Introduction
Pulmonary fibrosis is a chronic, progressive and
often fatal interstitial lung disorder of unknown etiology. It is
characterized by an abnormal accumulation of extracellular matrix
(ECM) molecules, and alternating areas of normal lung, interstitial
inflammation, fibroblast migration, proliferation and fibroblastic
foci. ECM remodeling is regulated by a complex interplay between
epithelial cells, fibroblasts, and inflammatory cells. Abnormal
wound healing in response to multiple microscopic sites of alveolar
epithelial cell (AEC) injury and activation may lead to idiopathic
pulmonary fibrosis (IPF). Disruption of the alveolar epithelium,
altered AEC phenotypes, AEC loss and failure of epithelial repair
are the distinctive features of pulmonary fibrosis. AECs are
capable of promoting the migration, proliferation and activation of
mesenchymal cells, along with the formation of
fibroblastic/myofibroblastic foci and excessive ECM accumulation.
Accumulation of ECM in the interstitial, alveolar spaces and
disruption of the basement membranes occur during progression of
the disease (1,2). An imbalance between the synthesis and
degradation of ECM molecules in the local lung microenvironment has
an important role in the pathogenesis of pulmonary fibrosis
(3).
Matrix metalloproteinases (MMPs) are a family of
zinc-endopeptidases that are associated with fibrocyte migration
throughout the basement membranes and the interstitial collagen
matrix, and also in fibrotic tissue remodeling. MMPs are capable of
degrading various components of connective tissue matrices, and are
believed to have an important role in remodeling following
parenchymal damage, leading to tissue destruction in pulmonary
diseases (4). MMPs have been shown
to be upregulated in pulmonary fibrosis (5,6).
Pivotal extracellular control of the catalytic activity of MMPs is
accomplished by members of a specific family of inhibitors, the
tissue inhibitors of metalloproteinases (TIMPs). There are four
known members of this family (TIMP-1 to -4) that bind to the active
site of MMPs in a 1:1 ration. TIMPs may act in the tissue
environment to neutralize used proteinases, thereby preventing
excessive and unwanted degradation away from the sites of
metalloproteinase production (7).
Following injury, the expression of MMPs is rapidly increased, and
gradually declines as the wound enters the remodeling phase. This
system is tightly regulated by TIMPs. Previous evidence has
suggested that abnormal alteration of the MMPs/TIMPs balance under
pathological conditions may lead to aberrant tissue repair,
accumulation of ECM and impairment of lung function (8).
Various cytokines and chemokines are also associated
with the fibrotic progress. Suppressor of cytokine signaling 1
(SOCS1) proteins are inhibitors of cytokine signaling (9). SOCS1 is usually expressed at low
levels, but may be induced by various cytokines and bind to Jaks,
in order to inhibit subsequent signal transduction. Shod et
al (10) demonstrated that
lower mRNA expression levels of SOCS1 were produced by fibroblasts
from the lungs of pulmonary fibrosis. The deficiency of SOCS1 in
murine fibroblasts resulted in the overproduction of collagen,
which was conversely suppressed by overexpression of SOCS1. However
the specific mechanisms remain unclear. The present study
hypothesized that the SOCS1 gene may have a role in the modulation
of ECM accumulation, by influencing the expression levels of
MMP/TIMP in AECs and fibroblasts, and SOCS1 may provide a novel
therapeutic strategy against pulmonary fibrosis. The expression
levels of MMPs and TIMPs in AECs and fibroblasts were determined
under the conditions of deficiency and overexpression of SOCS1.
Materials and methods
Construction of RNA interference
targeting SOCS1
A small hairpin (sh)RNA specifically targeting human
SOCS1 was produced, based on a published human SOCS1 gene sequence
(http://www.ncbi.nlm.nih.gov/gene/8651). A pair of
oligonucleotides synthesized by Biotelevector (Shanghai, China)
were annealed to the gene sequence, and the double-stranded shRNA
templates were diluted (1:50) to 200 nM, and further ligated to the
lentiviral vector pPll3.7, which was double digested with
XhoI/HpaI (Fermentas, Thermo Fisher Scientific,
Pittsburgh, PA, USA). The Pll3.7 vector lacking any insertion of
oligonucleotides was used as a negative control. JM 109
Escherichia coli (E. coli) competent cells were purchased
from the Cytology Center of the Chinese Academy of Science
(Shanghai, China) and were transformed with the plasmids. A colony
was selected and the recombinant plasmids were extracted for
sequencing using a gel extraction kit.
Plasmid transformation, selection and
extraction
Plasmid (1 ng) was added to 200 µl JM 109
E. coli competent cells, placed on ice for 30 min, then at
42°C for 90 sec, prior to the addition of 1 ml LB medium which was
then cultured for 1 h at 37°C and transferred to a 200-µl
coated plate (LB medium). For plasmid selection, a colony was
selected from the plate and added to 1 ml of the tube containing 5
ml LB medium, cultured at 37°C and the plasmids were subsequently
extracted from the bacteria using Plasmid Maxi Preperation kit
(Beyotime Institute of Biotechnology, Shanghai, China). Extraction
was achieved via the addition of 250 µl Solution I/RNaseA
mixture, which completely suspended the bacteria. Solution II (250
µl) was added to the resuspended mixture, followed by 350
µl Solution III. The mixture was inverted several times to
induce the formation of a white flocculent precipitate. This
mixture was subsequently centrifuged at 10,000 x g for 10 min, then
the supernatant was transferred to a collection tube containing the
Hi Bind DNA binding column, centrifuged at 10,000 x g for 1 min and
the liquid was discarded. Subsequently, 500 µl HB Buffer was
added and centrifuged at 10,000 x g for a further 1 min. The liquid
was discarded and 700 µl DNA wash buffer was added and
centrifuged at 10,000 x g for 1 min. Finally, the column was
mounted on a clean 1.5 ml tube, 30–50 µl elution buffer was
added to the column and centrifuged at 10,000 x g for 1 min to
elute the plasmid DNA.
Cloning of SOCS1 cDNA lentivirus
The A549 human epithelial lung carcinoma cell line
was purchased from the Cytology Center of the Chinese Academy of
Science. Fetal bovine serum (FBS) and Opti-MEM® were
obtained from Gibco Life Technologies (Carlsbad, CA, USA).
Dulbecco's modified Eagle's medium (DMEM) was purchased from
Sigma-Aldrich (St Louis, MO, USA). Penicillin and streptomycin were
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
Total RNA was extracted from 106 cells using
TRIzol® reagent (Invitrogen Life Technologies), and the
quality and quantity of the obtained RNA was determined by
spectrophotometry based on the absorbance at A260/A280. The RNA
product was then reverse transcribed into cDNA using a Fermentas
reverse transcription kit (Thermo Fisher Scientific). Total RNA (1
µg) and 1 µl oligo(dT) were added to a sterile,
nuclease-free tube on ice, followed by 1 µl DEPC water,
prior to incubation at 65°C for 5 min, chilling on ice and spinning
down back on ice. The following components were added: 4 µl
5X reaction buffer, 2 µl 10 mM dNTP mix, 1 µl
RevertAid™ M-MuLV reverse transcriptase and 1 µl RiboLock™
RNase inhibitor, and mixed gentley prior to centrifugation at 25°C
for 5 min, 42°C for 60min, 70°C for 5 min and 4°C for 5 min. The
SOCS1 gene was obtained by reverse transcription-polymerase chain
reaction (RT-PCR). The gene was digested by Sma1 and
Kpn1 enzymes (Fermentas, Burlington, ON, Canada):
Socs1-SmaI-F, 5′-TCC CCC GGG ATG GTA GCA CAC AAC CAG GTG-3′;
Socs1-KpnI-R, 5′-GGG GTA CCT CAA ATC TGG AAG GGG AAGG-3′
(Invitrogen Life Technologies, Shanghai, China). The reagent
included 8 µl 10X TE buffer + bovine serum albumin
(GaoChuang Medical Technology Co., Ltd., Shanghai, China), 50
µl SOCS1, 1 µl Smal, 1 µl Kpnl and
double-distilled H2O to a total of 80 µl, and was
kept overnight at 37°C. The target gene segment (7.5 L) was
connected with carrier ppic9K, in 1.5 µl 10X T4 buffer
solution and 1 µl T4 ligase, at 37°C for 3 h. The gene was
then transfected into DH5α competent cells (Cytology Center of the
Chinese Academy of Science), overnight at 37°C.
Lentiviral packaging and titration
Human embryonic kidney (HEK) 293T cells (Cytology
Center of the Chinese Academy of Science) were transfected with
each recombinant plasmid. For the transfection, 2×105
cells were seeded into six-well plates and an appropriate quantity
of virual suspension was added, and the cells were incubated at 37
°C for 24 hours. The medium containing the virus was subsequently
replaced with fresh complete medium. Following a 72 h incubation,
the supernatants were harvested and concentrated. The viral titers
were determined and calibrated in the HEK 293T cell lines.
Analysis of interference and
overexpression of SOCS1 gene
The supernatants of the three lentiviruses (100
µl) were added to wells containing the A549 cells and human
embryonic lung fibroblasts (HLFs; Cytology Center of the Chinese
Academy of Science), according to the following groups: Group A,
untreated cells; group B, cells transfected with the negative
control lentiviral plasmid; group C, cells transfected with the
SOCS1 interfering lentiviral plasmid; and group D: cells with an
overexpression of SOCS1. The supernatants were replaced with DMEM,
containing 10% FBS, after 24 h, and the cells were incubated for a
further 72 h. Western blot analysis was used to assess the
expression levels of the SOCS1 protein. Briefly, HLFs were lysed in
100 ml lysis buffer (150 mM NaCl, 50 mM Tris-Cl pH 7.4, 1 mM EDTA,
0.1% SDS, 1.0% deoxycholatic, 1.0% TritonX-100 and 1 mM PMSF).
Protein concentrations were quantified using the bicinchoninic acid
Protein Assay kit (Thermo Fisher Scientific) according to the
manufacturer's instructions. The proteins were separated on 12%
SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF)
membranes (EMD Millipore, Billerica, MA, USA). The PVDF membranes
were incubated with rabbit polyclonal anti-human SOCS1 (1:1,000;
Santa Cruz Biotechnology, Inc., Heidelberg, Germany) at 4°C
overnight. Human β-actin (Sigma-Aldrich) was used as an internal
control. The membranes were blocked with PBS containing 5% nonfat
milk for 2 h at room temperature. Chemiluminscent substrate (1 ml;
Thermo Fisher Scientific) was added and the signal was detected and
quantified using an enhanced chemiluminscence system (ImageQuant
LAS-4000 Mini; GE Healthcare Life Sciences, Little Chalfont, UK).
All samples were normalized to β-actin using Image J 1.4.3.67
software. The A549 cells and HLFs were treated using the same
protocol. To determine viral titres, 1×105 cells were
seeded into six-well plates and incubated for 24 h. Lentivirus was
added to the cells at concentrations ranging between
10−2 and 10−6 in a final volume of 1 ml and
incubated for 24 h. Following incubation, the medium containing the
virus was replaced with 2 ml complete medium and incubated for 24
h. The GFP signal was the detected (Axiovert 10; Zeiss, Oberkochen,
Germany) and the number of fluorescent clones were quantified. The
clones in the last two wells were assumed to be X and Y and the
titre (TU/ml) = (X + Y × 10) / 2×105.
Measurement of MMPs and TIMPs in A549
cells and HLFs
To examine the effects of the SOCS1 gene on the
expression levels of MMPs/TIMPs, the A549 cells and HLFs were
randomized into six groups: Cells left untreated; cells stimulated
with transforming growth factor-β1 (TGF-β1; R&D Systems,
Minneapolis, MN, USA) (2 ng/ml) alone; cells transfected with
negative lentiviral plasmid and stimulated with TGF-β1 (2 ng/ml);
cells transfected with a lentiviral plasmid overexpressing SOCS1
and stimulated with TGF-β1 (2 ng/ml); cells transfected with a
lentiviral plasmid interfering with SOCS1 expression and stimulated
with TGF-β1 (2 ng/ml); and cells treated with TGF-β1 (2 ng/ml) and
dexamethasone (DXM; 10−6 M; Biovision, San Francisco,
CA, USA). All of the cells were stimulated for ≥72 h. Quantitative
PCR was used to detect the mRNA expression levels of MMP-1, MMP-2,
MMP-9, TIMP-1, TIMP-2 and TIMP-4. The primer sequences for the PCR
are listed in Table I. PCR was
performed with a 7500 Fast Real-Time PCR System (Applied Biosystems
Life Technologies, Foster City, CA, USA) with FastStart Universal
SYBR Green (Roche Diagnostics, Basel, Switzerland) following cDNA
synthesis. PCR conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 40 cycles of amplification at 95°C for
15 sec and 60°C for 60 sec. Fold-change in the gene expression
levels were calculated using the 2−ΔΔCt method relative
to the internal reference gene GAPDH.
 | Table IPrimer sequences for the quantitative
polymerase chain reaction. |
Table I
Primer sequences for the quantitative
polymerase chain reaction.
| Target mRNA | Forward primer
(5′-3′) | Reverse primer
(3′-5′) |
|---|
| MMP-1 |
GCACAAATCCCTTCTACCCG |
ATGTCCTTGGGGTATCCGTG |
| MMP-2 |
GCGATGGATACCCCTTTGAC |
GTACTCCCCATCGGCGTTC |
| MMP-9 |
CAGAGATGCGTGGAGAGTCG |
GCAAGTCTTCCGAGTAGTTTTGG |
| TIMP-1 |
TGCGGATACTTCCACAGGTC |
GGGGATGGATAAACAGGGAA |
| TIMP-2 |
GCACCACCCAGAAGAAGAGC |
GCACAGGAGCCGTCACTTCT |
| TIMP-4 |
CTCCAGTGAGAAGGTAGTTCCG |
AGGGAAGAGTCAAAAGGCGT |
Statistical analysis
Statistical analyses were performed using a t-test
between every two groups using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Results of analysis of interference and
overexpression of SOCS1 gene
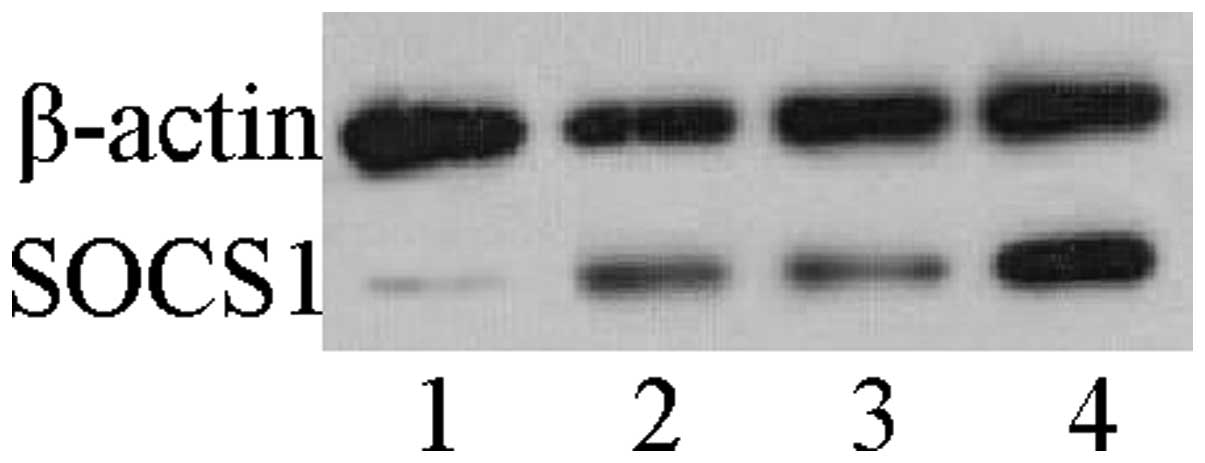
Western blot analysis was used to assess the
expression levels of the SOCS1 protein in HLF cells. The cells
deficient in SOCS1, had >50% reduction in SOCS1 protein
expression levels; whereas the cells overexpressing SOCS1 had a
significant upregulation of the protein. The other two groups had
no significant differences in the expression levels of the SOCS1
protein (Fig. 1). Identical
results were observed in A549 cells (data not shown).
Effects of SOCS1 gene on the mRNA
expression levels of MMPs in A549 cells and HLFs
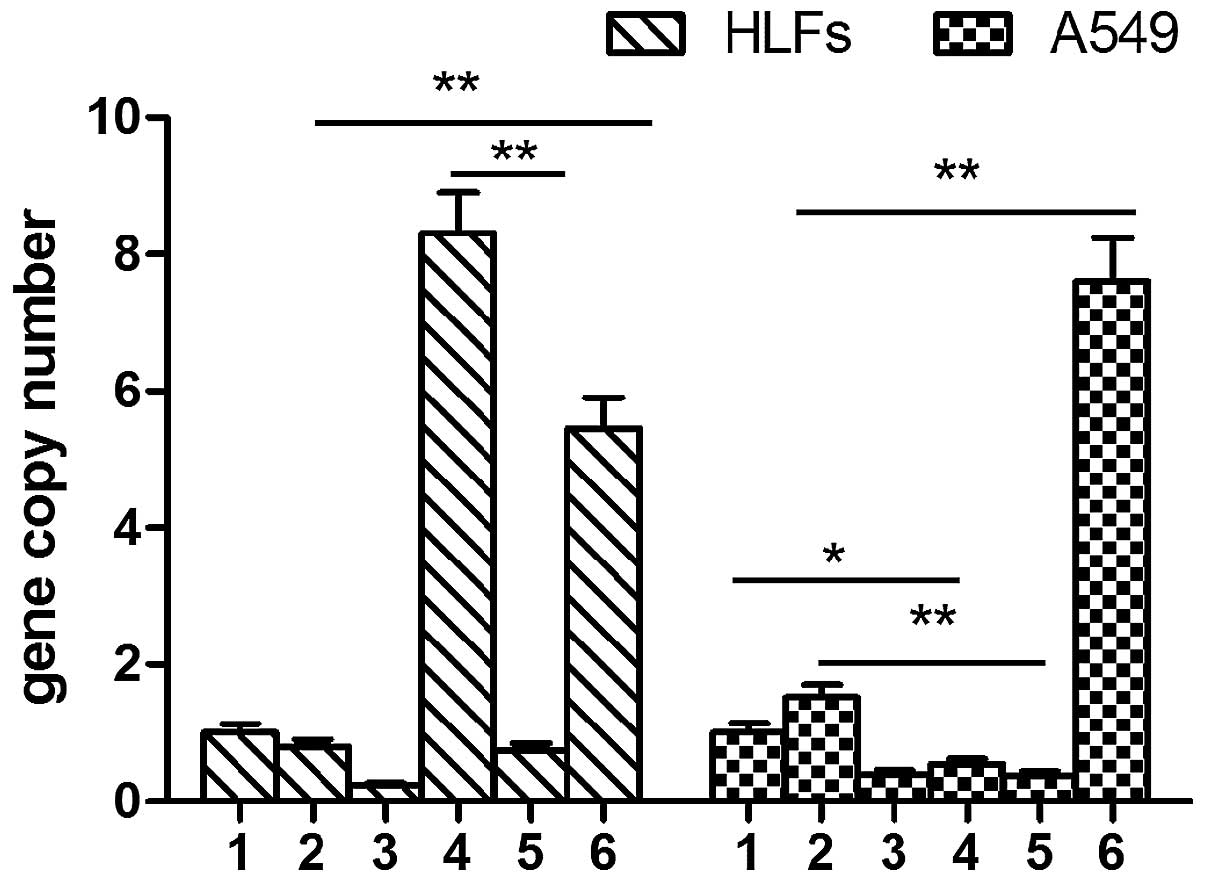
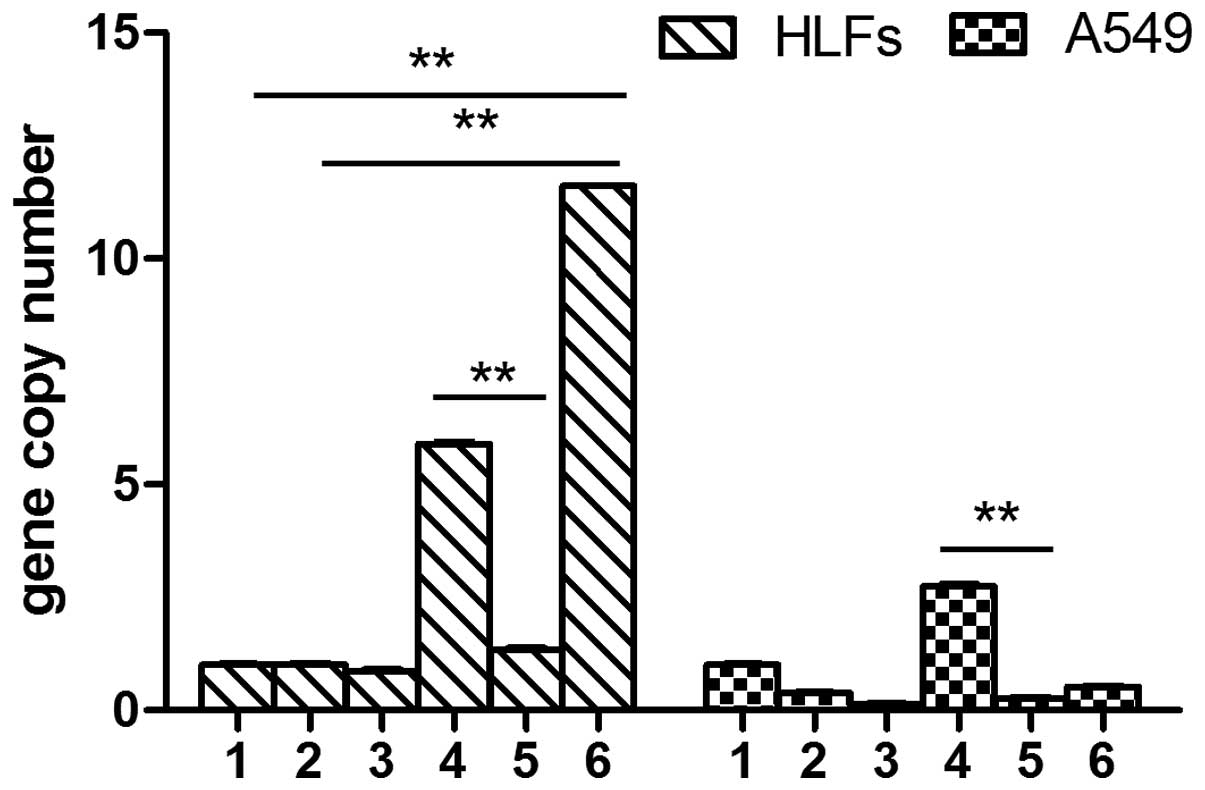
MMP-1 expression levels were slightly increased
following TGF-β1 stimulation in the A549 cells. MMP-1 expression
levels in the SOCS1-deficient group were significantly higher, as
compared with the A549 cells stimulated with TGF-β1 (7.61±0.63 vs.
1.52±0.19, P<0.01). DXM inhibited the expression levels of
MMP-1. Overexpression of SOCS1 in the A549 cells resulted in
significantly lower MMP-1 expression levels, as compared with the
cells stimulated with TGF-β1 alone (0.37±0.06 vs. 1.52±0.19,
P<0.01). The A549 cells transfected with the negative lentiviral
plasmid and stimulated with TGF-β1 had lower MMP-1 expression
levels, as compared with the untreated cells (0.54±0.08 vs.
1.01±0.13, P<0.05). Conversely, TGF-β1 did not induce the
expression of MMP-1 in the HLFs. The expression levels of MMP-1 in
the SOCS1-deficient HLFs was significantly higher, as compared with
the HLFs treated with TGF-β1 alone and the untreated HLFs
(5.46±0.44 vs. 0.79±0.10, P<0.01). The mRNA expression levels of
MMP-1 in the HLFs overexpressing SOCS1 were significantly
decreased, as compared with the HLFs transfected with the negative
lenti-virus (0.75±0.10 vs. 8.32±0.59, P<0.01) (Fig. 2).
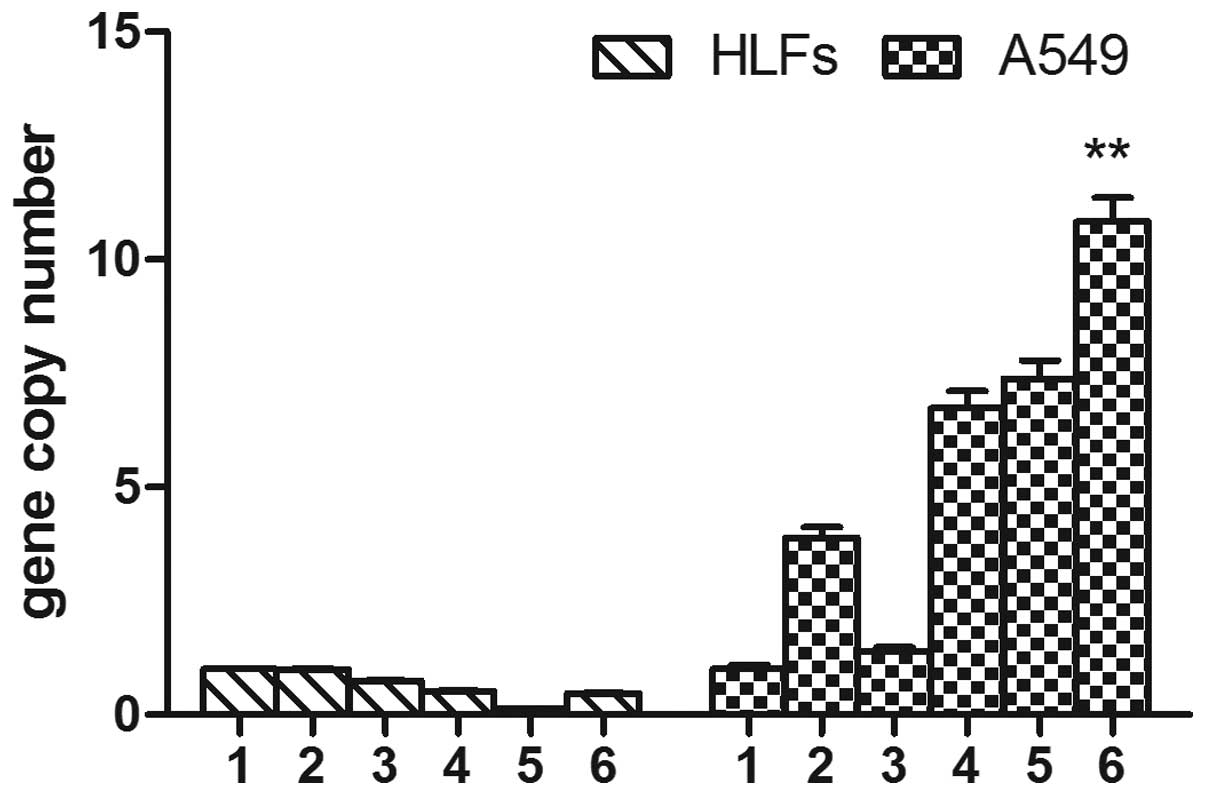
The mRNA expression levels of MMP-2 in the
SOCS1-deficient A549 cells were significantly higher, as compared
with the A549 cells stimulated with TGF-β1, and the A549 cells
transfected with the negative lentiviral plasmid and stimulated
with TGF-β1 (4.97±0.65 vs. 0.69±0.12, 1.51±0.24. P<0.01). The
A549 cells overexpressing SOCS1, similarly to the cells treated
with DXM, had lower expression levels of MMP-2, as compared with
the HLF cells transfected with the negative lentivirus and the
group stimulated with TGF-β1 (0.41±0.03 vs. 0.90±0.06, 1.82±0.08,
P<0.01). Conversely, TGF-β1 induced MMP-2 expression slightly
more in the HLFs, as compared with the SOCS1-deficient HLFs.
However, over-expression of SOCS1 reduced the expression levels of
MMP-2, as compared with the SOCS1-deficient HLFs (0.41±0.03 vs.
1.33±0.07, P<0.01) (Fig.
3).
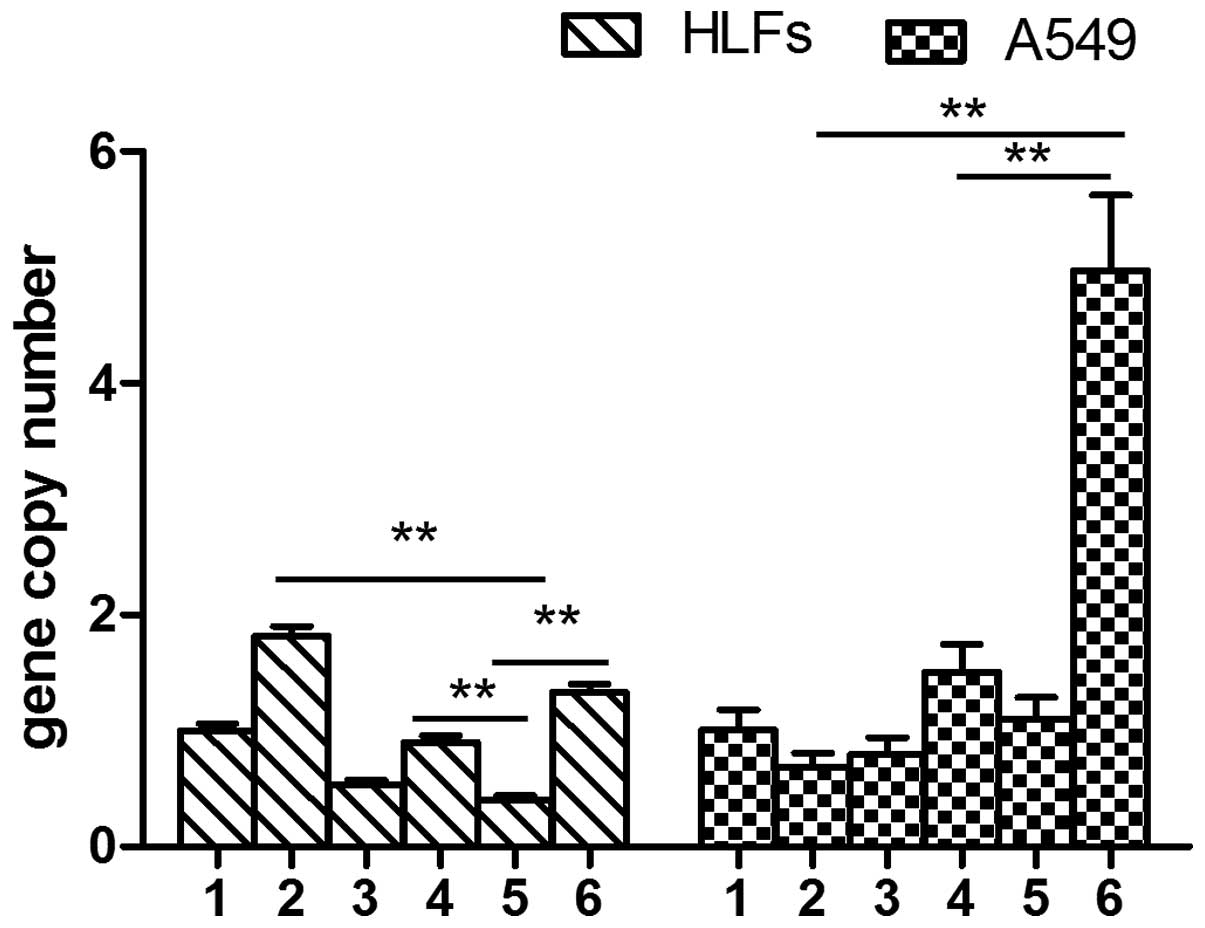
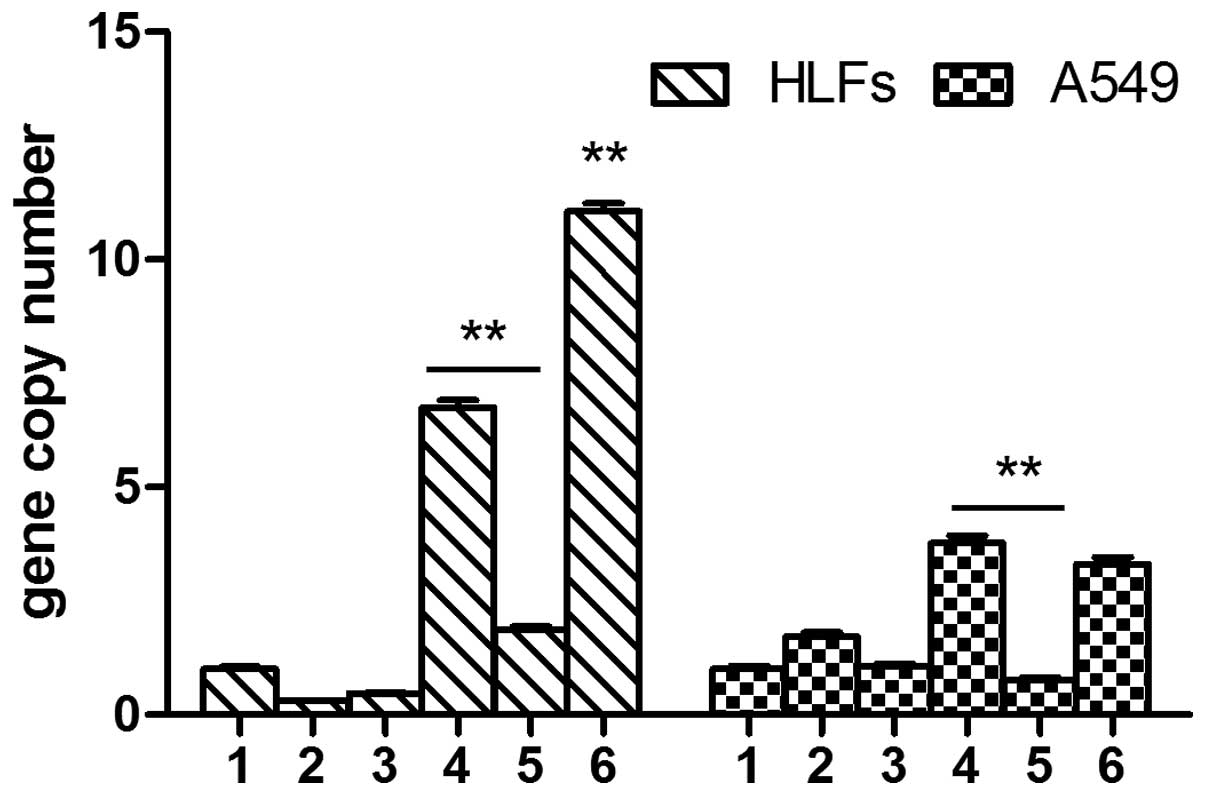
TGF-β1 did not induce the expression of MMP-9 in
either the A549 cells or the HLFs. There was no significant
difference between the A549 cells transfected with the negative
lentivirus and those transfected with the lentivirus interfering
with SOCS1 expression (P>0.05). DXM had no effect on the mRNA
expression levels of MMP-9 in the A549 cells. However, the mRNA
expression levels of MMP-9 in the A549 cells overexpressing SOCS1
were significantly decreased, as compared with the SOCS1-deficient
cells (0.68±0.09 vs. 3.91±0.34, P<0.01). The expression levels
of MMP-9 in the SOCS1-deficient HLFs were significantly increased,
as compared with all of the other groups (P<0.01). The mRNA
expression levels of MMP-9 in the HLFs overexpressing SOCS1 were
significantly decreased, as compared with the HLFs transfected with
the negative lentivirus (4.15±0.35 vs. 12.04±0.71, P<0.01)
(Fig. 4).
Effects of SOCS1 gene on the mRNA
expression levels of TIMPs in A549 cells and HLFs
TGF-β1 induced TIMP-1 expression in the A549 cells;
however, it had no effect on its expression in HLFs. The mRNA
expression levels of TIMP-1 were markedly higher in the
SOCS1-deficient A549 cells, as compared with the other groups
(P<0.01). Overexpression of SOCS1 partially reversed these
changes. The mRNA expression levels of TIMP-1 were low in the HLFs.
There were no significant changes to TIMP-1 expression following
TGF-β1 stimulation in the HLFs transfected with the negative
lentivirus, or in the SOCS1-deficient HLFs (P>0.05).
Furthermore, DXM did not affect the mRNA expression levels of
TIMP-1 in the HLFs following stimulation with TGF-β1. The
expression levels of TIMP-1 in the HLFs overexpressing SOCS1 were
decreased, as compared with the SOCS1-deficient HLFs following
TGF-β1 stimulation (0.14±0.01 vs. 0.47±0.01, P>0.05); however
these findings were not significant (Fig. 5).
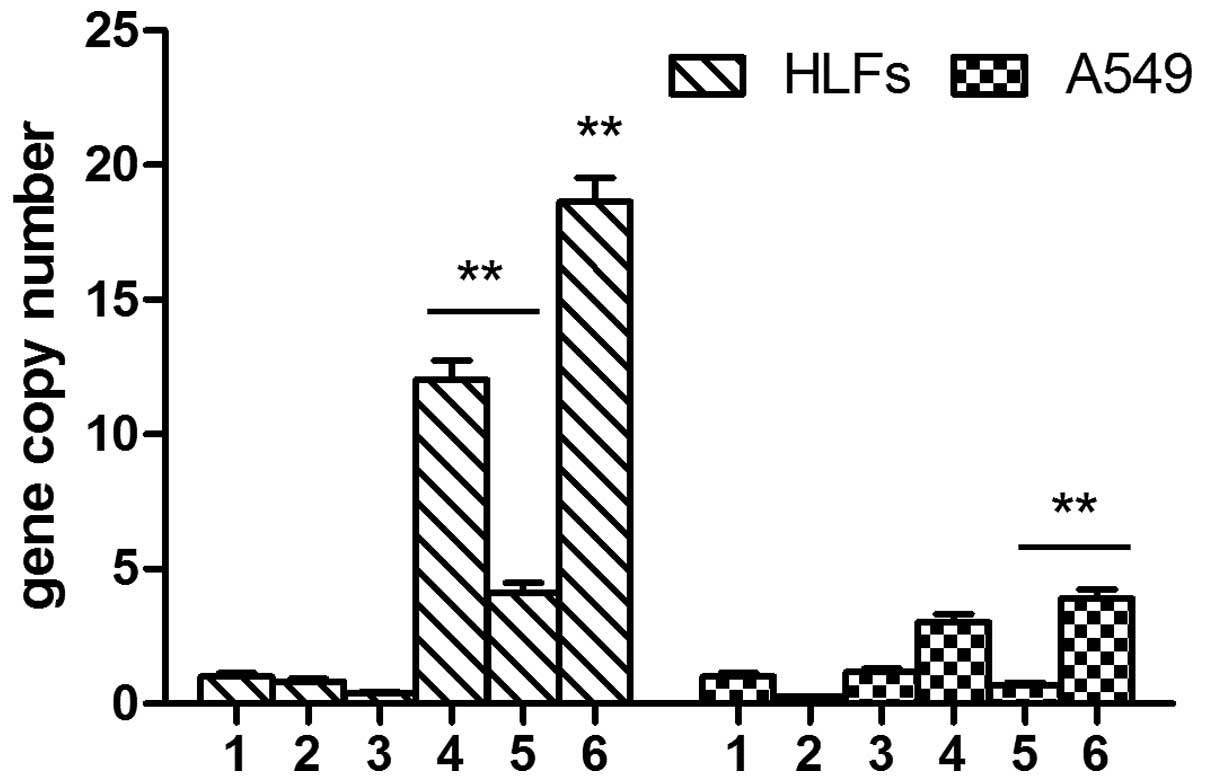
The mRNA expression levels of TIMP-2 were low in the
A549 cells and the expression was not elevated following TGF-β1
stimulation. The mRNA expression levels of TIMP-2 in the
SOCS1-deficient cells were slightly higher, as compared with all of
the other groups (P<0.05). However, the expression levels of
TIMP-2 were significantly decreased in the A549 cells
over-expressing SOCS1, as compared with the cells transfected with
the negative lentivirus and (0.27±0.02 vs. 2.74±0.05; P<0.01).
Furthermore, TGF-β1 could not induce TIMP-2 expression in the HLFs.
The expression levels of TIMP-2 were significantly higher in the
SOCS1-deficient HLFs, as compared with the cells stimulated with
TGF-β1 (11.6±0.01 vs. 1.00±0.03; P<0.01) and the untreated HLFs
(11.6±0.01 vs. 1.00±0.03; P<0.01). The expression levels of
TIMP-2 were significantly reduced in the HLFs overexpressing SOCS1,
as compared with the HLFs transfected with the negative control
lentivirus (1.34±0.04 vs. 5.89±0.05, P<0.01) (Fig. 6).
The mRNA expression levels of TIMP-4 were low in the
A549 cells. The expression levels of TIMP-4 were slightly lower in
the SOCS1-deficient group, as compared with the cells transfected
with the negative lentivirus (P>0.05). The cells overexpressing
SOCS1 and the cells treated with DXM had reduced expression levels
of TIMP-4, as compared with all of the other A549 groups
(P<0.05). TGF-β1 did not induced TIMP-4 expression in the HLFs.
However, TIMP-4 expression levels were elevated in the HLFs
following transfection with the SOCS1-deficient lentivirus. The
expression levels of TIMP-4 were significantly lower in the HLFs
overexpressing SOCS1, as compared with the cells transfected with
the negative control lentivirus and the SOCS1-deficient group
(Fig. 7).
Discussion
Despite recent advances the pathophysiology of IPF
remains not fully understood. However, injury to type II AECs is
considered to be the key event for the initiation of the
development of fibrosis (11).
Progressive pulmonary fibrosis is also thought to result from
dysregulated ECM control, which MMPs are believed to have important
roles in. MMPs degrade various components of connective tissue
matrices that are capable of remodeling following parenchymal
damage, resulting in tissue destruction or the induction of repair
processes in pulmonary diseases (12).
In the early stage of the disease, the expression
levels of MMP-1 (interstitial collagenase) in the epitheliocytes,
macrophages, fibroblasts, and myofibroblasts have been shown to be
higher, as compared with in the later stages of IPF (13). MMP-1 was previously detected in the
regenerated epithelial cells covering intra-alveolar fibrosis. In
the present study, A549 cells and HLFs were shown to express MMP-1
at low levels. MMP-1 expression levels slightly increased following
TGF-β1 stimulation in A549 cells, whereas TGF-β1 did not induce the
expression of MMP-1 in the HLFs. However, the mRNA expression
levels of MMP-1 in the SOCS1-deficient A549 cells and HLFs were
significantly higher, as compared with the other groups.
Furthermore, the A549 cells and HLFs overexpressing SOCS1 had
significantly decreased expression levels of MMP-1. These results
suggest that in different cells, the stimulator of MMP-1 may be
different. However, in the A549 cells and HLFs deficient in SOCS1,
which inhibits cytokine signaling, the MMP-1 expression levels were
increased.
MMP-2 is a 72 kDa gelatinase A, that degrades
collagen type IV, the major constituent of the basement membrane.
MMP-2 has been shown to be upregulated in pulmonary fibrosis, and
to be secreted by numerous kinds of cells in the lungs (6). The overexpression of MMP-2 is mainly
associated with its ability to provoke disruption of the AEC
basement membrane and enhance fibroblast invasion into the alveolar
spaces (14,15). The present study demonstrated that
A549 cells and HLFs express MMP-2. MMP-2 mRNA expression levels in
the SOCS1-deficient A549 cells were significantly higher, as
compared with the other groups, and treatment with DXM inhibited
MMP-2 expression. There were similar changes observed in the HLFs.
These results suggest that SOCS1 may be useful for attenuating
pulmonary fibrosis through the inhibition of MMP-2 production in
AECs and fibroblasts.
MMP-9 is a 92 kDa gelatinase B, which degrades
collagen type IV. Collagen type IV the major constituent of the
basement membrane. Suga et al (4) demonstrated increased MMP-9 activity
in IPF, particularly in those with rapid progression. In the lung
parenchyma, MMP-9 was shown to be predominantly expressed in the
inflammatory cells at the early stage of the disease. In later
stages, type II pneumocytes and AECs at the periphery of the
fibrotic foci retained MMP-9 expression, which was more prominent
in the cells, suggesting that MMP-9 may have a role in the process
of repair (16). The present study
showed that SOCS1-deficient A549 cells and HLFs express higher
levels of MMP-9, as compared with the control groups, and
overexpression of SOCS1 inhibited the expression of MMP-9.
Matrix remodeling ensues with initiation of the
disease with an MMPs/TIMPs imbalance favoring over activity of the
enzyme to stimulate fibrogenesis (17). Previous studies have shown that
upregulation of MMPs, and the imbalance with TIMPs, may lead to the
release of growth factors from fibroblasts (18,19).
Numerous studies have detected elevated expression levels of MMP-1
and MMP-9, which occur in conjunction with alterations in the
levels of their soluble inhibitors, TIMP-1 and -2, in IPF (3,5,6,8).
Therefore, the present study investigated the changes to TIMP-1,
TIMP-2 and TIMP-4 expression levels. TIMP-1 expression levels were
low in AECs and fibroblasts. TGF-β1 induced TIMP-1 expression in
A549 cells; however, it had no effect on TIMP-1 expression in HLFs.
Treatment with DXM did not alter the mRNA expression levels of
TIMP-1 in the HLFs following TGF-β1 stimulation, however DXM
inhibited TIMP-1 expression in A549 cells. A lack of SOCS1
expression resulted in increased expression levels of TIMP-1, in
the A549 cells, and overexpression of SOCS1 partially reversed
these changes. SOCS1 had little effect on the expression of TIMP-1
in HLFs. These results suggest that SOCS1 may regulate the
expression of TIMP-1 in A549 cells, but not in fibroblasts.
TIMP-2 was shown to be almost exclusively associated
with fibroblast foci. Fibroblast foci are distributed throughout
the lung parenchyma and indicate that fibrosis is actively ongoing
(12). The present study
demonstrated that TGF-β1 could not induce TIMP-2 expression in AECs
and fibroblasts. However, the expression levels were significantly
higher in the SOCS1-deficient group, as compared with the HLFs
stimulated with TGF-β1. The expression levels of TIMP-2 were
signifi-cantly lower in the cells overexpressing SOCS1. Conversely,
the expression levels of TIMP-2 were slightly higher in the
SOCS1-deficient A549 cells, as compared with all of the other
groups. Therefore, it may be hypothesized that fibroblasts are the
major sources of TIMP-2, and SOCS1 regulated fibrosis by
influencing TIMP-2.
TIMP-4 is abundantly expressed in human
cardiovascular structures, whereas all other tissues in the normal
state, including lung parenchyma, are characterized by low or
absent expression (20). The
present study showed that the mRNA expression levels of TIMP-4 were
low in the A549 cells. The expression levels were slightly higher
in the SOCS1-deficient group, as compared with the cells
transfected with the negative lentivirus. Overexpression of SOCS1
and treatment with DXM decreased the expression levels of TIMP-4,
as compared with all of the other A549 groups. TGF-β1 did not
induce TIMP-4 expression in the HLFs. However, TIMP-4 expression
levels were elevated in the HLFs transfected with the
SOCS1-deficient lentivirus. The expression levels of TIMP-4 were
significantly lower in the HLFs overexpressing SOCS1, as compared
with the cells transfected with the negative control lentivirus and
the SOCS1-deficient lentivirus.
Nakashima et al (21) previously suggested that SOCS1
expression was significantly reduced in the lung tissue from
patients with IPF, as compared with that in non-IPF patients.
Furthermore, SOCS1 expression was significantly reduced in severe
fibrotic lesions, as compared with in less fibrotic lesions.
SOCS1-deficient murine embryonic fibroblasts (MEF) spontaneously
produced significantly higher amounts of type I collagen, as
compared with the wild type MEF. Conversely, the effects of
overexpression of SOCS1 were inhibition of type I collagen
production from fibroblasts. In the present study, overexpression
of SOCS1 regulated MMP-1, MMP-2, and MMP-9, and TIMP-1, TIMP-2 and
TIMP-4 expression in AECs and fibroblasts. These results suggest
that SOCS1 may act as a suppressor of pulmonary fibrosis, by
reducing expression of MMPs and TIMPs. Therefore, SOCS1 may be a
potential therapeutic target of IPF treatment.
Acknowledgments
The present study was supported by grants from the
Science and Technology Commission of Shanghai (no. 09411951400) and
the National Natural Science Foundation of China (no.
81200027).
References
|
1
|
Selman M, King TE, Pardo A, et al:
Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses
about its pathogenesis and implications for therapy. Ann Intern
Med. 134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green FH: Overview of pulmonary fibrosis.
Chest. 122(6 Suppl): 334S–339S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selman M, Ruiz V, Cabrera S, et al:
TIMP-1, -2, -3 and -4 in idiopathic pulmonary fibrosis. A
prevailing nondegradative lung microenvironment? Am J Physiol Lung
Cell Mol Physiol. 279:L562–L574. 2000.PubMed/NCBI
|
|
4
|
Suga M, Iyonaga K, Okamoto T, et al:
Characteristic elevation of matrix metalloproteinase activity in
idiopathic interstitial pneumonias. Am J Respir Crit Care Med.
162:1949–1956. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nkyimbeng T, Ruppert C, Shiomi T, et al:
Pivotal role of matrix metalloproteinase 13 in extracellular matrix
turnover in idiopathic pulmonary fibrosis. PLoS One. 8:e732792013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dancer RC, Wood AM and Thickett DR:
Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J.
38:1461–1467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagase H and Brew K: Designing TIMP
(tissue inhibitor of metalloproteinases) variants that are
selective metalloproteinase inhibitors. Biochem Soc Symp.
70:201–212. 2003.PubMed/NCBI
|
|
8
|
Wang BL, Tu YY, Fu JF, et al: Unbalanced
MMP/TIMP-1 expression during the development of experimental
pulmonary fibrosis with acute paraquat poisoning. Mol Med Rep.
4:243–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakashima T, Yokoyama A, Onari Y, et al:
Suppressor of cytokine signaling 1 inhibits pulmonary inflammation
and fibrosis. J Allergy Clin Immunol. 121:1269–1276. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shoda H, Yokoyama A, Nishino R, et al:
Overproduction of collagen and diminished SOCS1 expression are
causally linked in fibroblasts from idiopathic pulmonary fibrosis.
Biochem Biophys Res Commun. 353:1004–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Margaritopoulos GA, Giannarakis I,
Siafakas NM and Antoniou KM: An update on idiopathic pulmonary
fibrosis. Panminerva Med. 55:109–120. 2013.PubMed/NCBI
|
|
12
|
Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M
and Yamanaka N: Localization of matrix metalloproteinases-1, -2,
and -9 and tissue inhibitor of metalloproteinase-2 in interstitial
lung diseases. Lab Invest. 78:687–698. 1998.PubMed/NCBI
|
|
13
|
Henry MT, McMahor K, Mackarel AJ, et al:
Matrix metalloproteinases and tissue inhibiter of
metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J.
20:1220–1227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McKeown S, Richter AG, O'Kane C, McAuley
DF and Thickett DR: MMP expression and abnormal lung permeability
are important determinants of outcome in IPF S. Eur Respir J.
33:77–84. 2009. View Article : Google Scholar
|
|
15
|
Kim JY, Choeng HC, Ahn C and Cho SH: Early
and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary
fibrosis. Yonsei Med J. 50:68–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouchi H, Fujita M, Ikegame S, et al: The
role of collagenases in experimental pulmonary fibrosis. Pulm
Pharmacol Ther. 21:401–408. 2008. View Article : Google Scholar
|
|
17
|
Ramos C, Montaño M, García-Alvarez J, et
al: Fibroblasts from idiopathic pulmonary fibrosis and normal lungs
differ in growth rate, apoptosis, and tissue inhibitor of
metalloproteinases expression. Am J Respir Cell Mol Biol.
24:591–598. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruiz V, Ordóñez RM, Berumen J, et al:
Unbalanced colla-genases/TIMP-1 expression and epithelial apoptosis
in experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol.
285:L1026–L1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pardo A, Selman M and Kaminski N:
Approaching the degradome in idiopathic pulmonary fibrosis. Int J
Biochem Cell Biol. 40:1141–1155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koskivirta I, Rahkonen O, Mäyränpää M, et
al: Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in
inflammatory processes of human cardiovascular pathology. Histochem
Cell Biol. 126:335–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakashima T, Yokoyama A, Onari Y, et al:
Suppressor of cytokine signaling 1 inhibits pulmonary inflammation
and fibrosis. J Allergy Clin Immunol. 121:1269–1276. 2008.
View Article : Google Scholar : PubMed/NCBI
|