Introduction
Hepatocellular carcinoma (HCC) is one of the most
malignant types of cancer and is listed as the second most frequent
cause of cancer-associated mortality in males, and the sixth most
frequent cause in females worldwide (1). Surgical resection is the most
effective way of treating HCC, however, the majority of patients
present at an advanced, inoperable stage. These patients have a
poor prognosis and require other types of treatment (2). Palliative treatments, including
chemotherapy and radiation therapy only contribute to 6% of the
overall 5-year-survival rate in HCC (3). Therefore there is an urgent
requirement to develop novel therapeutic strategies for HCC.
It is understood that solid tumors cannot continue
to grow without the formation of new vessels (4). Unlimited tumor expansion requires
continuous angiogenesis to acquire sufficient oxygen and nutrients.
Angiogenesis, the formation of new blood vessels from the original
vasculature, is a complicated multi-step process, which involves a
number of signal transduction pathways (5). Tumors secrete a variety of
pro-angiogenic factors, among which, vascular endothelial growth
factor (VEGF) has been investigated in the most depth for its
association with tumor angiogenesis (6). VEGF binds to vascular endothelial
growth factor receptor (VEGFR), usually VEGFR-2, and activates
receptor tyrosine kinase. This causes a signal transduction series,
which induces and promotes endothelial cell proliferation and
migration, respectively, eventually leading to neovascularization
(6). Microvessel density (MVD) is
considered a golden standard in evaluating tumor angiogenesis
(7). To quantify the angiogenic
status, markers of endothelial cells, including Factor VIII, CD31
and CD34, have been used (8). With
the identification of novel biological markers of cancer, molecular
targeted therapies are considered the most promising strategies for
the management of patients with progressive HCC.
Heat shock protein 90 (HSP90) is a molecular
chaperone, which comprises 1–2% total cellular protein content and
regulates the correct conformation, activity, function and
stability of >200 client proteins (9). A variety of receptor tyrosine
kinases, including VEGFR, insulin-like (I)GFR, and epidermal GFR
are client proteins of HSP90, which depend on HSP90 to achieve
active conformation or to increase stability (9). The classic HSP90 inhibitors are
benzoquinone and ansamycins, including geldanamycin and its
derivative 17-allylamino-17-demethoxygeldanamcyin (17-AAG). The
inhibition of HSP90 has been achieved using the novel, low
molecular weight, adenosine triphosphate (ATP)-competitive
non-geldamycin HSP90 inhibitor, AUY922. This compound has been
considered to offer advantages over ansamycin and benzoquinone
HSP90 inhibitors, including 17-AAG, based on independence from the
metabolism of NAD(P)H quinone oxidoreductase 1, expression of
P-glycoprotein expression and favorable aqueous solubility
(10,11). In the present study, the expression
of HSP90 and MVD in HCC was investigated, and the antitumor
efficacy of the novel AUY922 HSP90 inhibitor, AUY922, in inhibiting
HCC cell proliferation and migration was evaluated.
Materials and methods
Patients and tissue samples
The present study was approved by the ethics
committee of the First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China). The pathology specimens and medical
records were reviewed from the First Affiliated Hospital of
Xinjiang Medical University database. The preoperative diagnosis
was based on the clinical history, symptoms, signs, endocrine
evaluation, imagine examination, including magnetic resonance
imaging and computed tomography. Paraffin-embedded pathological
specimens from 76 patients (51 males/25 females; mean age,
59.57±9.16 years old; age range between 42 and 69 years) with HCC
were obtained from the archives of the Department of Oncology,
First Affiliated Hospital of Xinjiang Medical University between
2010 and 2013. Adjacent non-tumorous liver tissues were obtained
from 12 of the patients (5 cm to the tumor area), to serve as
normal controls. The patients involved in offering samples for the
investigation signed informed consent forms.
Immunohistochemical analysis
To evaluate the expression levels of HSP90 and MVD
in ACC, immunohistochemical analyses were performed using an
EnVision method (DAKO, Glostrup, Denmark). Antigen retrieval was
achieved by microwave at 750 W for 15 min, and the sections were
incubated with 10% normal goat serum at room temperature for 10 min
to block non-specific reactions. This was followed by washing with
phosphate-buffered-saline (PBS) and incubation with polyclonal
mouse anti-human HSP90 antibody (Abcam, Cambridge, MA, USA) diluted
to 1:100 for 12 h at 4°C. CD34 was a monoclonal mouse anti-human
antibody (Novocastra Laboratories, Ltd., Newcastle-Upon-Tyne, USA),
diluted to 1:200. The positive controls were gastric carcinoma and
colon carcinoma, with positive expression levels of HSP90 and CD34.
PBS was used instead of the primary antibodies as a negative
control. The localization of immunostaining was demonstrated by
incubation with the EnVision-peroxidase system.
Evaluation of the immunohistochemical
results
The immunohistochemical analyses designated a result
as positive for HSP90 if purple-brown granules were located
diffusely in the cytoplasm of the tumor cells. The lack of any
purple-brown or brown-red pigmentation in the cytoplasm of a tumor
cell was considered negative (12). For HSP90 semi-quantitative
immunoanalysis, the percentage of positive staining in the tumor
cells was determined. A total of five representative high-power
fields were selected, and the number of positive-staining cells was
calculated. The following categories were used: Negative (−); weak
(+)=1–10%; moderate (++)=11–50%; strong (+++) ≥51%. The results
were scored by two independent pathologists in a blinded-manner
into tumor subtypes. A single microvessel was defined as any brown
or brownish yellow CD34-immunostained endothelial cell. The MVD was
evaluated using a method previously described by Weider (7): High vascular density areas were
selected under a low power objective and the numbers of vascular
cells stained with CD34 were determined in three visual fields
under a high power microscope (magnification, ×400). The average
value was regarded as the MVD value of the tumor sample.
Cell culture and reagents
A total of five HCC cell lines, SK-Hep1, HepG2,
HEK-293T, HCCLM3 and HuH7, and one normal liver cell line (L-02)
were obtained from American Type Culture Collection (ATCC,
Manassas, VA, USA) and were maintained in a humidified atmosphere
containing 5% CO2 at 37°C, which were cultured and
passaged in RPMI-1640 medium (Hyclone, Thermo Fisher Scientific,
Logan, UT, USA) containing 10% fetal bovine serum (FBS; Invitrogen
Life Technologies). The cells at logarithmic growth phase were
harvested for subsequent experiments once the cells had reaced 80%
confluence. The HCC cell lines were maintained in Dulbecco’s
modified Eagle medium (DMEM) (Invitrogen, Life Technologies)
supplemented with 10% FBS, 100 U/ml penicillin G and 100 mg/ml
streptomycin sulphate (Sigma-Aldrich, St. Louis, MO, USA). The
AUY922 HSP90 inhibitor was purchased from Selleck (Houston, TX,
USA). The compounds were dissolved at 10 mM in dimethylsulf-oxide
(DMSO) at stock solutions and stored at −20°C. Mouse anti-human
HSP90 antibody was obtained from Abcam.
Western blot analysis
Western immunoblot analyses were performed with
protein lysates obtained from snap-frozen HCC tissue samples and
cell lines. The protein levels were determined using a
Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology, Inc.
Rockford IL, USA). The respective tissue protein (30 µg)
were separated by 10% SDS-PAGE (using 10% gels) and transferred
onto polyvinylidene fluoride membranes (Millipore, Billerica, MA).
The membranes were blocked with 5% nonfat milk and then incubated
with monoclonal mouse anti-human HSP90 polyclonal antibody (1:100;
cat. no. ab13492; Abcam) and monclonal mouse anti-actin (1:10,000;
cat. no. 0869100; MP Biomedicals, Santa Ana, CA, USA). The
membranes were washed three times for 10 min each with
Tris-buffered saline (50 mM Tris, pH 7.4, 0.9% NaCl) containing
0.05% Tween-20 (TBS-T; Biohao, Beijing, China) and incubated with
phycoerythrin-conjugated secondary antibodies (1:2,000; donkey
anti-mouse IgG H&L; cat. no. ab7003; Abcam). Membranes were
then washed again three times for 10 min each with TBS-T. The
target protein bands were visualized using the Pierce enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific,
Waltham, MA, USA). All western blot analyses were performed three
times.
Cell viability measurement
The viability of the cells was analyzed by Thiazolyl
blue (MTT; Sigma-Aldrich). The MTT assay examines the activity of
metabolic enzymes in the mitochondria of live cells. Therefore MTT
can reflect cell proliferation. Cells that were grown to 70–80%
confluency in 96 well plates were treated with AUY922 at a final
concentration of 1, 2.5, 5, 10, 25, 50, 100 nM for 24, 48 or 72 h,
respectively. Cells treated with 5 mg/l cisplatin (DDP) were used
as positive control. After the reaction with the drugs for 24, 48,
72 h, cells were then treated with MTT (10 ml/well) for 4 h at
37°C. The cells were subjected to an absorbance reading at 570 nm
using a 96-well microplate reader (CKX41SF; Olympus, Tokyo, Japan).
The optical density (OD) values were normalized to those of the
cells treated with 0 nmol/l AUY922. The percentage of residual cell
viability was determined as [(OD experiment group − OD blank group)
/ OD negative group − OD blank group)] ×100%. Each assay was
performed three times. In order to calculate IC50, a
dose-responsive curve was fitted using a nonlinear regression model
with a sigmoidal dose response. The IC50 was
automatically produced by GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA).
Cell migration assay
The motility capabilities of the cells in
vitro were measured using Transwell chambers (Corning, Corning
Incorporated, Corning, NY, USA). Subsequently, four groups of cells
(5×105) were seeded on the upper wells with serum-free
medium. Medium with 20% FBS was plated in the bottom wells as
chemoattractants. After 48 h incubation, the cells were fixed with
90% methanol and stained with 1% crystal violet (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 30 min at 37°C. Cells
staying on the upper side of the membranes were wiped, while those
on the lower side were counted and photographed with
microscope.
Statistical analysis
Data analyses were performed using SPSS statistical
package 15.0. Phenotypic differences in quantitative traits were
assessed by genotype using Student’s t-test or analysis of
variance. Differences in the distribution of qualitative traits by
genotype were assessed using standard χ2-square analysis
and Fisher’s exact test. To evaluate the correlation of HSP90 and
MVD, Spearman’s correlation test was used. P<0.05 was considered
to indicate statistically significant difference.
Results
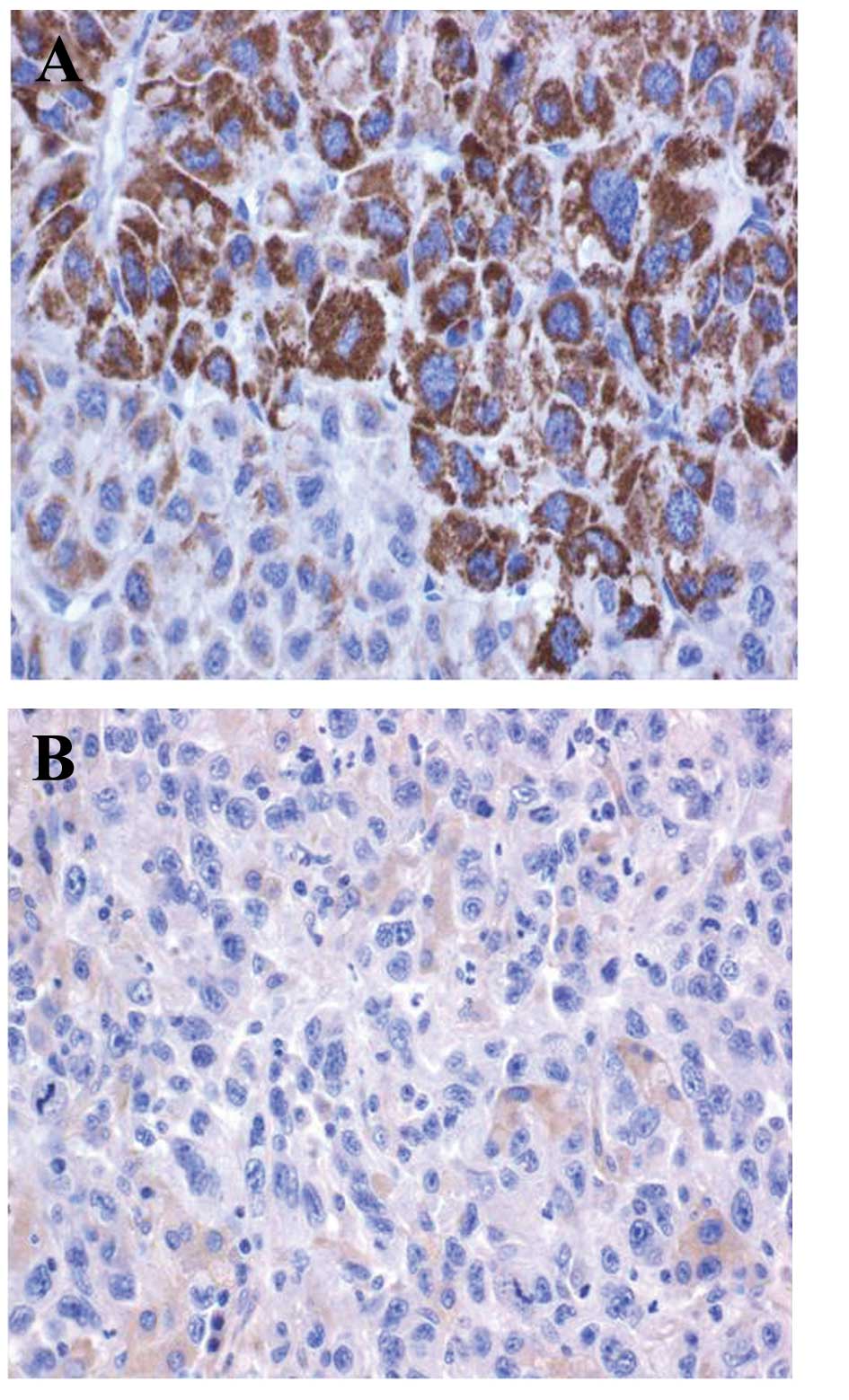
HSP90 is overexpressed in HCC tissues
compared with normal tissues
The expression of HSP90 was higher in 67 of the 76
randomly selected positive human HCC tissues compared with the
adjacent normal tissues. Staining of HSP90 was observed in 88.16%
(67/76) of the HCC tissues, compared with 16.67% (4/24) of the
normal tissues, and this difference between the expression of HSP90
between the HCC and normal tissues was statistically significant
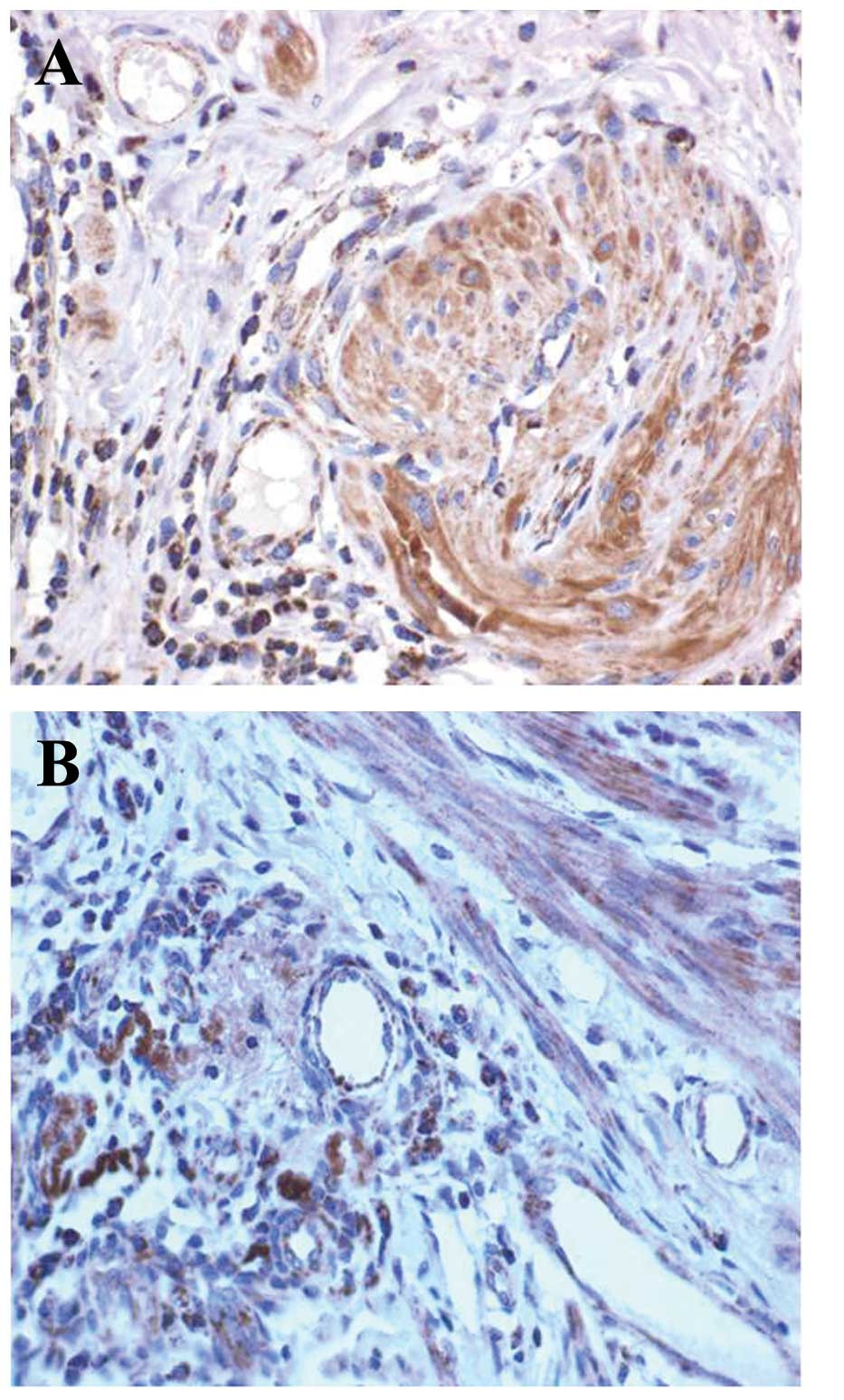
(P<0.001). Representative images of the HSP90 and MVD are shown
in Figs. 1 and 2. The tumor tissues with positive
expression of HSP90 had a significantly higher MVD compared with
their HSP90-negative counterparts (82.8±12.44 vs. 23.8±8.07,
respectively; P<0.001). The expression levels of HSP90 were
positively correlated with MVD in all the samples (r_s=0.714;
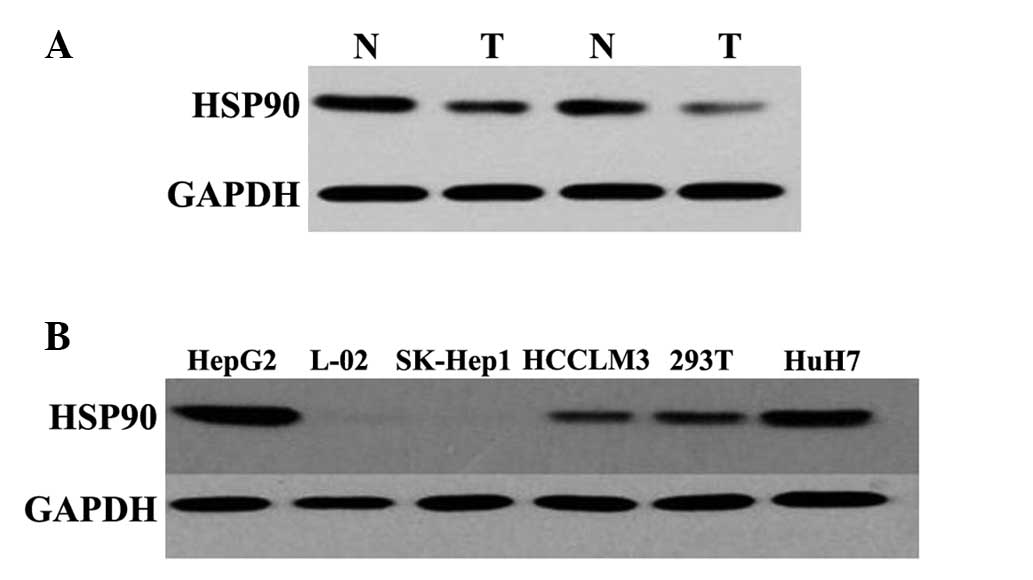
P<0.001). A total of 30 tissue samples of HCC and adjacent
normal tissues were examined to detect the protein expression of
HSP90 using western blot analysis. The protein expression level of
HSP90 was also overexpressed in the HCC tissues compared with the
normal tissues, and the difference between the two groups was
statistically significant (P<0.01; Fig. 3A). In addition, the expression of
HSP90 was detected in five HCC cell lines and one normal liver cell
using western blot analysis, the results of which revealed that the
expression of HSP90 was high in the HepG2 cells and low in the HuH7
cells(Fig. 3B). Therefore, the
HepG2 cell line was selected for use in the subsequent
investigations.
HSP90 inhibitor, AUY922, has an
inhibitory role in the proliferation of HepG2 cells
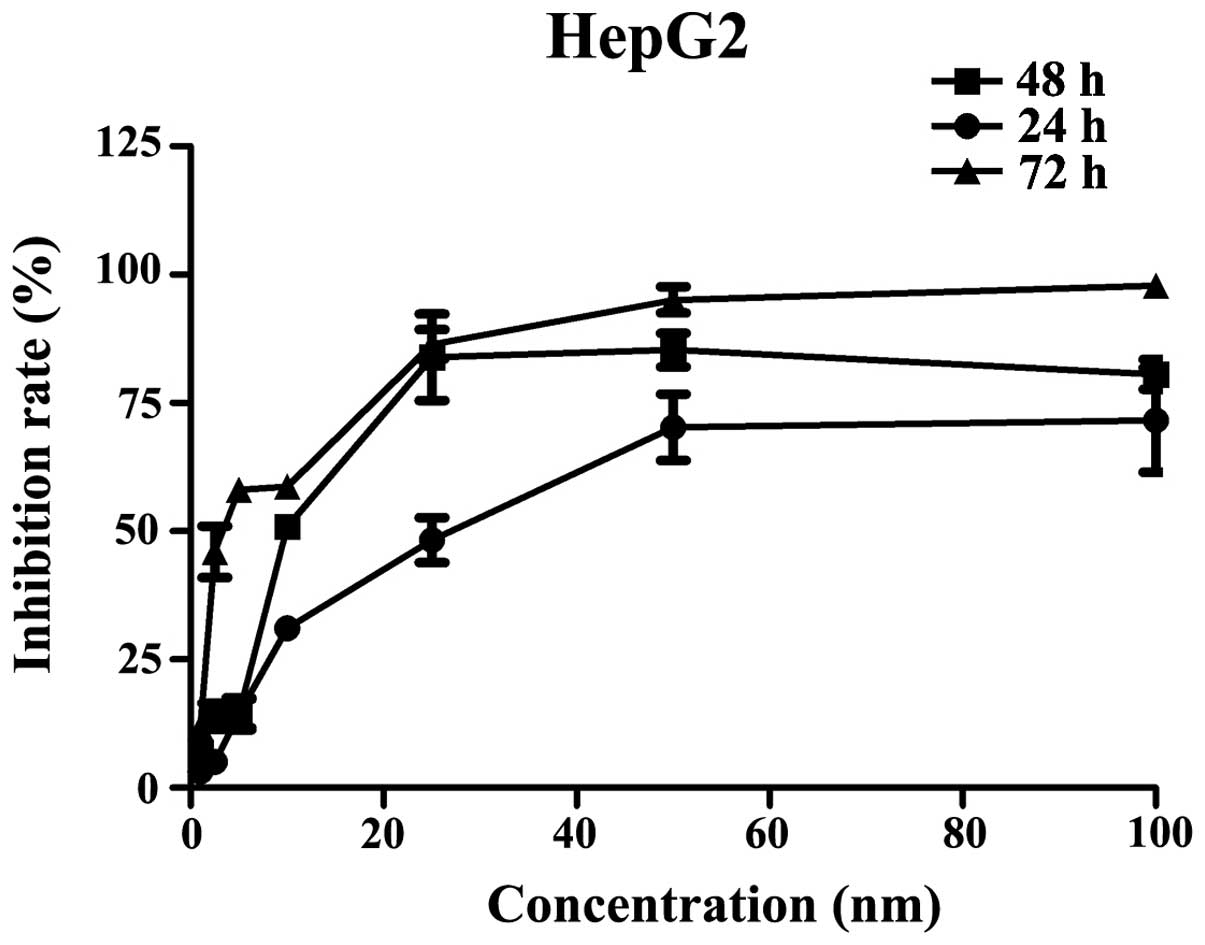
The HepG2 cells were treated with 1, 2.5, 5, 10, 25,
50, or 100 nM AUY922 for 24, 48 or 72 h, and cell proliferation was
measured using an MTT assay. As shown in Fig. 4, the MTT assay demonstrated that
AUY922 significantly inhibited cell proliferation in a time- and
concentration-dependent manner. A AUY922 concentration of 50 nM
exhibited similar efficacy as 5 mg/l DDP. The half maximal
inhibitory concentrations at 24, 48, and 72 h were 27., 10.1 and
3.4 nM, respectively.
Inhibition of the migration of HepG2
cells by AUY922
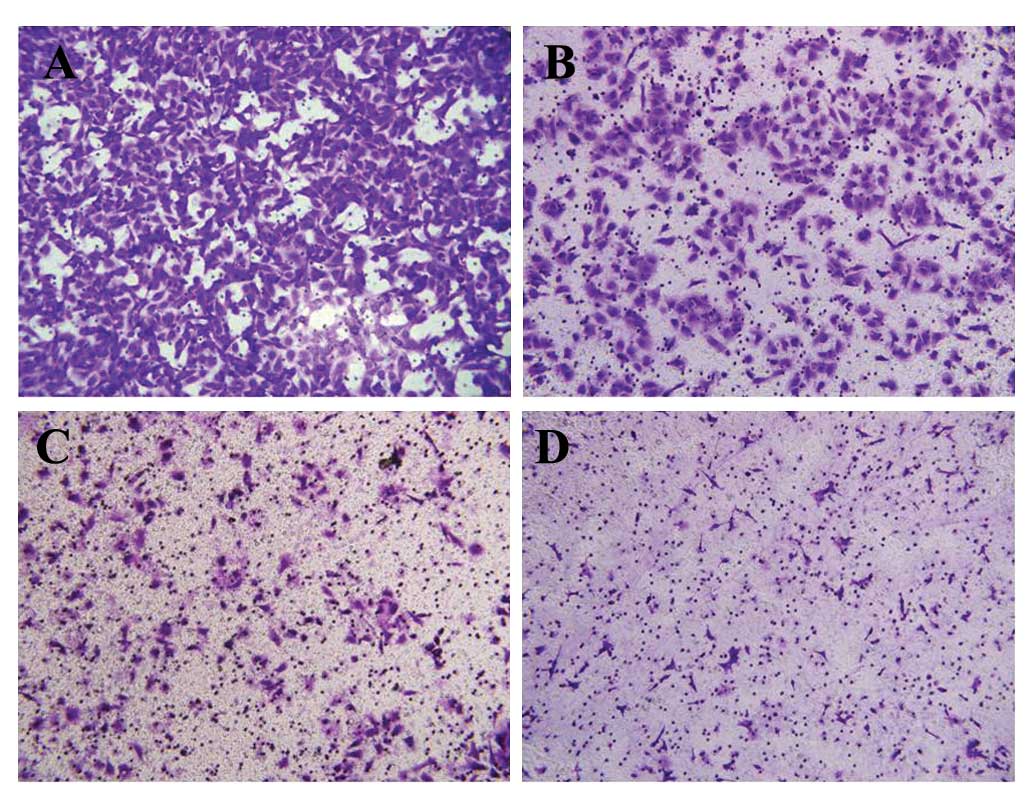
A Transwell assay was used to verify the effect of
AUY922 on the migration of HCC cells in vitro. The results
of the migration assay demonstrated that the number of HepG2 cells,
which penetrated through the membrane in the AUY922-treated groups
was significantly lower than that observed in the negative control
group, and fewer HepG2 cells penetrated through the polycarbonate
membrane in the 100 nM group compared with the 10 nM group
(P<0.05; Fig. 5).
Discussion
HCC is the sixth most common type of malignancy
worldwide and is the third most common cause of cancer-associated
mortality (1). HCC is a highly
vascularized tumor, which emphasizes the importance of
investigating its angiogenesis. Angiogenesis, an indispensable step
in the progression of a variety of solid tumors, is a key event in
numerous pathological and physiological conditions (5). MVD has been regarded as a gold
standard in assessing significant angiogenesis in tumors (7). The MVD in tumor tissues is determined
by evaluating tumor-derived vascular endothelial cells using
monoclonal antibodies, including CD-31, CD-34 and FactorVIII
(13). One of the most potent
endothelial mitogens and mediators of angiogenesis is VEGF, and the
induction of VEGF in cancer cells can be mediated through
activation of various signaling pathways, including
phosphoinositide 3-kinase (PI3K)/Akt (14).
HSP90 is the most abundant cytosolic HSP and
regulates the maturation and stability of various proteins, which
are essential for multiple cell signaling processes (15). In several types of cancer,
including breast cancer, non-small-cell lung cancer, and prostate
cancer, HSP90 is overexpressed and may contribute to tumour cell
survival by mediating the maturation and stability of a variety of
client proteins, including the IGF1 receptor and elements of the
PI3/Akt, signal transducer and activator of transcription 3 and
mitogen-activated protein kinase signalling pathways (15,16).
These client proteins of HSP90 are available to promote growth
factor independence, resistance to drugs, proliferation, tissue
invasion, metastasis and angiogenesis, which are all critical for
tumor progression and survival (17). The aim of the present study was to
investigate the expression and function of HSP90 in HCC to examine
the effects on the oncogenetic process. The expression of HSP90 and
the MVD were measured in tissue samples from 76 samples of HCC
tissue and 24 samples of adjacent normal hepatic tissues using
immunohistochemistry. The results demonstrated positive staining of
HSP90 in 88.16% (67/76) of the HCC tissue samples, compared with
16.67% (4/24) of the normal tissue samples, and this difference in
the expression of HSP90 between the HCC and normal tissues was
statistically significant (P<0.001). The tumors exhibiting
positive expression of HSP90 had a significantly higher MVD than
their HSP90-negative counterparts (82.8±12.44 vs. 23.8±8.07,
respectively; P<0.001). The expression of HSP90 expression was
positively correlated with MVD in all the specimens (r_s=0.724;
P<0.001). Therefore, a statistically significant correlation was
observed between HSP90 and MVD in HCC. Accordingly, the expression
levels of HSP90 in five HCC cell lines and one normal liver cell
was detected using western blot analysis. It was hypothesized that
HSP90, involved in angiogenesis, may be a potential molecular
target for the treatment of HCC.
The inhibition of HSP90 has become one of the most
popular areas of investigation (18). A previous found that, in cancer
cells, HSP90 exhibits higher binding affinity for 17-AAG
exclusively, and forms 17-AAG-sensitive HSP90-containing
‘superchaperone’ complexes in malignant cells, whereas normal
cells, with predominantly uncomplexed HSP90, are significantly less
sensitive to these types of inhibitors (19). By contrast, HSP90 inhibitors
preferentially accumulate in tumor cells rather than normal cells
(20), and the client proteins,
which are most sensitive to HSP90 inhibition are preferentially
expressed in tumor cells. Nguyen et al (21) observed H358 cell and rat tumor cell
lines in vitro and demonstrated that 17-AAG markedly
inhibits the production of VEGF, which confirmed that 17-AAG is
effective in regulating the expression of the genes of VEGF. Hur
et al (22) also
demonstrated that 17-AAG can reduce the angiogenesis of tumor cells
by inhibiting the genes of VEGF.
HSP90 inhibitors have improved considerably. AUY922
is part of the isoxazole HSP90-inhibitor family and is a
non-geldanamycin analog, which offers prolonged target inhibition
and has not been associated with the same degree of hepatotoxicity
as its geldanamycin counterparts (23). AUY922 exerts its effects by binding
to the ATPase domain of the HSP90 N-terminal, preventing HSP90 from
its chaperone functions. This leads to the proteasomal degradation
of several relevant client proteins (11). Single agent AUY922 has been
observed to exhibit potent preclinical anticancer activity in
vitro and in vivo against a range of histologic cell
types, including head and neck squamous cell carcinomas,
pancreatic, prostate, lung, cervical, colorectal and breast
carcinomas, myelomas and melanomas (24–28).
In the present study, HepG2 cells were treated with 1, 2.5, 5, 10,
25, 50, or 100 nM AUY922 for 24, 48 or 72 h. The cell survival rate
was measured using an MTT assay to assess the inhibitory effect of
AUY922 on the HCC cells. The results revealed that AUY922
significantly inhibited the proliferation of the HepG2 cells in a
time- and concentration-dependent manner. AUY922 can inhibit
proliferation in various types of tumor cells, according to
previous reports (26), therefore,
the results of the present study confirm HSP90 as an important
target in HCC. The presumptive tumor suppressor function of AUY922
in human HCC was further investigated using a Transwell assay. The
results demonstrated that the migratory ability of the cells was
significantly suppressed by AUY922, with fewer HepG2 cells
penetrating the polycarbonate membrane in the AUY922-treated group
compared with the negative control group. This occurred in a
dose-dependent manner.
In conclusion, the results of the present study
demonstrated that HSP90 was overexpressed in HCC, and that the
HSP90 inhibitor, AUY922 inhibited HepG2 cell proliferation and
migration in a time- and dose-dependent manner. Treatment involving
the inhibition of HSP90 may provide a promising strategy for
antitumor therapy in HCC, with HSP90 offering a novel target.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ades S: Adjuvant chemotherapy for colon
cancer in the elderly: moving from evidence to practice. Oncology
(Williston Park). 23:162–167. 2009.
|
|
3
|
Frangov T, Gaĭdarski R, Dimitrova V, Popov
V, Grozeva K and Rusenov D: Prognostic factors for survival in
primary liver cancer. Khirurgiia (Sofiia). 6:36–39. 2007.In
Bulgarian.
|
|
4
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 280:C1358–C1366. 2001.PubMed/NCBI
|
|
7
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
8
|
Xu YZ, Zhu Y, Shen ZJ, Sheng JY, He HC, Ma
G, et al: Significance of heparanase-1 and vascular endothelial
growth factor in adrenocortical carcinoma angiongenesis: Potential
for therapy. Endocrine. 40:445–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KH, Lee JH, Han SW, Im SA, Kim TY, Oh
DY and Bang YJ: Antitumor activity of NVP-AUY922, a novel heat
shock protein 90 inhibitor, in human gastric cancer cells is
mediated through proteasomal degradation of client proteins. Cancer
Sci. 102:1388–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eccles SA, Massey A, Raynaud FI, Sharp SY,
Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall
F, Aherne W, et al: NVP-AUY922: A novel heat shock protein 90
inhibitor active against xenograft tumor growth, angiogenesis and
metastasis. Cancer Res. 68:2850–2860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Zhang C, Chen D, Zhao J, Shen Z, Wu
Y and Zhu Y: Effect of HSP90 inhibitor in pheochromocytoma PC12
cells: an experimental investigation. Tumour Biol. 34:4065–4071.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laforga JB and Aranda FI: Angiogenic
Index: A new method for assessing microvascularity in breast
carcinoma with possible prognostic implications. Breast J.
6:103–107. 2000. View Article : Google Scholar
|
|
14
|
Geng J, Li X, Zhou Z, Wu CL, Dai M and Bai
X: EZH2 promotes tumor progression via regulating VEGF-A/AKT
signaling in non-small cell lung cancer. Cancer Lett. 359:275–287.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearl LH, Prodromou C and Workman P: The
Hsp90 molecular chaperone: an open and shut case for treatment.
Biochem J. 410:439–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pratt WB and Toft DO: Regulation of
signaling protein function and trafficking by the hsp90/hsp70-based
chaperone machinery. Exp Biol Med (Maywood). 228:111–133. 2003.
|
|
17
|
Powers MV and Workman P: Targeting of
multiple signalling pathways by heat shock protein 90 molecular
chaperone inhibitors. Endocr Relat Cancer. 13(Suppl 1): 125–135.
2006. View Article : Google Scholar
|
|
18
|
Gartner EM, Silverman P, Simon M, Flaherty
L, Abrams J, Ivy P and Lorusso PM: A phase II study of
17-allylamino-17-demethoxygeldanamycin in metastatic or locally
advanced, unresectable breast cancer. Breast Cancer Res Treat.
131:933–937. 2012. View Article : Google Scholar
|
|
19
|
Eiseman JL, Lan J, Lagattuta TF, et al:
Pharmacokinetics and pharmacodynamics of 17-demethoxy
17-[[(2-dimethylamino) ethyl]amino]geldanamycin (17DMAG, NSC
707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer
xenografts. Cancer Chemother Pharmacol. 55:21–32. 2005. View Article : Google Scholar
|
|
20
|
Moulick K, Ahn JH, Zong H, Rodina A,
Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F,
Hatzi K, Vu LP, et al: Affinity-based proteomics reveal
cancer-specific networks coordinated by Hsp90. Nat Chem Biol.
7:818–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen DM, Lorang D, Chen GA, Stewart JH
IV, Tabibi E and Schrump DS: Enhancement of paclitaxel-mediated
cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in
vitro and in vivo analysis. Ann Thorac Surg. 72:371–378; discussion
378–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hur E, Kim HH, Choi SM, et al: Reduction
of hypoxia-induced transcription through the repression of
hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear
translocator DNA binding by the 90-kDa heat-shock protein inhibitor
radicicol. Mol Pharmacol. 62:975–982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pacey S, Gore M, Chao D, Banerji U, Larkin
J, Sarker S, et al: A Phase II trial of 17-allylamino,
17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with
metastatic melanoma. Invest New Drugs. 30:341–349. 2012. View Article : Google Scholar
|
|
24
|
Eccles SA, Massey A, Raynaud FI, Sharp SY,
Box G, Valenti M, et al: NVP-AUY922: a novel heat shock protein 90
inhibitor active against xenograft tumor growth, angiogenesis and
metastasis. Cancer Res. 68:2850–2860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jensen MR, Schoepfer J, Radimerski T,
Massey A, Guy CT, Brueggen J, et al: NVP-AUY922: a small molecule
HSP90 inhibitor with potent antitumor activity in preclinical
breast cancer models. Breast Cancer Res. 10:R332008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moser C, Lang SA, Hackl C, Wagner C,
Scheiffert E, Schlitt HJ, et al: Targeting HSP90 by the novel
inhibitor NVP-AUY922 reduces growth and angiogenesis of pancreatic
cancer. Anticancer Res. 32:2551–2561. 2012.PubMed/NCBI
|
|
27
|
Chatterjee M, Andrulis M, Stuhmer T,
Müller E, Hofmann C, Steinbrunn T, et al: The PI3K/Akt signalling
pathway regulates the expression of Hsp70, which critically
contributes to Hsp90-chaperone function and tumor cell survival in
multiple myeloma. Haematologica. 98:1132–1141. 2013. View Article : Google Scholar :
|
|
28
|
Ueno T, Tsukuda K, Toyooka S, Ando M,
Takaoka M, Soh J, et al: Strong anti-tumor effect of NVP-AUY922, a
novel Hsp90 inhibitor, on non-small cell lung cancer. Lung Cancer.
76:26–31. 2012. View Article : Google Scholar
|