorescein) was 65–85
relative fluorescent units (R.F.U.) (30,31).
Enzymatic assays
Following exposure of the cultured cells to
melatonin (10−6 M), ghrelin (10−8 M) or
leptin (10−6 M) for 24 h, the antioxidative enzyme
activity of MnSOD, CuZnSOD, GSH-Px and CAT, and the levels of MDA
were measured in the cell supernatants.
The method of Paglia and Valentine (32) was used to determine GSH-Px activity
with minor modifications (33).
Briefly, the HCT 116 cells were pooled to a density of
5×106 cells/µl. Following centrifugation at 600 ×
g for 5 mins, the cell pellet was mixed with 200 µl cell
lysis buffer, containing 2.5 M NaCl, 100 mM Na2-EDTA, 10
mM Tris-HCl, 1% Triton X-100 (POCH, Gliwice, Poland) and sonicated
for 10 sec. Cell lysates were obtained by centrifugation at 17,000
× g for 30 min at 4°C. The protein concentration was then measured
using Bio-Rad protein assay dye reagent (cat.no. 500-0006; Bio-Rad
Laboratories, Hercules, CA, USA). Equal volumes of each sample,
containing 30 µg protein, were mixed with 2.68 ml of 0.05 M
phosphate buffer (pH 7.0) supplemented with 0.005 M EDTA. The
following solutions were then added sequentially: 100 µl of
0.0084 M NADPH (a reduced form of nicotinamide adenine dinucleotide
phosphate), 10 µl glutathione reductase, 10 µl of
1.125 M sodium nitrate, and 100 µl of 0.15 M reduced
glutathione. The enzymatic reaction was initiated by the addition
of 100 µl of 0.0022 M H2O2. The
conversion of NADPH to oxidized NADP+ was monitored by
continuous recording of the absorbance at a wavelength of 340 nm
using a microplate reader (Dynex Technologies, VA, USA), between 2
and 4 min after the initiation of the reaction. The control
measurements were recorded in a simultaneous assay, where the
sample was replaced with an equal volume of cell lysis buffer. One
IU GSH-Px enzyme activity is defined as 1 mM NADPH converted to
NADP+ per mg of protein (IU/mg p.).
The SOD activity assay was conducted based on a
procedure described by Paoletti and Mocali (34), with minor modifications (33). The preparation of cell lysates and
the measurement of protein concentration were the same as that
described prior to the GSH-Px assay. Equal amounts of protein (30
µg) from each sample were mixed with 800 µl of 1X
triethanolamine-diethanolamine buffer (pH 7.4), 40 µl of 7.5
mM NADPH, and 25 µl of 100 mM EDTA-MnCl2. The
reaction was initiated by the addition of 0.1 ml of 10 mM
mercaptoethanol. The decrease in absorbance was measured at a
wavelength of 340 nm over 20 min, at room temperature. The control
consisted of a reaction mixture in which the sample was replaced
with an equal volume of cell lysis buffer.
For the determination of MnSOD activity, CuZnSOD
activity was inhibited by incubating the samples with 5 mM
potassium cyanide (KCN) for 30 min. The samples were assayed for
MnSOD activity within 2 h of the addition of KCN. Total specific
SOD and MnSOD (following inhibition of CuZnSOD with KCN) activity
levels were measured, and then CuZnSOD activity was calculated. The
total activity of SOD was detected using methods of Paoletti and
Mocali without KCN inhibition, according to absorbance obtained in
cell lysates. The activity of MnSOD was calculated by reading the
absorbance in cell lysates following treatment with the CuZnSOD
inhibitor, KCN. The activity of CuZnSOD was calculated using the
formula: CuZnSOD activity = total SOD - MnSOD activity. The
enzymatic activity of both SOD isoenzymes was expressed in Nitric
Units (NU) per mg of protein (NU/mg p.). One NU represents 50%
inhibition by SOD of the nitrosol ion formation under these
conditions.
Catalase (CAT) activity was measured according to
the kinetic method of Aebi (35),
and was expressed as kIU per mg of protein (kIU/mg p.).
Concentrations of MDA were determined according to the colorimetric
method described by Ohkawa et al (36) using a reaction with thiobarbituric
acid. Levels of MDA were expressed as µmol MDA per mg of
protein (µmol MDA/mg p.)
Assessment of cell viability
Cell viability was evaluated using
alamarBlue® reagent (Invitrogen Life Technologies,
Paisley, UK), according to the manufacturer’s instructions. The
cells were seeded in 96-well culture plates at a density of
1.5×104 cells/well. The control cells were incubated in
McCoy’s 5A medium supplemented with 5% FBS, whereas the treated
cells were treated with ghrelin (10−8 M), leptin
(10−6 M) or melatonin (10−6 M), separately or
in combination for 24 h. The medium was refreshed daily, with new
medium and new test compounds. The alamarBlue® stock
solution was aseptically added to the wells, following 24 h of
culture, in amounts equal to 10% of the incubation volume.
Resazurin reduction was determined following a 4 h incubation by
measuring the fluorescence at a wavelength of 560 nm
(excitation)/590 nm (emission), using an Astroscan Cytofluor
microplate reader (Millipore).
Statistical analysis
The normality of distribution was evaluated by means
of Shapiro-Wilk’s test. Data are expressed as the mean ± standard
deviation from four independent experiments, each performed in
triplicate (n=12; four experiments performed in tripilicate).
Statistical analyses were performed using Statistical 7.0 software
(Statsoft, Krakow, Poland). The cell viability assay data were
analyzed by one-way analysis of variance (ANOVA), followed by
Tukey’s honestly significant difference multiple range test. For
all enzymatic assays, data were analyzed with one-way ANOVA with
post-hoc Bonferroni corrections. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ghrelin, leptin and melatonin
on ROS levels, antioxidant enzyme activity and MDA levels in
HCT-116 cells
Treatment with leptin (10−6 M) did not
affect the production of ROS after 24 h, whereas treatment with
melatonin at a 10−6 M concentration resulted in
increased ROS production by ~60% in the tested HCT 116 cells, as
compared with the control group (P<0.05). Conversely,
administration of ghrelin (10−8 M) resulted in a
decrease in the intracellular levels of ROS by ~20% in the tested
cells, as compared with the untreated cells (P<0.05).
Significant differences were also detected between ROS levels in
the HCT 116 cells stimulated solely with ghrelin or melatonin, as
compared with all of the remaining studied groups (P<0.05). The
intracellular ROS levels of the HCT 116 cells treated with the
various substances are shown in Fig.
1.
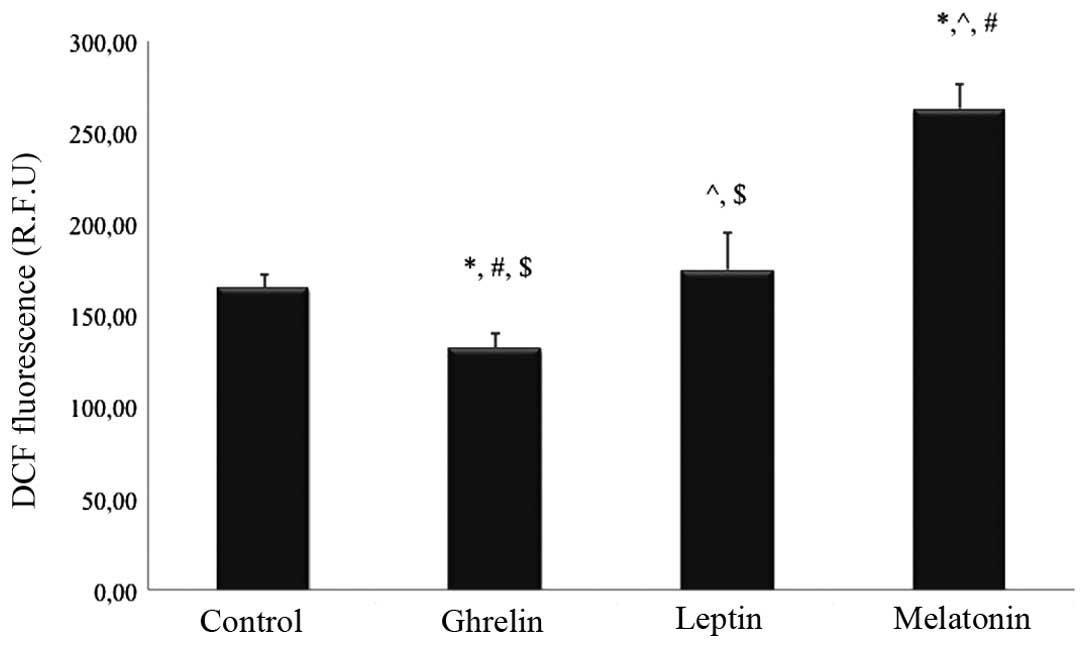 | Figure 1Effects of 24 h ghrelin
(10−8 M), leptin (10−6 M) and melatonin
(10−6 M) treatment on the intracellular ROS levels of
HCT 116 human colorectal cancer cells. DCF-detectable ROS were
measured in all of the study and control (untreated cells) groups.
Values are expressed as R.F.U, and were analyzed by one-way
analysis of variance with post-hoc Bonferroni correction. Data are
expressed as the mean ± standard deviation (n=12).
*P<0.05, vs. control; ^P<0.05, vs. the
ghrelin-treated group; #P<0.05, vs. the
leptin-treated group; $P<0.05, vs. the
melatonin-treated group. ROS, reactive oxygen species; DCF, 2′,
7′-dichlorofluorescein; R.F.U, relative fluorescent units. |
The present study also evaluated the activity of
selected antioxidant enzymes in HCT 116 cells following treatment
with ghrelin, leptin or melatonin. Compared with the control cells,
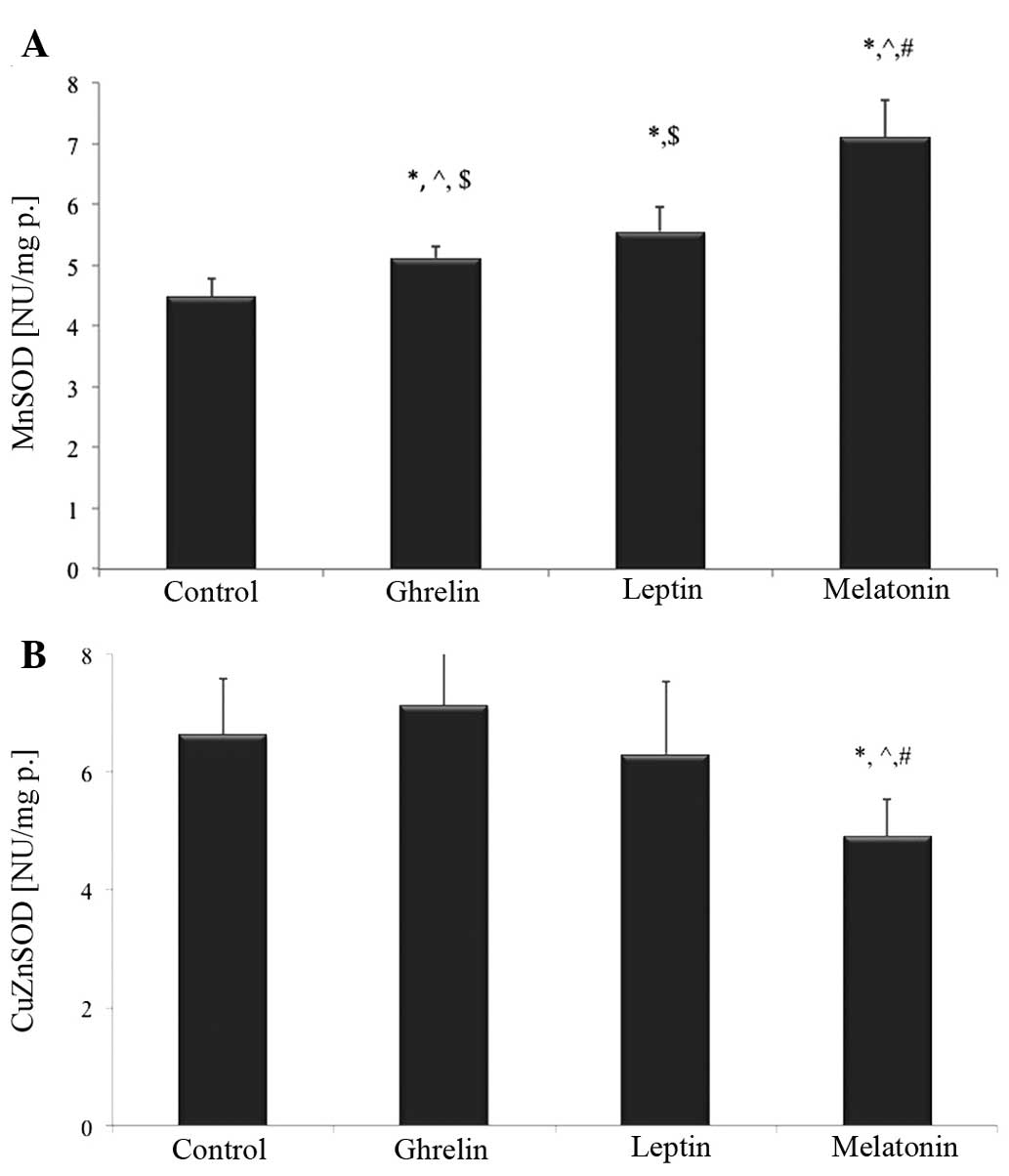
MnSOD activity was significantly higher following treatment with
melatonin, ghrelin and leptin by ~52, 14 and 24%, respectively
(P<0.05; Fig. 2A).
Notably, treatment with melatonin alone resulted in
a significant decrease in CuZnSOD activity by ~28%, as compared
with the control group (P<0.05). CuZnSOD activity was also ~40%
lower in the melatonin-treated group, as compared with the cells
exposed to ghrelin in combination with leptin (P<0.05). There
were no statistically significant differences in CuZnSOD activity
following treatment with ghrelin or leptin, as compared with the
untreated cells (Fig. 2B).
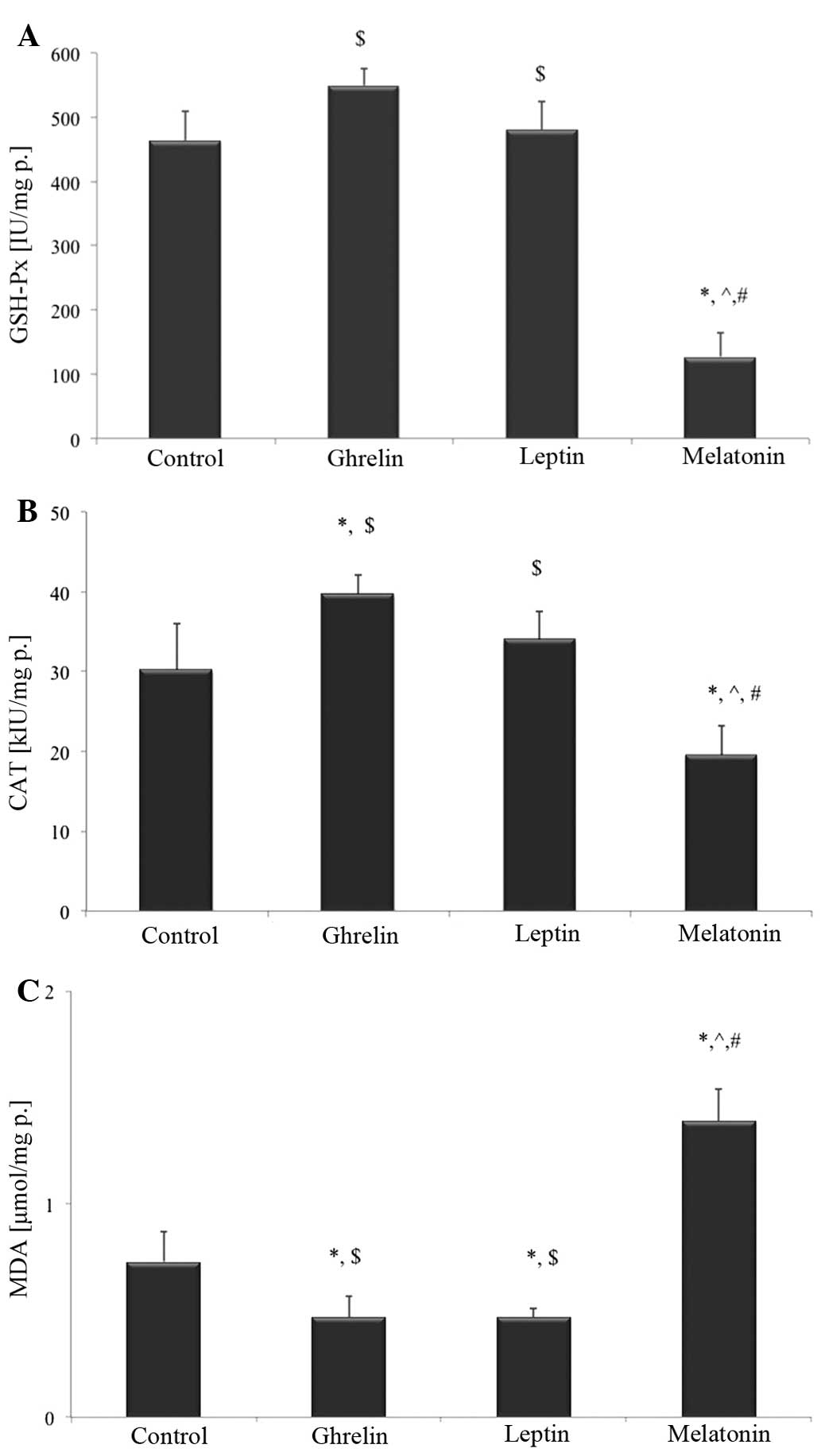
No statistically significant differences were
detected in GSH-Px activity following treatment with ghrelin or
leptin, as compared with the control group. Treatment with
melatonin decreased GSH-Px activity by ~3-times, as compared with
the control group (P<0.05; Fig.
3A). Furthermore, incubation with melatonin resulted in
decreased CAT activity by ~40%, as compared with the control group
(P<0.05). CAT activity was also decreased, as compared with all
of the remaining studied groups (P<0.05). Conversely, treatment
with ghrelin resulted in a significant increase in CAT activity by
~30%, as compared with the untreated cells (P<0.05). CAT
activity was also increased in the HCT 116 cells treated solely
with ghrelin, as compared with the melatonin-treated cells
(P<0.05). Leptin administration did not cause any statistically
significant changes in CAT activity in the HCT 116 cells, as
compared with the control cells (Fig.
3B).
Following 24 hours of treatment, ghrelin and leptin
decreased MDA levels by ~38%, as compared with the untreated cells
(P<0.05). MDA levels were ~2-fold higher in the
melatonin-treated cells, as compared with the control cells
(P<0.05; Fig. 3C).
Effects of ghrelin, leptin and melatonin
on HCT 116 cell viability
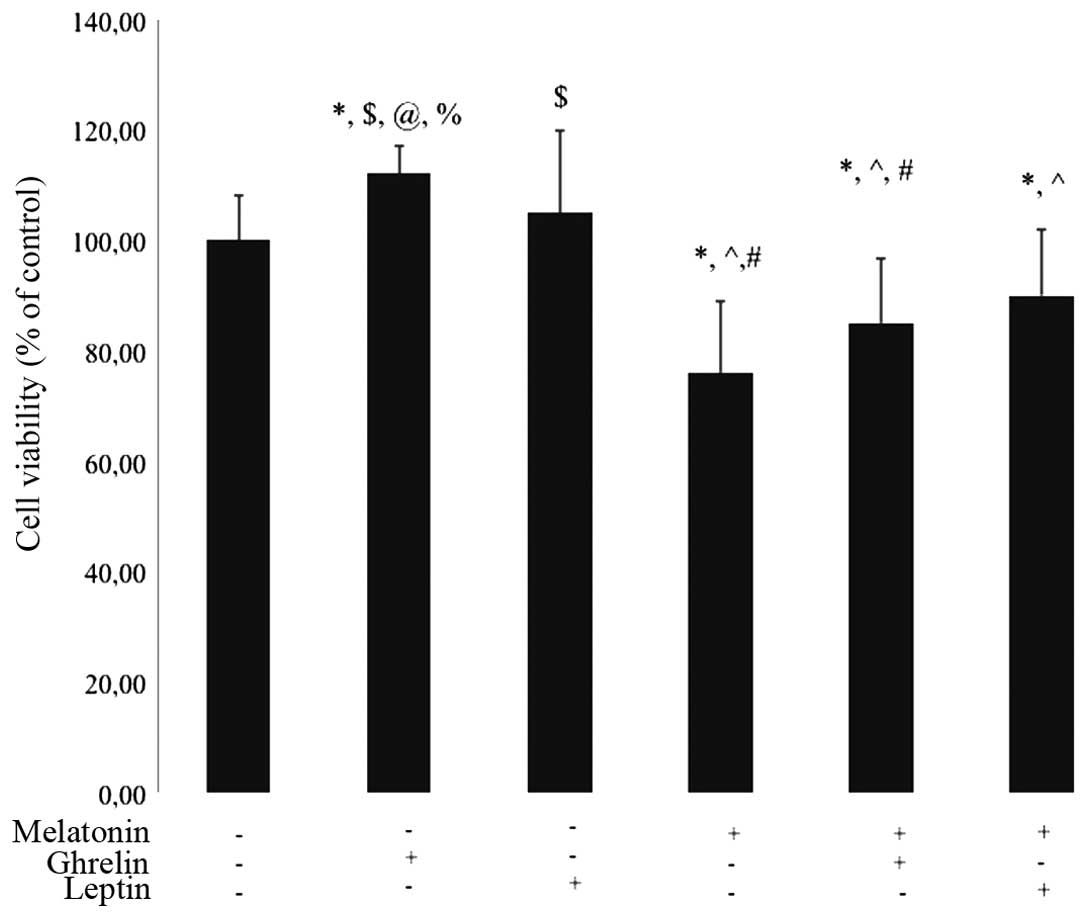
The growth of HCT 116 cells was determined in
response to treatment with melatonin, ghrelin and leptin, either
separately or in combination, for 24 h. Cell viability was
decreased following treatment with melatonin (10−6 M) at
24 h (76% of control; P<0.05). In the group treated with
melatonin (10−6 M) in combination with ghrelin
(10−8 M) the cells also exhibited decreased cell
viability, as compared with the control group (85% of control;
P<0.05). No statistical differences were observed between the
cell viability of HCT 116 cells treated solely with leptin for 24
h, as compared with the control cells. Conversely, ghrelin
administration alone (10−8 M) resulted in a slight
increase in cell viability, as compared with the untreated cells
(112% of control; P<0.05; Fig.
4). In addition, there was a significant increase in cell
viability in the cells treated solely with ghrelin, as compared
with the groups treated with the following combinations: Ghrelin +
melatonin, and leptin + melatonin (P<0.05).
Discussion
Obesity represents a significant risk factor for the
development of cancer; however, the mechanisms underlying this
association remain to be determined. It has been suggested that
certain adipose tissue-derived hormones may significantly influence
the growth and proliferation of tumorous stroma and malignant cells
(1). Recent evidence has
demonstrated that key mechanisms of cancer are linked via
endogenous stress-induced DNA damage, which is caused by ROS
(37). Oxidative stress induced by
a cellular redox imbalance has been detected in various types of
cancer cells (38). Certain
enzymatic antioxidants, including MnSOD, CuZnSOD, CAT and GSH-Px,
are able to remove ROS and prevent oxidative stress.
The present study evaluated the antioxidant enzyme
activities, ROS levels and cell viability of human derived
colorectal carcinoma HCT 116 cells following treatment with
melatonin, ghrelin and leptin.
In obese individuals, serum levels of leptin are
markedly increased, and a recent study indicated that leptin is
overexpressed in human colorectal cancer, suggesting that leptin
may contribute to the development and progression of colorectal
cancer (39). The majority of
studies have demonstrated that leptin may increase the growth of
cancer cells in vitro (40–44).
It has also been shown that leptin increases the production of ROS
in various cancer cell lines (45,46).
Conversely, in the present study, treatment with leptin
(10−6 M) did not affect the production of ROS, and had
no effect on HCT 116 cell viability. Lack of ROS overproduction in
the present study may be explained by the fact that leptin
treatment resulted in increased activity of selected antioxidant
enzymes, which are able to remove ROS from the cells. The results
of the present study demonstrated that treatment with leptin
significantly increased MnSOD activity, and as a result, decreased
the concentration of MDA. In the present study, leptin had no
effect on the activities of CAT and GSH-Px. However, in our
previous findings treatment with leptin (10−6 M)
markedly increased SOD, CAT and GSH-Px activity in 3T3-L1
pre-adipocytes, after 24 h of exposure. Furthermore, leptin
stimulation also failed to influence the cell count of tested cells
(47). The dose of leptin used in
the present study was able to induce the activity of selected
antioxidant enzymes, without altering HCT 116 cell viability.
Conversely, Balasubramaniyan et al (48) demonstrated a decrease in viability
of HepG2 hepatocellular cancer cells alongside elevated SOD and CAT
activity following leptin treatment; however, the dose of leptin in
this previous study was ~10-times greater than in the present
study. In the present study, leptin and ghrelin decreased MDA
levels in HCT 116 cells. MDA is the end product of the lipid
peroxidation process, and the amount of MDA can express the degree
of oxidative stress in living cells. The lipid peroxidation process
has previously been implicated in the mechanism of carcinogenesis.
MDA is mutagenic in bacterial and mammalian cells, and is
carcinogenic in rats (38). Our
previous findings suggested that another adipose tissue-derived
adipokine, visfatin, may be able to trigger redox adaptation
responses, leading to an increased antioxidant capacity alongside
decreased levels of lipid peroxidation in Me45 melanoma cells.
Furthermore, visfatin led to a significantly increased rate of
proliferation and viability of Me45 melanoma cells (33).
Ghrelin has been suggested to possess both
antioxidant and anti-inflammatory effects (7,12).
studies using human polymorphonuclear cells incubated with ghrelin,
demonstrated that ghrelin inhibited ROS generation (14). The present study also observed
similar ROS depletion (~20 %) in HCT 116 cells treated with
ghrelin. Conversely, Li et al (48) demonstrated that ghrelin did not
reduce the levels of intracellular ROS in human endothelial cells
treated with H2O2. This different observation
may be explained by variations in experimental design regarding
cell type, concentration of ghrelin and duration of incubation, as
well as the number of receptors activated. In the present study the
HCT 116 cells were incubated with ghrelin at a 10−8 M
concentration. The system used in the present study allowed the
measurement of ROS (mainly H2O2) using the
oxidant sensitive H2DCFDA probe, whereas the system used
by Li et al (49), measured
complete ROS level by chemiluminescence.
The present study also observed a slight increase in
HCT 116 cell viability (112% of control) following treatment with
ghrelin (10−8 M) for 24 h, and this may be due to ROS
depletion. Concordant with the results of the present study, Tian
and Fan (11) previously
demonstrated that the same concentration of ghrelin enhanced the
viability of AGS gastric cancer cells in vitro. The
pro-proliferative role of ghrelin in AGS cells was accompanied by
the increased percentage of cells in S phase of the cell cycle, as
compared with the untreated cells (59.2 vs. 43.2%, respectively)
(11). Both studies suggest that
ghrelin may have a role in the development and progression of
gastric and colon cancer. The role of ghrelin in the initiation and
progression of gastrointestinal cancer requires further
investigation.
In the present study ghrelin also significantly
increased CAT and MnSOD activity. CAT and MnSOD are endogenous
antioxidant enzymes that protect against ROS-induced damage. MnSOD
is one of the most important intracellular antioxidant enzymes, and
CAT is the most important enzyme that metabolizes exogenous
H2O2 into water and molecular oxygen
(10). The significantly decreased
capacity of various types of tumor for detoxifying
H2O2 has previously been linked to a
decreased level of CAT (50).
Similarly, in non-cancerous H9c2 cardiomyocytes, ghrelin at a
10−8 M concentration significantly increased the mRNA
expression levels and activity of MnSOD and CAT after 24 h of
incubation (51). Furthermore, the
present study demonstrated that ghrelin had no effect on GSH-Px;
however, it was able to decrease the concentration of MDA.
Kheradmand et al (52)
demonstrated that ghrelin was able to enhance SOD activity in an
animal model of ovarian cancer; however, it failed to induce overt
changes in GSH-Px activity. In addition, ghrelin markedly increased
SOD activity, which is a key antioxidant enzyme that targets
oxidative damage, in rat ovarian tissue by >3-fold, as compared
with the control group (52). The
findings of the present study were concordant with the results
obtained from normal, non-cancerous cells and tissues in other
studies (51,52), and suggest that ghrelin may enhance
the endogenous antioxidant defense mechanisms through the
upregulation of intracellular antioxidant enzymes, including CAT
and MnSOD. In the present study, ghrelin protected the HCT 116
cells against oxidative stress and lipid peroxidation.
The results of the present study also demonstrated
that melatonin had an effect on antioxidant enzyme activities, ROS
levels and HCT 116 cell viability, after a 24 h incubation.
Treatment with melatonin decreased CAT and GSH-Px activity, and
increased MDA and ROS levels. Free radical-induced cell damage may
be quantitatively assessed by measuring MDA levels, which are an
indicator of lipid peroxidation. The present study suggested that
melatonin had pro-oxidant properties in HCT 116 cells. Osseni et
al (28), was the first to
demonstrate that melatonin can have a pro-oxidant effect, depending
on its concentration and the duration of incubation in HepG2
hepatocarcinoma cells. Furthermore, ROS overproduction and
increased MDA levels may directly influence HCT 116 cell viability.
In the present study, melatonin at a 10−6 M
concentration had pro-oxidant properties, which resulted in
decreased HCT 116 cell viability (76% of control, untreated cells).
These results are concordant with the observations of other studies
(28,53). Roth et al (53), also revealed that melatonin, at
analogous experimental concentrations, resulted in decreased PC-12
prostate cancer cell viability, as compared with the control cells
(85.5% of control). The antiproliferative effects of melatonin have
also been investigated in numerous cell culture systems, including
the growth of chick skeletal muscle cells (54), ME-180 human cervical cancer cells
(55), AH 130 rat hepatoma cells
(56), human melanoma cells
(57), human benign (58) and malignant prostate cells
(59), and ovarian carcinoma cells
(29).
The pro-oxidant and growth inhibitory effects of
melatonin may be explained by the increased levels of intracellular
ROS, as well as the decreased antioxidant capacity exhibited in the
melatonin-treated cells. These observations are concordant with the
findings of other studies, which revealed that melatonin was able
to stimulate the production of ROS in tumor cells (60,61),
resulting in apoptosis (62).
Pirozhok et al (61)
demonstrated that an analogous dose of melatonin (10−6
M) was able to inhibit the proliferation of PC-3 human prostate
cells, but did not alter the viability of the DU-145 prostate
cancer cell line. Furthermore, numerous studies have reported that
antioxidants, such as melatonin, may enhance the cytotoxic effects
of chemotherapeutic agents on cancer cells, depending on the dose,
form and type of cancer (63). For
instance, Kim et al (63),
demonstrated that combined treatment of cisplatin with melatonin
synergistically inhibited the viability of SK-OV-3 ovarian cancer
cells in vitro. Conversely, melatonin exhibited a protective
effect against cisplatin-induced cytotoxicity in OSEN normal
ovarian epithelial cells. Furthermore, Lamson and Brignall
(64) showed that treatment with
melatonin increased survival time and tumor response in patients
treated with tamoxifen, cisplatin and etoposide, thus suggesting
that melatonin may increase the chemotherapeutic effect of these
drugs (64). The modulation of
cell signaling pathways by antioxidants may aid in the prevention
of cancer by preserving normal cell cycle regulation, inhibiting
tumor invasion and angiogenesis, inhibiting proliferation, inducing
apoptosis and stimulating phase II detoxification enzyme activity
(37). Previous findings by the
authors of the present study demonstrated that another antioxidant,
vitamin E (α-tockopherol), also induced antioxidant enzyme activity
in AT478 carcinoma cells in vitro. AT478 cells subjected to
the action of vitamin E were shown to be more resistant to
exogenous oxidative stress, as compared with cells exposed solely
to a magnetic field (65).
In conclusion, the present study demonstrated that
ghrelin, leptin and melatonin have various affects on the
antioxidant capacity of HCT 116 cells. Compared with the
adipokines, treatment with melatonin increased ROS levels and
decreased cellular viability of tested cells. The present study
also had several limitations. The in vitro setting may not
fully reflect the more complex assocaitions in organisms, as a
whole, and therefore, the setting of the study may be kept in mind.
It is possible that the result may not be similar to what is
observed in living subjects
Acknowledgments
The present study was supported by a grant from the
Medical University of Silesia in Katowice (grant no.
KNW-1-011/N/1/0).
References
|
1
|
Housa D, Housová J, Vernerová Z and
Haluzík M: Adipocytokines and cancer. Physiol Res. 55:233–244.
2006.
|
|
2
|
Pischon T, Nöthlings U and Boeing H:
Obesity and cancer. Proc Nutr Soc. 67:128–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani G, Macciò A, Madeddu C, et al:
Reactive oxygen species, antioxidant mechanisms and serum cytokine
levels in cancer patients: Impact of an antioxidant treatment. J
Cell Mol Med. 6:570–582. 2002. View Article : Google Scholar
|
|
4
|
Wiecek A, Kokot F, Chudek J and Adamczak
M: The adipose tissue - a novel endocrine organ of interest to the
nephrologist. Nephrol Dial Transplant. 17:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Lely AJ, Tschöp M, Heiman ML and
Ghigo E: Biological, physiological, pathophysiological, and
pharmacological aspects of ghrelin. Endocr Rev. 25:426–457. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki H, Matsuzaki J and Hibi T: Ghrelin
and oxidative stress in gastrointestinal tract. J Clin Biochem
Nutr. 48:122–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X-T, Fan X-M and Zha X-L: Ghrelin
inhibits 5-fluorouracil-induced apoptosis in colonic cancer cells.
J Gastroenterol Hepatol. 26:1169–1173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nanzer AM, Khalaf S, Mozid AM, et al:
Ghrelin exerts a proliferative effect on a rat pituitary
somatotroph cell line via the mitogen-activated protein kinase
pathway. Eur J Endocrinol. 151:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gnanapavan S, Kola B, Bustin SA, et al:
The tissue distribution of the mRNA of ghrelin and subtypes of its
receptor, GHS-R, in humans. J Clin Endocrinol Metab. 87:29882002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian PY and Fan XM: The proliferative
effects of ghrelin on human gastric cancer AGS cells. J Dig Dis.
13:453–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barazzoni R, Zanetti M, Semolic A, Cattin
MR, Pirulli A, Cattin L and Guarnieri G: High-fat diet with
acyl-ghrelin treatment leads to weight gain with low inflammation,
high oxidative capacity and normal triglycerides in rat muscle.
PLoS One. 6:e262242011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konturek PC, Brzozowski T, Pajdo R, et al:
Ghrelin-a new gastroprotective factor in gastric mucosa. J Physiol
Pharmacol. 55:325–336. 2004.PubMed/NCBI
|
|
14
|
El Eter E, Al Tuwaijiri A, Hagar H and
Arafa M: In vivo and in vitro antioxidant activity of ghrelin:
Attenuation of gastric ischemic injury in the rat. J Gastroenterol
Hepatol. 22:1791–1799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aloulou N, Bastuji-Garin S, Le Gouvello S,
et al: Involvement of the leptin receptor in the immune response in
intestinal cancer. Cancer Res. 68:9413–9422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trayhurn P and Beattie JH: Physiological
role of adipose tissue: White adipose tissue as an endocrine and
secretory organ. Proc Nutr Soc. 60:329–339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watson AM, Poloyac SM, Howard G and Blouin
RA: Effect of leptin on cytochrome P-450, conjugation, and
antioxidant enzymes in the ob/ob mouse. Drug Metab Dispos.
27:695–700. 1999.PubMed/NCBI
|
|
19
|
Yuan Y, Zhang J, Cai L, et al: Leptin
induces cell proliferation and reduces cell apoptosis by activating
c-myc in cervical cancer. Oncol Rep. 29:2291–2296. 2013.PubMed/NCBI
|
|
20
|
Wu X, Yan Q, Zhang Z, Du G and Wan X:
Acrp30 inhibits leptin-induced metastasis by downregulating the
JAK/STAT3 pathway via AMPK activation in aggressive SPEC-2
endometrial cancer cells. Oncol Rep. 27:1488–1496. 2012.PubMed/NCBI
|
|
21
|
Yoon K-W, Park S-Y, Kim J-Y, et al:
Leptin-induced adhesion and invasion in colorectal cancer cell
lines. Oncol Rep. 31:2493–2498. 2014.PubMed/NCBI
|
|
22
|
Brydon L, Petit L, Delagrange P, Strosberg
AD and Jockers R: Functional expression of MT2 (Mel1b) melatonin
receptors in human PAZ6 adipocytes. Endocrinology. 142:4264–4271.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reiter RJ: Melatonin: Lowering the high
price of free radicals. News Physiol Sci. 15:246–250. 2000.
|
|
24
|
Baydaş G, Erçel E, Canatan H, Dönder E and
Akyol A: Effect of melatonin on oxidative status of rat brain,
liver and kidney tissues under constant light exposure. Cell
Biochem Funct. 19:37–41. 2001. View Article : Google Scholar
|
|
25
|
Rodriguez C, Mayo JC, Sainz RM, Antolín I,
Herrera F, Martín V and Reiter RJ: Regulation of antioxidant
enzymes: A significant role for melatonin. J Pineal Res. 36:1–9.
2004. View Article : Google Scholar
|
|
26
|
Martín-Renedo J, Mauriz JL, Jorquera F,
Ruiz-Andrés O, González P and González-Gallego J: Melatonin induces
cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line.
J Pineal Res. 45:532–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan L-L, Sun G-P, Wei W, Wang Z-G, Ge L,
Fu W-Z and Wang H: Melatonin and doxorubicin synergistically induce
cell apoptosis in human hepatoma cell lines. World J Gastroenterol.
16:1473–1481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osseni RA, Rat P, Bogdan A, Warnet JM and
Touitou Y: Evidence of prooxidant and antioxidant action of
melatonin on human liver cell line HepG2. Life Sci. 68:387–399.
2000. View Article : Google Scholar
|
|
29
|
Petranka J, Baldwin W, Biermann J, Jayadev
S, Barrett JC and Murphy E: The oncostatic action of melatonin in
an ovarian carcinoma cell line. J Pineal Res. 26:129–136. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
LeBel CP, Ischiropoulos H and Bondy SC:
Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of
reactive oxygen species formation and oxidative stress. Chem Res
Toxicol. 5:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bułdak RJ, Polaniak R, Bułdak L, et al:
Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced
oxidative response in AT478 murine squamous cell carcinoma cells.
Bioelectromagnetics. 33:641–651. 2012. View Article : Google Scholar
|
|
32
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
33
|
Bułdak RJ, Bułdak Ł, Polaniak R, et al:
Visfatin affects redox adaptative responses and proliferation in
Me45 human malignant melanoma cells: An in vitro study. Oncol Rep.
29:771–778. 2013.
|
|
34
|
Paoletti F and Mocali A: Determination of
superoxide dismutase activity by purely chemical system based on
NAD(P)H oxidation. Methods Enzymol. 186:209–220. 1990.PubMed/NCBI
|
|
35
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984.PubMed/NCBI
|
|
36
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anisimov VN, Popovich IG, Zabezhinski MA,
Anisimov SV, Vesnushkin GM and Vinogradova IA: Melatonin as
antioxidant, geroprotector and anticarcinogen. Biochim Biophys
Acta. 1757:573–589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valko M, Leibfritz D, Moncol J, Cronin
MTD, Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
39
|
Koda M, Sulkowska M, Kanczuga-Koda L,
Surmacz E and Sulkowski S: Overexpression of the obesity hormone
leptin in human colorectal cancer. J Clin Pathol. 60:902–906. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aparicio T, Kotelevets L, Tsocas A, et al:
Leptin stimulates the proliferation of human colon cancer cells in
vitro but does not promote the growth of colon cancer xenografts in
nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut.
54:1136–1145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou J, Lei W, Shen L, Luo H-S and Shen
Z-X: Primary study of leptin and human hepatocellular carcinoma in
vitro. World J Gastroenterol. 14:2900–2904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Catalano S, Marsico S, Giordano C, et al:
Leptin enhances, via AP-1, expression of aromatase in the MCF-7
cell line. J Biol Chem. 278:28668–28676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang S, Hursting SD, Contois JH, et al:
Leptin and prostate cancer. Prostate. 46:62–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gonzalez RR, Cherfils S, Escobar M, et al:
Leptin signaling promotes the growth of mammary tumors and
increases the expression of vascular endothelial growth factor
(VEGF) and its receptor type two (VEGF-R2). J Biol Chem.
281:26320–26328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamagishi SI, Edelstein D, Du XL, Kaneda
Y, Guzmán M and Brownlee M: Leptin induces mitochondrial superoxide
production and monocyte chemoattractant protein-1 expression in
aortic endothelial cells by increasing fatty acid oxidation via
protein kinase A. J Biol Chem. 276:25096–25100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu F-P, Chen M-S, Wang Y-Z, Yi Q, Lin S-B,
Chen AF and Luo J-D: Leptin induces hypertrophy via
endothelin-1-reactive oxygen species pathway in cultured neonatal
rat cardiomyocytes. Circulation. 110:1269–1275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zwirska-Korczala K, Adamczyk-Sowa M, Sowa
P, et al: Role of leptin, ghrelin, angiotensin II and orexins in
3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J
Physiol Pharmacol. 58(Suppl 1): 53–64. 2007.PubMed/NCBI
|
|
48
|
Balasubramaniyan V, Shukla R, Murugaiyan
G, Bhonde RR and Nalini N: Mouse recombinant leptin protects human
hepatoma HepG2 against apoptosis, TNF-alpha response and oxidative
stress induced by the hepatotoxin-ethanol. Biochim Biophys Acta.
1770:1136–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li WG, Gavrila D, Liu X, et al: Ghrelin
inhibits proinflammatory responses and nuclear factor-kappaB
activation in human endothelial cells. Circulation. 109:2221–2226.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tong X-X, Wu D, Wang X, et al: Ghrelin
protects against cobalt chloride-induced hypoxic injury in cardiac
H9c2 cells by inhibiting oxidative stress and inducing autophagy.
Peptides. 38:217–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kheradmand A, Alirezaei M and Birjandi M:
Ghrelin promotes antioxidant enzyme activity and reduces lipid
peroxidation in the rat ovary. Regul Pept. 162:84–89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roth JA, Rosenblatt T, Lis A and Bucelli
R: Melatonin-induced suppression of PC12 cell growth is mediated by
its Gi coupled transmembrane receptors. Brain Res. 919:139–146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lamosová D, Zeman M and Juráni M:
Influence of melatonin on chick skeletal muscle cell growth. Comp
Biochem Physiol C Pharmacol Toxicol Endocrinol. 118:375–379. 1997.
View Article : Google Scholar
|
|
55
|
Chen LD, Leal BZ, Reiter RJ, et al:
Melatonin’s inhibitory effect on growth of ME-180 human cervical
cancer cells is not related to intracellular glutathione
concentrations. Cancer Lett. 91:153–159. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cini G, Coronnello M, Mini E and Neri B:
Melatonin’s growth-inhibitory effect on hepatoma AH 130 in the rat.
Cancer Lett. 125:51–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Roberts JE, Wiechmann AF and Hu DN:
Melatonin receptors in human uveal melanocytes and melanoma cells.
J Pineal Res. 28:165–171. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gilad E, Laudon M, Matzkin H, et al:
Functional melatonin receptors in human prostate epithelial cells.
Endocrinology. 137:1412–1417. 1996.PubMed/NCBI
|
|
59
|
Lupowitz Z and Zisapel N: Hormonal
interactions in human prostate tumor LNCaP cells. J Steroid Biochem
Mol Biol. 68:83–88. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dziegiel P, Podhorska-Okolow M, Surowiak
P, Ciesielska U, Rabczynski J and Zabel M: Influence of exogenous
melatonin on doxorubicin-evoked effects in myocardium and in
transplantable Morris hepatoma in rats. In Vivo. 17:325–328.
2003.PubMed/NCBI
|
|
61
|
Pirozhok I, Meye A, Hakenberg OW, Fuessel
S and Wirth MP: Serotonin and melatonin do not play a prominent
role in the growth of prostate cancer cell lines. Urol Int.
84:452–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jang SS, Kim WD and Park W-Y: Melatonin
exerts differential actions on X-ray radiation-induced apoptosis in
normal mice splenocytes and Jurkat leukemia cells. J Pineal Res.
47:147–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim J-H, Jeong S-J, Kim B, Yun S-M, Choi Y
and Kim S-H: Melatonin synergistically enhances cisplatin-induced
apoptosis via the dephosphorylation of ERK/p90 ribosomal S6
kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res.
52:244–252. 2012. View Article : Google Scholar
|
|
64
|
Lamson DW and Brignall MS: Antioxidants in
cancer therapy; their actions and interactions with oncologic
therapies. Altern Med Rev. 4:304–329. 1999.PubMed/NCBI
|
|
65
|
Polaniak R, Bułdak RJ, Karoń M, et al:
Influence of an extremely low frequency magnetic field (ELF-EMF) on
antioxidative vitamin E properties in AT478 murine squamous cell
carcinoma culture in vitro. Int J Toxicol. 29:221–230. 2010.
View Article : Google Scholar : PubMed/NCBI
|