Introduction
Suicide is a major public health problem that has
received increasing attention worldwide (1). In the majority of studies regarding
death by suicide, ~9 out of every 10 individuals appear to have had
a psychiatric disorder at the time of their death (2). Depression is the most common
psychiatric disorder in people who commit suicide (3). Depression is strongly associated with
suicide and non-fatal suicidal behaviors, and requires an ongoing
assessment due to suicide risk. Patients with major affective
disorders, such as major depressive disorder (MDD), are highly
vulnerable to suicidal behavior (4).
Serotonin (5-HT) is a monoamine implicated in
various physiological processes, which functions as a
neurotransmitter and a peripheral hormone. 5-HT is associated with
the pathophysiology of depressive disorders. The peripheral and
cerebral biosynthesis of 5-HT is initiated by two distinct
tryptophan hydroxylases (TPH): TPH1 and TPH2 (5). TPH1 is predominantly expressed in the
periphery and the pineal gland, whereas TPH2 is thought to be
neuron-specific and is predominantly expressed in the brain;
however, it may also be expressed or induced to be expressed in
peripheral tissues (6). Of note,
numerous studies have indicated that TPH2 gene expression is highly
inducible and is closely associated with depressive disorders
(7,8).
Previous studies have linked TPH2 genetic variance
to various behavioral traits and disorders (9-11).
It has been hypothesized that functional polymorphisms involving
the promoter region of TPH2, which affect gene expression, may
explain this finding (12,13). The presence of a functional
cis-acting polymorphism, with high frequency in normal human
subjects, results in increased expression levels of TPH2, and has
been shown to be associated with major depression and suicide
(14). A study demonstrated that
the 5′-untranslated region (UTR) and common polymorphisms in the
5′-regulatory region of human TPH2 have a significant impact on
gene expression (15).
Non-synonymous single nucleotide polymorphisms (SNPs) in rhesus
monkey TPH2 have been shown to affect mRNA stability, thus
suggesting that non-synonymous SNPs affect TPH2 function and gene
expression (16). The TPH2 SNPs
rs1386482 and rs1386486 have previously been associated with
bipolar affective disorder (17).
In addition, the rs4570625 SNP of TPH2 were suggested to have an
important role in the development of positive symptoms in Han
Chinese patients with schizophrenia (18). A previous study by our group
indicated that TPH2 rs7305115A remained a significant protective
predictor of suicide attempts (19). However, little is currently known
regarding the impact of TPH2 gene variants on expression (20,21).
Recent evidence regarding DNA methylation
alterations within distinct genes and pathways provided novel
insight into the pathophysiology of psychiatric disorders (22,23).
DNA methylation, even in peripheral tissues, appears to be an
informative reflection of environmental exposure of the genome, and
may have potential as a biomarker for the early prevention of
depressive disorders (24). The
TPH2 promoter contains no CpG island; however, it does contain
numerous scattered CpG sites and an enriched signal of DNA
hypomethylation at the 5′-UTR locus (6). To identify the molecular basis of
gene expression variation, the present study conducted association
studies between TPH2 promoter methylation and TPH2 transcription
levels in 50 patients with MDD who had attempted suicide and 75
control subjects.
Patients and methods
Patients and controls
The subjects of the present study consisted of 125
unrelated patients with MDD, who were recruited from the Han
population of Jiangsu (China) between 2010 and 2013. All patients
(58 males, 67 females) were hospitalized in the Department of
Geriatric Psychiatry, Wuxi Psychiatric Hospital (Wuxi, China).
Diagnosis of MDD was confirmed using the Mini International
Neuropsychiatric Interview, and by a minimum score of Hamilton
Depression Rating Scale (HDRS) (25,26).
All cases met the DSM-IV diagnostic criteria for MDD and were
severe enough to require follow-up in a specialized psychiatric
outpatient clinic at the Department of Geriatric Psychiatry, Wuxi
Psychiatric Hospital (27,28). The HAMA scale was used to assess
the severity of symptoms (29) by
means of structured questionnaires, information on specific
demographic and clinical variables, including family history of
suicide was obtained.
A total of 50 MDD + suicide patients (defined as the
MDD + suicide attempts group; 23 males, and 27 females) who were
consecutively admitted to our psychiatric departments following a
suicide attempt, were included in the present study. Suicide was
defined as intentional self-harm, in order to end one’s life. The
remaining 75 MDD control patients (defined as the MDD group; 35
males, and 40 females) who had not attempted suicide were selected
for the present study. Written informed consent was obtained from
each patient, and the protocol of the study was approved by the
local ethics committee of Nanjing Medical University (Nanjing,
China).
Bisulfite modification and
methylation-specific polymerase chain reaction (MSP)
Venous blood was collected from the patients and
immediately frozen in aliquots at −80°C or below, prior to
analysis. For genotyping, genomic DNA was extracted from
EDTA-supplemented blood samples using a commercial DNA extract kit
(Wizard® Genomic DNA Purification kit; Promega Corp.,
Madison, WI, USA).
Genomic DNA (3 μg) from the cells was
denatured with 0.3 M NaOH (Sigma-Aldrich, Shanghai, China) at 37°C
for 10 min. Bisulfite treatment (Sigma-Aldrich) was performed as
described previously (30).
Primers specific for TPH2 MSP were designed using MethPrimer
(31) and synthesized by Sangon
Biotech Co. (Shanghai, China). Bisulfite-treated DNA was used for
amplification of the TPH2 promoter. Primers specific for TPH2 were
as follows: Unmethylated (U) TPH2 sense,
5′-TTTGTAATTTGATTGTGGTTATTGG-3′ and anti-sense,
5′-ACAATCAACTACCTACTTAAAACACT-3′; and methylated (M) TPH2 sense,
5′-GGTTTGTAATTTGATTGTGGTTATC-3′ and anti-sense,
5′-CGATCAACTACCTACTTAAAACGCT-3′, which amplify a 164 bp product and
a 165 bp product, respectively. MSP consisted of 38 cycles at 95° C
for 45 sec, 60°C for 50 sec and 72°C for 30 sec, followed by a
10-min extension at 72°C in a DNA Thermocycler (Agilent
Technologies, Inc., Santa Clara, CA, USA). The amplification
products were separated by 2% agarose gel electrophoresis (Sangon
Biotech Co.) and visualized by ethidium bromide staining (Sangon
Biotech Co.) and ultraviolet transillumination (FR 2000 UV
transillumination; Shanghai Furi Science & Technology Co.,
Ltd., Shanghai, China). Methylation was defined as M/(M+U)≥0.5; and
unmethylation was defined as M/(M+U)<0.5.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using SYBR Green I chemistry
(Invitrogen Life Technologies, Carlsbad, CA, USA). Specific primers
were designed to target human TPH2 (PrimerBank ID: 169234956c2; 130
bp). Total RNA was isolated from the blood samples. The
first-strand cDNA was synthesized from a moloney murine leukemia
virus-reverse transcriptase kit using 2 μg total RNA
according to the manufacturer’s instructions (Takara Bio, Inc.,
Dalian, China). Primer sequences of TPH2 for the RT-qPCR reaction
were as follows: Forward, 5′-CAAAAATGACGACAAAGGCAACA-3′ and
reverse, 5′-CCTCAGTGCTTTTACCAATCCA-3′. Primer sequences of β-actin
for the RT-qPCR reaction were as follows: Forward,
5′-CTGGGACGAATGGAGAAA-3′ and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′.
The qPCR was performed using the Mx3000P qPCR system (Stratagene,
La Jolla, CA, USA) and the PCR reaction mixture consisted of cDNA
in 20 μl SYBR Premix Ex Taq. The qPCR was performed under
the following conditions: 5 min at 95°C, followed by 40 cycles of
30 sec at 95°C, 30 sec at 58°C and 50 sec at 72°C. As an internal
control for qPCR, β-actin mRNA expression was amplified from the
same cDNA samples. All of the results were normalized to β-actin.
Cycle threshold (Ct) values for triplicate reactions were averaged
and relative TPH2 expression levels were determined using the
comparative CT method (32), using
the average Ct values for TPH2 and β-actin.
Statistical analysis
All data were generated without knowledge of the
clinical status of the samples, and were analyzed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Differences between the
groups were calculated using the unpaired t-test. Associations
between categorical variables were examined using Pearson’s
χ2 and Fisher’s exact tests. Data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
Demographic and clinical characteristics of the
subjects of the present study are presented in Table I. The study population consisted of
50 MDD + suicide patients [mean age ± standard deviation (SD),
36.8±10.2 years] and 75 matched MDD patients (mean age ± SD,
35.3±11.0 years). For the MDD + suicide group, 22 patients
attempted suicide by drug overdose, hanging and drowning, and 28
patients attempted suicide by violent methods, such as through
several deep cuts. No statistically significant differences were
observed between the MDD + suicide and MDD groups for age, age at
onset, course of disease or HDRS scores.
 | Table IClinical characteristics of patients
with MDD, with or without suicidal behavior. |
Table I
Clinical characteristics of patients
with MDD, with or without suicidal behavior.
| Characteristic | MDD + suicide | MDD | P value |
|---|
| Gender
(male/female) | 23/27 | 35/40 | 0.554 |
| Age, range (mean ±
SD) in years | 14–71
(36.8±10.2) | 13 70
(35.3±11.0) | 0.645 |
| Age at onset, range
(mean ± SD) in years | 11–60
(25.2±11.5) | 10 56
(26.6±12.1) | 0.843 |
| Course of disease,
range (mean ± SD) | 2 weeks-24 years
(3.73±3.82 years) | 2 weeks-26 years
(3.69±4.04 years) | 0.082 |
| HDRS scores | 47.32±9.31 | 48.65±9.14 | |
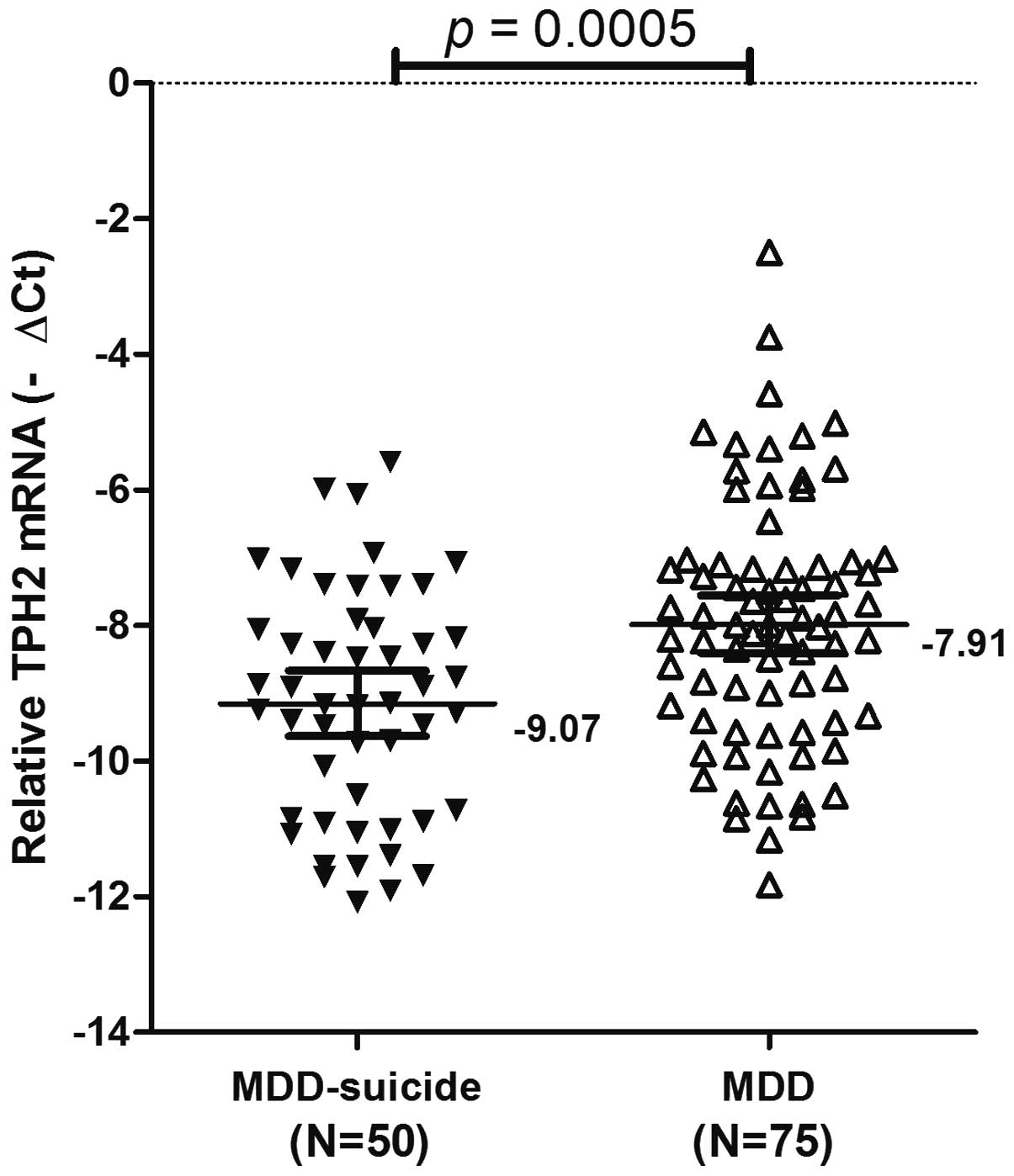
mRNA expression levels of TPH2 in the MDD
+ suicide and MDD groups
To accurately quantify the relative mRNA expression
levels of TPH2, an RT-qPCR assay was conducted on samples from the
MDD + suicide and MDD groups. The overall results are summarized in
Fig. 1. TPH2 mRNA expression
levels were lower in the MDD + suicide group [mean−∆Ct ± standard
error (SE),−9.07±0.24], as compared with the MDD group (mean−∆Ct ±
SE,−7.91±0.21). The difference between TPH2 expression levels in
the MDD + suicide and MDD groups was significant (P=0.0005;
Fig. 1). These results suggested
that the downregulation of TPH2 gene expression may have an
important role in the development of suicidal behaviors.
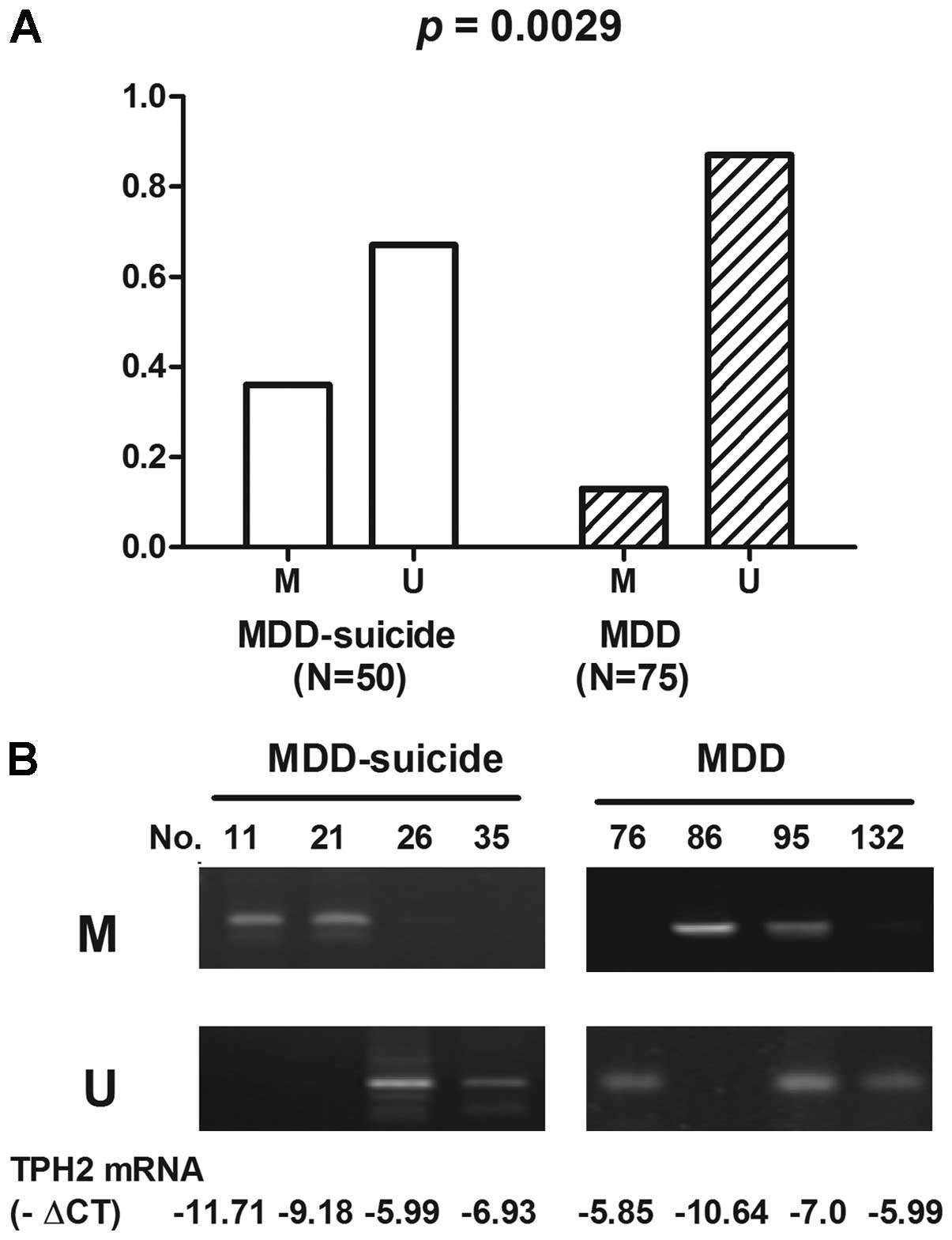
Methylation status of the TPH2 promoter
in the MDD + suicide and MDD groups
The methylation status of the TPH2 promoter region
was analyzed in the MDD + suicide and MDD groups. The TPH2 promoter
was methylated in 36.0% (18/50) of MDD + suicide patients, as
compared with in 13.0% (10/75) of MDD patients. Furthermore, the
TPH2 promoter was unmethylated in 64.0% (32/50) of MDD + suicide
patients, as compared with 87.0% (65/75) of MDD patients. The
differences in TPH2 methylation status between the MDD + suicide
and MDD groups were statistically significant (P=0.0029; Fig. 2).
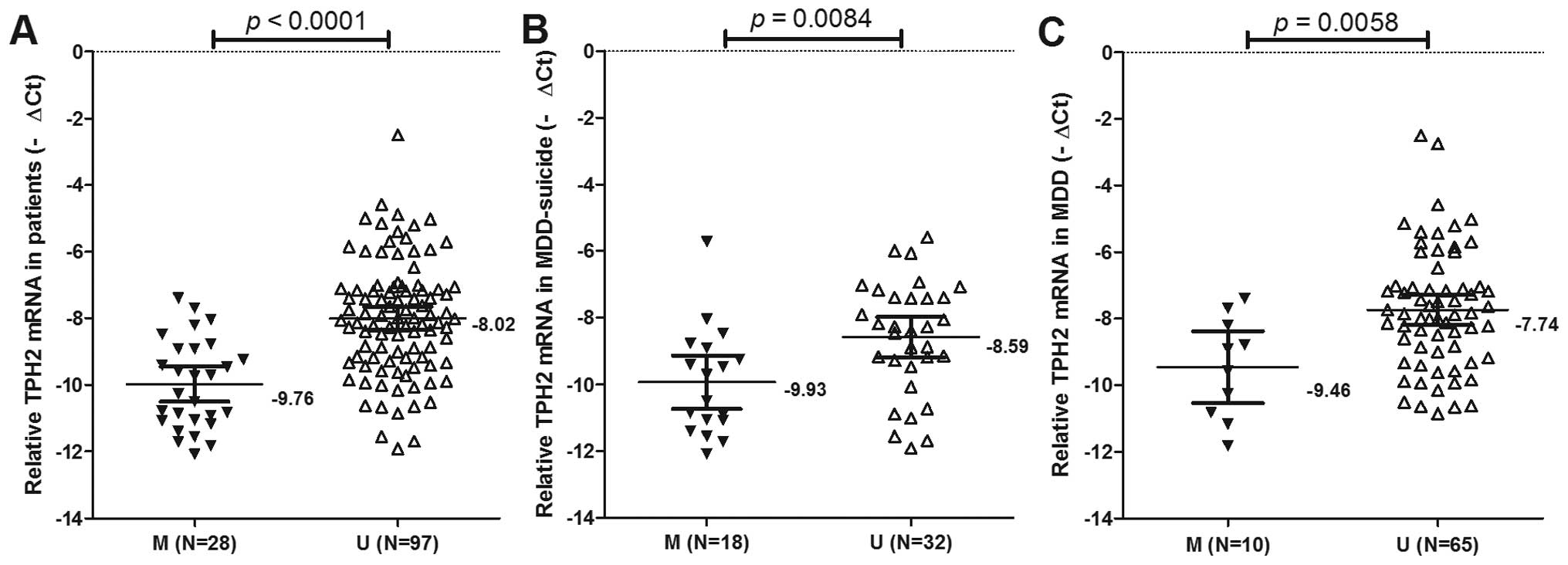
Association of TPH2 methylation with TPH2
mRNA expression levels in the MDD + suicide and MDD groups
To determine whether TPH2 promoter methylation was
correlated with the suppression of TPH2 mRNA expression in MDD,
qPCR was used to detect the mRNA expression levels of TPH2 in all
patients (Fig. 3). The mRNA
expression levels of TPH2 were significantly decreased in the
patients with TPH2 promoter methylation, as compared with those
possessing an unmethylated TPH2 promoter (mean−∆Ct ± SE, −9.76±0.25
and −8.02±0.17, respectively; P<0.0001; Fig. 3A).
In the MDD + suicide group, the mRNA expression
levels of TPH2 were −9.93±0.38 in patients possessing a methylated
TPH2 promoter and −8.59±0.30 in those with an unmethylated TPH2
promoter (P=0.0084; Fig. 3B).
In the MDD group, the mRNA expression levels of TPH2
were −9.46±0.48 in the patients with a methylated TPH2 promoter,
and −7.74±0.23 in those possessing an unmethylated TPH2 promoter
(P=0.0058; Fig. 3C). These results
suggested that in patients with MDD exhibiting methylation of the
TPH2 promoter, TPH2 mRNA expression is likely to be
downregulated.
Correlation of the methylation status of
the TPH2 promoter with clinicopathological parameters in the MDD +
suicide and MDD groups
The methylation of specific gene promoters is
considered a marker for various types of psychiatric disorder
(33). The correlation of TPH2
methylation with the clinicopathological parameters of the MDD +
suicide and MDD groups is shown in Table II. The methylation status of the
TPH2 promoter was associated with depression, hopelessness and
cognitive impairment (P=0.011, 0.022 and 0.015, respectively) in
the MDD + suicide group. In addition, methylation of TPH2 was only
associated with depression (P=0.014) in the MDD group. Methylation
of TPH2 was not associated with the remaining clinicopathological
parameters evaluated, including gender, diurnal change, sleep
disorders, anxiety and slow movement.
 | Table IIMethylation of the TPH2 gene promoter
and clinical characteristics of patients with MDD. |
Table II
Methylation of the TPH2 gene promoter
and clinical characteristics of patients with MDD.
| MDD suicide
| P-value | MDD
| P-value |
|---|
| M (n=18) | U (n=32) | M (n=10) | U (n=65) |
|---|
| Gender | | | 0.869 | | | 0.649 |
| Male | 8 | 15 | | 4 | 31 | |
| Female | 10 | 17 | | 6 | 34 | |
| Course of
disease | | | 0.351 | | | 0.722 |
| <1 year | 8 | 10 | | 4 | 29 | |
| ≥1 year | 10 | 22 | | 6 | 36 | |
| Age at onset | | | 0.522 | | | 0.221 |
| <35 years | 9 | 13 | | 3 | 33 | |
| ≥35 years | 9 | 19 | | 7 | 32 | |
| Family history of
mental illness | | | 0.584 | | | 0.961 |
| Yes | 11 | 22 | | 7 | 45 | |
| No | 7 | 10 | | 3 | 20 | |
| Depression | | | 0.011 | | | 0.014 |
| HDRS <35 | 4 | 19 | | 2 | 40 | |
| HDRS ≥35 | 14 | 13 | | 8 | 25 | |
| Hopelessness | | | 0.022 | | | 0.488 |
| HDRS <6 | 7 | 23 | | 5 | 40 | |
| HDRS ≥6 | 11 | 9 | | 5 | 25 | |
| Cognitive
impairment | | | 0.015 | | | 0.101 |
| HDRS <12 | 6 | 22 | | 2 | 31 | |
| HDRS ≥12 | 12 | 10 | | 8 | 34 | |
| Diurnal change | | | 0.941 | | | 0.716 |
| HDRS <1 | 6 | 11 | | 4 | 30 | |
| HDRS ≥1 | 12 | 21 | | 6 | 35 | |
| Sleep
disorders | | | 0.053 | | | 0.488 |
| HDRS <2 | 5 | 18 | | 3 | 27 | |
| HDRS ≥2 | 13 | 14 | | 7 | 38 | |
| Anxiety | | | 0.352 | | | 0.855 |
| HAMA <21 | 6 | 15 | | 4 | 28 | |
| HAMA ≥21 | 12 | 17 | | 6 | 37 | |
| Slow movement | | | 0.309 | | | 0.820 |
| HDRS <8 | 8 | 19 | | 5 | 35 | |
| HDRS ≥8 | 10 | 13 | | 5 | 30 | |
Discussion
Suicide has been identified as a serious public
health problem, which is a result of an interplay between distal
(e.g. genetic loading or family history of suicide) and proximal
factors (e.g. presence of psychiatric disorders or events
conferring acute stress) (1).
Disorders of emotional regulation, including anxiety disorders and
depression, are common and debilitating. Accumulating evidence has
suggested an involvement of 5-HT in the regulation of emotion
(34). The recent advent of TPH2
knockout mice, which lack the rate-limiting enzyme for 5-HT
synthesis in the brain, has provided further insight into the brain
serotonergic system and its role in emotional dysregulation
(35). With increased
understanding of the regulation of TPH2 activity, TPH2 may be used
as a target for the development of novel treatments or for
optimization of current therapies, which are expected to markedly
improve the prevention and treatment of depression disorders
(36).
TPH2 synthesizes neuronal 5-HT and its genetic
variance is associated with numerous behavioral traits and
psychiatric disorders. An association has been identified between
TPH2 and genetically defined behavioral variations, and TPH2
genetic variation may be used to predict the risk of affective
disorders and the sensitivity to anti-depressant therapeutics
(5,37). TPH2 gene expression is
tissue-specific and responsive to stressors, including adverse
experiences during early life and adulthood (9). These findings suggested that
epigenetic mechanisms may have important roles in the regulation of
TPH2 expression (38). Epigenetic
modifications, such as DNA methylation, are able to induce lasting
and stable changes in gene expression, and have therefore been
implicated in promoting the adaptive behavioral and neuronal
changes that accompany depression (39).
The present study aimed to compare the gene
expression levels of TPH2 between patients with MDD who had
attempted suicide and those who had not (controls), and to identify
epigenetic methylation involved in TPH2 regulation. The results of
the present study demonstrated that the frequency of TPH2
methylation was 36.0% in the MDD + suicide group, as compared with
13.0% in the MDD group. The mRNA expression levels of TPH2 were
significantly decreased in the patients with methylated TPH2
promoters, as compared with those with unmethylated TPH2 promoters.
These results suggested that methylation of the TPH2 promoter may
silence TPH2 mRNA expression in patients with MDD. Furthermore, a
significant correlation was shown between the methylation status of
the TPH2 promoter and depression, hopelessness and cognitive
impairment in the MDD + suicide group. The results of the present
study demonstrated that TPH2 expression may be regulated by DNA
methylation in the promoter region in patients with MDD.
Epigenetic modification of gene expression provides
a mechanism for understanding the link between long-term effects of
adverse life events and the alterations in gene expression that are
associated with depression. Although epigenetics remain a
developing field, future studies on epigenetic modifications of
gene expression may provide novel biomarkers to predict future
susceptibility and/or onset of MDD, improve diagnosis and aid in
the development of epigenetics based therapies for depression
(23). TPH2 transcription and
protein expression are modulated by neuronal differentiation in the
central nervous system-originated cell line A1 mes-c-myc, which
endogenously expresses TPH2, and promoter activity strongly
increases with cell differentiation upon mutation of the
neuron-restrictive silencer factor (NRSF)/RE1-silencing
transcription factor responsive element (40). The upstream segment of the TPH2
5′-UTR contains a binding motif for NRSF, which mediates
transcriptional repression. Therefore, it is likely that the 5′-UTR
serves as an ‘on-off’ switch for the regulation of TPH2 expression
(41).
In conclusion, the results of the present study
provided novel insight into the epigenetic mechanisms of MDD.
Increasing knowledge regarding the regulation of TPH2 expression
will not only improve the understanding of 5-HT-stress interaction
and the pathophysiology of neuropsychiatric disorders, but may also
provide novel strategies for the treatment of stress-associated
diseases.
Acknowledgments
The present study was supported by the Science and
Technology Planning Project of Wuxi Municipality, China (grant no.
CSE01N1118), the Medical Technology Project of Wuxi Hospital
Management Center (grant no. YGZX1116), the Natural Science
Foundation of China (grant no. 81372212), the Jiangsu Provincial
Special Program of Medical Science (grant no. BL2013012) and the
Health Talents Project for Jiangsu, China (grant nos. LJ201157 and
RC2011038).
References
|
1
|
Hawton K and van Heeringen K: Suicide.
Lancet. 373:1372–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGirr A, Renaud J, Séguin M, Alda M and
Turecki G: Course of major depressive disorder and suicide outcome:
A psychological autopsy study. J Clin Psychiatry. 69:966–970. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cavanagh JT, Carson AJ, Sharpe M and
Lawrie SM: Psychological autopsy studies of suicide: A systematic
review. Psychol Med. 33:395–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pompili M, Innamorati M, Raja M, et al:
Suicide risk in depression and bipolar disorder: Do
impulsiveness-aggressiveness and pharmacotherapy predict suicidal
intent? Neuropsychiatr Dis Treat. 4:247–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matthes S, Mosienko V, Bashammakh S,
Alenina N and Bader M: Tryptophan hydroxylase as novel target for
the treatment of depressive disorders. Pharmacology. 85:95–109.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen GL and Miller GM: Advances in
tryptophan hydroxylase-2 gene expression regulation: New insights
into serotonin-stress interaction and clinical implications. Am J
Med Genet B Neuropsychiatr Genet. 159B:152–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Must A, Tasa G, Lang A, et al: Variation
in tryptophan hydroxylase-2 gene is not associated to male
completed suicide in Estonian population. Neurosci Lett.
453:112–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai SJ, Hong CJ, Liou YJ, et al:
Tryptophan hydroxylase 2 gene is associated with major depression
and antidepressant treatment response. Prog Neuropsychopharmacol
Biol Psychiatry. 33:637–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waider J, Araragi N, Gutknecht L and Lesch
KP: Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive
control and emotion regulation: A perspective.
Psychoneuroendocrinology. 36:393–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del’Guidice T, Lemay F, Lemasson M, et al:
Stimulation of 5 HT2C receptors improves cognitive deficits induced
by human tryptophan hydroxylase 2 loss of function mutation.
Neuropsychopharmacology. 39:1125–1134. 2014. View Article : Google Scholar
|
|
11
|
Carkaci Salli N, Salli U, Tekin I, et al:
Functional character ization of the S41Y (C2755A) polymorphism of
tryptophan hydroxylase 2. J Neurochem. 130:748–758. 2014.
View Article : Google Scholar
|
|
12
|
Arango V, Huang YY, Underwood MD and Mann
JJ: Genetics of the serotonergic system in suicidal behavior. J
Psychiatr Res. 37:375–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gutknecht L, Jacob C, Strobel A, et al:
Tryptophan hydroxylase-2 gene variation influences personality
traits and disorders related to emotional dysregulation. Int J
Neuropsychopharmacol. 10:309–320. 2007. View Article : Google Scholar
|
|
14
|
Lim JE, Pinsonneault J, Sadee W and Saffen
D: Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of
TPH2 mRNA expression in human pons. Mol Psychiatry. 12:491–501.
2007.PubMed/NCBI
|
|
15
|
Chen GL, Vallender EJ and Miller GM:
Functional characterization of the human TPH2 5′ regulatory region:
Untranslated region and polymorphisms modulate gene expression in
vitro. Hum Genet. 122:645–657. 2008. View Article : Google Scholar
|
|
16
|
Chen GL and Miller GM: Rhesus monkey
tryptophan hydroxylase-2 coding region haplotypes affect mRNA
stability. Neuroscience. 155:485–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roche S and McKeon P: Support for
tryptophan hydroxylase 2 as a susceptibility gene for bipolar
affective disorder. Psychiatr Genet. 19:142–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Li Z, Shao Y, et al: Association
study of tryptophan hydroxylase-2 gene in schizophrenia and its
clinical features in Chinese Han population. J Mol Neurosci.
43:406–411. 2011. View Article : Google Scholar
|
|
19
|
Zhang Y, Zhang C, Yuan G, et al: Effect of
tryptophan hydroxylase-2 rs7305115SNP on suicide attempts risk in
major depression. Behav Brain Funct. 6:492010. View Article : Google Scholar
|
|
20
|
Perroud N, Neidhart E, Petit B, et al:
Simultaneous analysis of serotonin transporter, tryptophan
hydroxylase 1 and 2 gene expression in the ventral prefrontal
cortex of suicide victims. Am J Med Genet B Neuropsychiatr Genet.
153B:909–918. 2010.PubMed/NCBI
|
|
21
|
De Luca V, Likhodi O, Van Tol HH, Kennedy
JL and Wong AH: Gene expression of tryptophan hydroxylase 2 in
post-mortem brain of suicide subjects. Int J Neuropsychopharmacol.
9:21–25. 2006. View Article : Google Scholar
|
|
22
|
Domschke K, Tidow N, Schwarte K, et al:
Serotonin transporter gene hypomethylation predicts impaired
antidepressant treatment response. Int J Neuropsychopharmacol.
17:1167–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dalton VS, Kolshus E and McLoughlin DM:
Epigenetics and depression: Return of the repressed. J Affect
Disord. 155:1–12. 2014. View Article : Google Scholar
|
|
24
|
Klengel T, Pape J, Binder EB and Mehta D:
The role of DNA methylation in stress-related psychiatric
disorders. Neuropharmacology. 80:115–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams JB: A structured interview guide
for the Hamilton Depression Rating Scale. Arch Gen Psychiatry.
45:742–747. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung CM, Wing YK, Kwong PK, Lo A and Shum
K: Validation of the Chinese-Cantonese version of the hospital
anxiety and depression scale and comparison with the Hamilton
Rating Scale of Depression. Acta Psychiatr Scand. 100:456–461.
1999. View Article : Google Scholar
|
|
27
|
Baca-Garcia E, Perez Rodriguez MM,
Basurte-Villamor I, et al: Diagnostic stability of psychiatric
disorders in clinical practice. Br J Psychiatry. 190:210–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spitzer RL and Wakefield JC: DSM-IV
diagnostic criterion for clinical significance: Does it help solve
the false positives problem? Am J Psychiatry. 156:1856–1864.
1999.PubMed/NCBI
|
|
29
|
Hamilton M: The assessment of anxiety
states by rating. Br J Med Psychol. 32:50–55. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling Y, Zhang C, Xu Y, et al: Promoter
methylation-associated silencing of p27kip1 gene with metastasis in
esophageal squamous cell carcinoma. Mol Med Rep. 9:1075–1079.
2014.PubMed/NCBI
|
|
31
|
Li LC and Dahiya R: MethPrimer: Designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Aberg KA, McClay JL, Nerella S, et al:
Methylome-wide association study of schizophrenia: identifying
blood biomarker signatures of environmental insults. JAMA
Psychiatry. 71:255–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meneses A and Liy-Salmeron G: Serotonin
and emotion, learning and memory. Rev Neurosci. 23:543–553.
2012.PubMed/NCBI
|
|
35
|
Araragi N and Lesch KP: Serotonin (5-HT)
in the regulation of depression-related emotionality: Insight from
5-HT transporter and tryptophan hydroxylase-2 knockout mouse
models. Curr Drug Targets. 14:549–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen GL and Miller GM: Tryptophan
hydroxylase-2: An emerging therapeutic target for stress disorders.
Biochem Pharmacol. 85:1227–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Popova NK and Kulikov AV: Targeting
tryptophan hydroxylase 2 in affective disorder. Expert Opin Ther
Targets. 14:1259–1271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gardner KL, Hale MW, Oldfield S, Lightman
SL, Plotsky PM and Lowry CA: Adverse experience during early life
and adulthood interact to elevate tph2 mRNA expression in
serotonergic neurons within the dorsal raphe nucleus. Neuroscience.
163:991–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahgoub M and Monteggia LM: Epigenetics
and psychiatry. Neurotherapeutics. 10:734–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gentile MT, Nawa Y, Lunardi G, Florio T,
Matsui H and Colucci-D’Amato L: Tryptophan hydroxylase 2 (TPH2) in
a neuronal cell line: Modulation by cell differentiation and
NRSF/rest activity. J Neurochem. 123:963–970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel PD, Bochar DA, Turner DL, Meng F,
Mueller HM and Pontrello CG: Regulation of tryptophan hydroxylase-2
gene expression by a bipartite RE-1 silencer of
transcription/neuron restrictive silencing factor (REST/NRSF)
binding motif. J Biol Chem. 282:26717–26724. 2007. View Article : Google Scholar : PubMed/NCBI
|