Introduction
Vestibular schwannomas (VS) are benign tumors of the
Schwann cell sheath, which originate on the vestibular branches of
cranial nerve VIII. VS occur either sporadically or as the hallmark
tumor of neurofibromatosis type 2 (NF2), which is an hereditary
disease caused by loss of the NF2 gene. Current treatment
options for VS are surgical excision, stereo-tactic radiation and
observation. Knowledge of the molecular biology of VS and the
development of novel medical therapeutic methods have advanced
markedly in previous years (1).
Tanshinone IIA (Tan IIA) is a key component of
Danshen, a traditional herbal medicine isolated from Salvia
miltiorrhiza Bunge, which is used to treat cardiovascular
diseases (2). Tan IIA exhibits
anti-inflammatory and antioxidative properties, and inhibits
various human cancer cell lines by inducing apoptosis or inhibiting
angiogenesis (3–5). However, the inhibitory effects of Tan
IIA on VS cells remain to be elucidated.
Hypoxia-inducible factor-1 (HIF-1) is a
transcription factor composed of α and β subunits. The expression
of HIF-1α is regulated by low oxygen tension, whereas HIF-1β is
expressed constitutively (6). As
intratumoral hypoxia is a common characteristic of solid tumors,
HIF-1α is overexpressed in various types of human cancer (7). The activity of HIF-1α in cells
correlates with tumor growth and angiogenesis (8); therefore, HIF-1α represents an
attractive therapeutic target for the treatment of numerous types
of cancer (9). However, its effect
in benign tumors is unclear and, to the best of our knowledge,
there are no studies regarding the association between VS and
HIF-1α.
The present study aimed to assess the effect of Tan
IIA on VS cells and evaluate the functional targets of Tan IIA.
Materials and methods
Isolation of Tan IIA
Tan IIA was isolated from extracts of the roots of
Salvia miltiorrhiza Bunge (Danshen) using methanol. Briefly,
2 kg Danshen root was extracted with 4 liters methanol for five
days. The supernatant was subsequently filtered through Whatman
Grade 4 filter paper (Sigma-Aldrich, St. Louis, MO, USA). The
filtrate was concentrated under reduced pressure and the residue
was dissolved in ethyl acetate. The ethyl acetate soluble fractions
were concentrated under a vacuum and the obtained residue (125 g)
was subjected to column chromatography with silica gel 60
(Sigma-Aldrich). The column was packed with methylene chloride and
the sample was loaded in methylene chloride. The sample was
subsequently eluted with hexane, which was followed by consecutive
elution with hexane, containing 2, 5, 8 and 10% ethyl acetate. Tan
IIA was purified by recrystallization in hexane and ethyl acetate.
The structure of the compound was determined using nuclear magnetic
resonance (1H and 13C NMR; JEOL Ltd., Tokyo,
Japan) and by comparison with authentic samples.
Cell culture
The HEI-193 cells, an immortalized VS cell line, and
Nf2-/-mouse Schwann (SC4) cells were obtained from the House
Research Institute (Los Angeles, CA, USA) and were maintained in
Dulbecco's modified Eagle's medium (DMEM), containing 10% fetal
bovine serum (FBS) and 100 U/ml penicillin/streptomycin. To prevent
changes in cell morphology, the schwannoma cells were used at
passage six. The cell number was calculated using a hemocytometer
(Marienfeld-Superior, Lauda-Königshofen, Germany). Hypoxic
conditions were induced in an incubator chamber
(Billups-Rothenberg, Inc., San Diego, CA, USA), which was flushed
with a mixture of 1% O2, 5% CO2 and 94%
N2. For normoxic incubation, the gas contained 20%
O2, 5% CO2 and 75% N2.
Cell proliferation assay
HEI-193 cells and SC4 cells were seeded into 96-well
plates at 5×103 cells per well. Following incubation for
24 h, the culture medium was replaced with medium containing Tan
IIA at 1, 3, 5, 7 or 10 µg/ml, with four wells per
concentration. Following 24 h treatment, one of the plates was
removed and 40 µl fresh
3-[4,5-Dimethylthiazol]-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) in 5 g/l phosphate-buffered saline (PBS) was added
to each well. Following incubation for 4 h at 37°C, the culture
medium was discarded and 100 µl dimethyl sulfoxide was added
to each well. The plates were agitated at room temperature for 30
min to dissolve the formazan crystals and the optical density was
measured at 595 nm using a micro-plate reader (VersaMax Microplate
Reader with SoftMax Pro Software; Molecular Devices, LLC,
Sunnyvale, CA, USA).
Annexin V/propidium iodide (PI)
staining
Apoptosis was analyzed using an Annexin V assay kit
(BD Pharmingen, San Diego, CA, USA). Briefly, the HEI-193 cells
were plated at 7×103 cells per well into six-well
plates. Following overnight attachment, the cells were treated with
2 or 4 µg/ml Tan IIA for 48 h. The floating cells in the
medium were combined with the attached cells, which had been
harvested by trypsinization. The cells were washed with cold PBS
and resuspended in 1X binding buffer, containing 10 mM HEPES/NaOH
(pH 7.4; BD Pharmingen), 140 mM NaCl and 2.5 mM CaCl2,
at a density of 1×106 cells/ml. Next, the cell
suspension was stained with Annexin V-fluorescein isothiocyanate
(FITC) and PI, provided with the kit, at room temperature for at
least 10 min in the dark. The cells were analyzed using a FACScan
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) within 1 h
of staining. The data were analyzed using WinList 5.0 software
(Verity Software House, Topsham, ME, USA).
Measurement of caspase 3 activity
The enzymatic activity of capase-3 was assessed
using a caspase colorimetric assay kit (BioVision Inc., Milpitas,
CA, USA), according to the manufacturer's instructions. Briefly,
the cells were rinsed twice with ice-cold PBS and lysed in lysis
buffer (50 mM Tris-HCl, pH 8.0; 150 MM sodium citrate; 1% NP-40;
0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate) for 10 min
on ice. The lysed cells were centrifuged at 10,000 × g for 5 min
and an equal quantity of total protein in each lysate was
quantified using a Lowry protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cell lysates were incubated with 50 µl
2X reaction buffer, containing 10 mM dithiothreitol and 200
µM DEVD-pNA substrate at 37°C for 1.5 h. The absorbance was
measured at a wavelength of 405 nm on a spectrophotometer.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
To assess the mRNA expression levels, total RNA was
isolated using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer's instructions.
Total RNAs (3 µg) were reverse-transcribed at 55°C for 30
min and the cDNAs were amplified by 35 cycles in a PCR mixture
(Qiagen Inc., Valencia, CA, USA). The oligo-nucleotide primers used
in the PCR reactions were determined from the human HIF-1α sequence
and β-actin, and were as follows: Forward,
5′-CCCCAGATTCAGGATCAGACA-3′ and reverse, 5′-CCATCATGTTCCATTTTTCG-3′
for HIF-1α, (701 bp fragment). The mRNA expression levels were
normalized against β-actin. The PCR products were electro-phoresed
on 1% agarose gels containing ethidium bromide and scanned using a
PCR system (2720 Thermal Cycler; Applied Biosystems Life
Technologies, Foster City, CA, USA).
Western blot analysis
To assess the protein expression levels, the cells
at 80–90% confluence were starved by culturing in 0.2% FBS in DMEM
for a further 20 h. The cells were stimulated with Tan IIA for 48 h
and then rinsed twice in PBS (pH 7.4). The cells were lysed on ice
for 30 min in radioim-munoprecipitation buffer [50 mM Tris (pH
7.5), 50 mM NaCl, 0.1% SDS, 1% Triton X-100 and 1% sodium
deoxycholate], centrifuged at 11,700 x g for 30 min at 4°C and the
insoluble materials were collected. The protein concentration was
determined using the Lowry protein assay (Bio-Rad Laboratories,
Inc.). The supernatant (30 µg) was separated on 6–12%
acryl-amide gels (Bio-Rad Laboratories, Inc.) and the proteins were
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked for 30 min at room
temperature in Tris-buffered saline, containing 5% non-fat milk and
0.1% Tween 20. The proteins were detected by immunoblotting using
the following specific primary antibodies: Monoclonal mouse
anti-human HIF-1α (1:500; cat. no. 610958; BD Biosciences),
polyclonal rabbit anti-human caspase-3 (1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc., Danvers, MA, USA) polyclonal
rabbit anti-human phosphorylated-AKT (1:1,000; cat. no. sc-7985R;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), polyclonal rabbit
anti-human total AKT (1:1,000; cat. no. 9272; Cell Signaling
Technology, Inc.), polyclonal rabbit anti-human
phosphorylated-extracellular signal-regulated kinases (ERKs)
(1:1,000; cat. no. 9101; Cell Signaling Technology, Inc.),
polyclonal rabbit anti-human total ERK (1:1,000; cat. no. 9102;
Cell Signaling Technology, Inc.), and monoclonal mouse anti-human
α-tubulin (1:5,000; cat. no. CP06; EMD Millipore) overnight at 4°C.
Following incubation with the primary antibodies, the membranes
were washed three times with Tris-buffered saline containing 0.1%
Tween 20, and incubated with horseradish peroxidase-conjugated goat
anti-rabbit (1:3,000; cat. no. sc-2004) and goat anti-mouse
(1:3,000; cat. no. sc-2005) secondary antibodies (Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Detection was
performed using an ECL-Plus western blotting detection system (GE
Healthcare Life Sciences, Piscataway, NJ, USA).
Luciferase reporter assay
To examine the transcription of HIF-1, the HEI-193
cells were transfected with the firefly lucif-erase reporter
plasmid, pGL 4.42 [luc2P/HRE/Hygro]. The HEI-193 cells were plated
into DMEM, containing 10% FBS, in a 12-well plate. The cells were
transiently transfected with 1 µg luciferase reporter
plasmid (Promega Corporation, Madison, WI, USA), along with the
desired combination of expression plasmids, using Lipofectamine
Plus (Invitrogen Life Technologies), according to the
manufacturer's instructions. Following incubation for 6 h, the
cells were rinsed and provided fresh 10% FBS/DMEM. Following
stabilization, the cells were treated for 24 h with Tan IIA (1 or 3
µg/ml) and were subsequently washed with PBS, lysed with
passive lysis buffer and the luciferase activity was measured. The
experiments were performed a minimum of three times. The luciferase
activity was measured using the dual-luciferase assay system
(Promega Corporation) using a luminometer (PerkinElmer, Inc.,
Waltham, MA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation from three independent experiments. Statistical analysis
by Student's t-test was performed using SPSS 13.0 software for
Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibitory effect of Tan IIA on
schwannoma cells
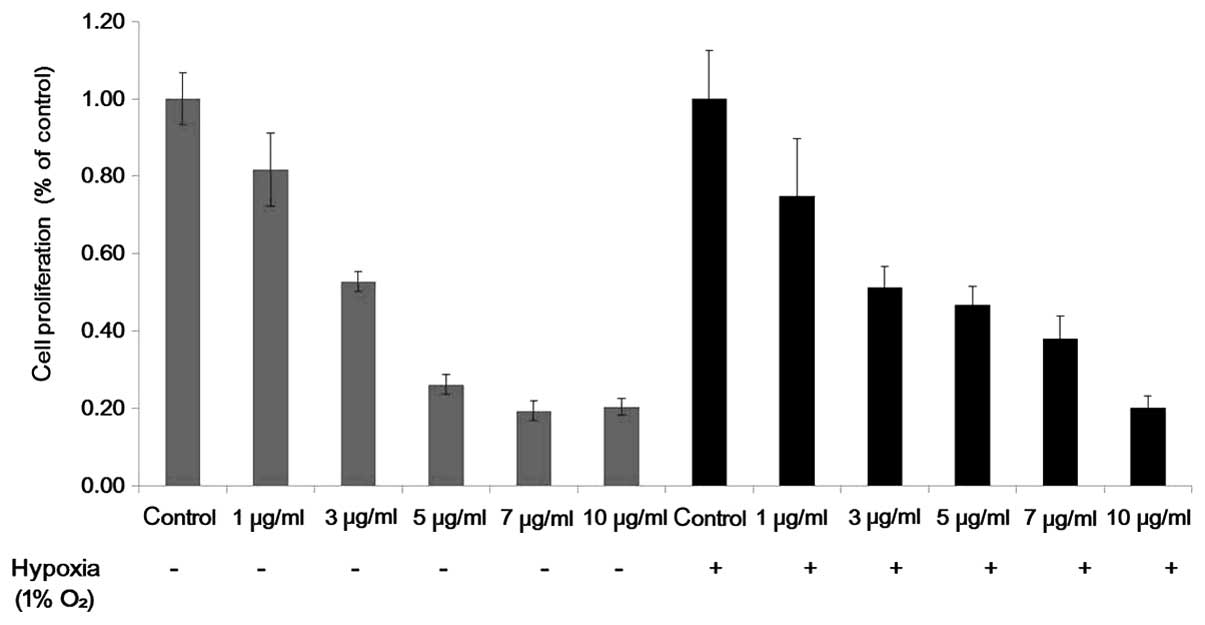
An MTT assay was used to detect the inhibitory
effect of Tan IIA on the HEI-193 and SC4 cells. Cell proliferation
of the HEI-193 and SC4 cells was inhibited as the concentration of
Tan IIA increased from 1 to 10 µg/ml under normoxic and
hypoxic conditions (Fig. 1). All
experiments were repeated a minimum of three times and revealed
similar results.
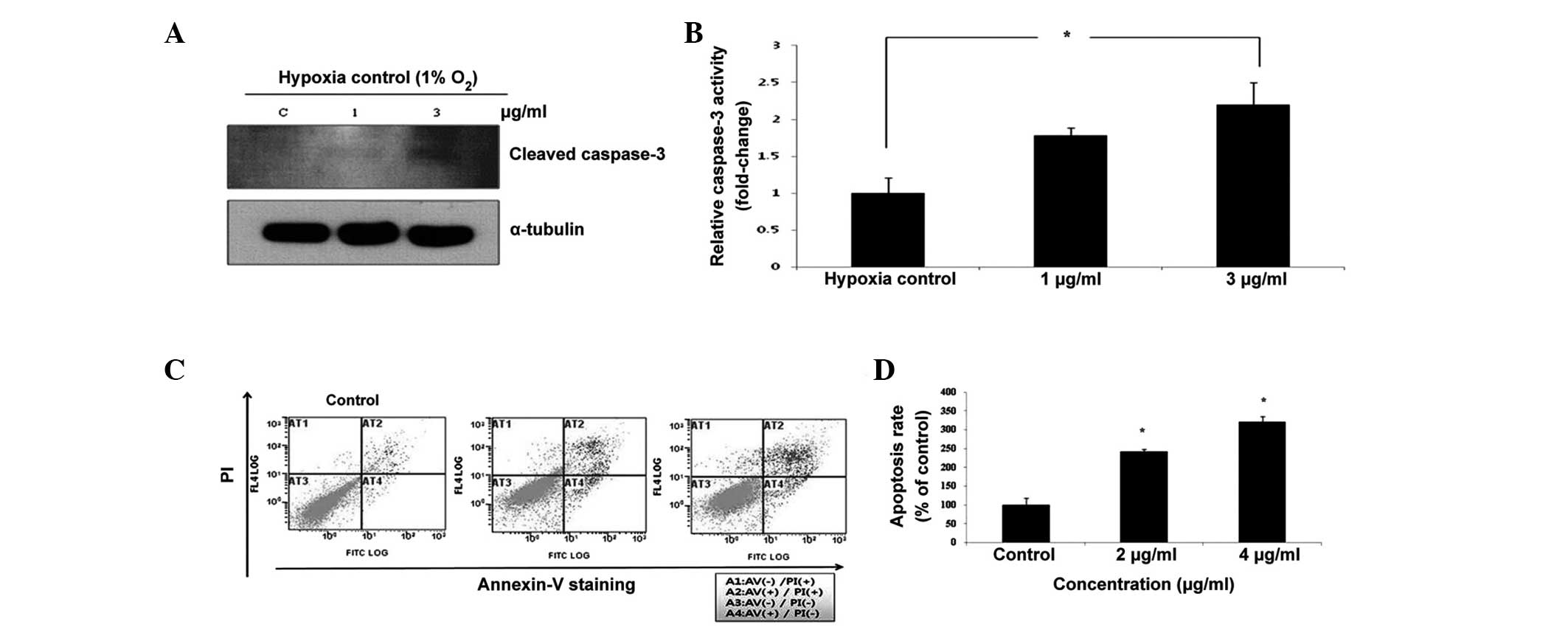
Tan IIA induces apoptosis in schwannoma
cells
To demonstrate the induction of apoptosis by Tan
IIA, western blotting and flow cytometric analysis with Annexin
V/PI staining were performed. Caspase-3 has previously been
associated with apoptotic changes in Tan IIA-induced cell death.
Tan IIA activated the cleavage of caspase-3 under hypoxic
conditions, which is a hallmark of apoptotic activation (Fig. 2A). Exposure to 1 or 3 µg/ml
Tan IIA increased the activity of caspase-3 when compared with the
untreated control (Fig. 2B).
Following flow cytometric analysis, the PI fluorescence was plotted
over the Annexin V-FITC fluorescence (Fig. 2C). Using this method, healthy cells
exhibited low levels of FITC and PI fluorescence, whereas early
apoptotic cells exhibited strong FITC fluorescence, but low PI
fluorescence. By contrast, late apoptotic cells exhibited strong
FITC and PI fluorescence, whereas dead cells exhibited low levels
of FITC fluorescence and strong PI fluorescence. The proportions
(%) of apoptotic cells (Q2 + Q4) were significantly higher in the
HEI-193 cells that were exposed to 2 and 4 µg/ml Tan IIA
(Fig. 2D).
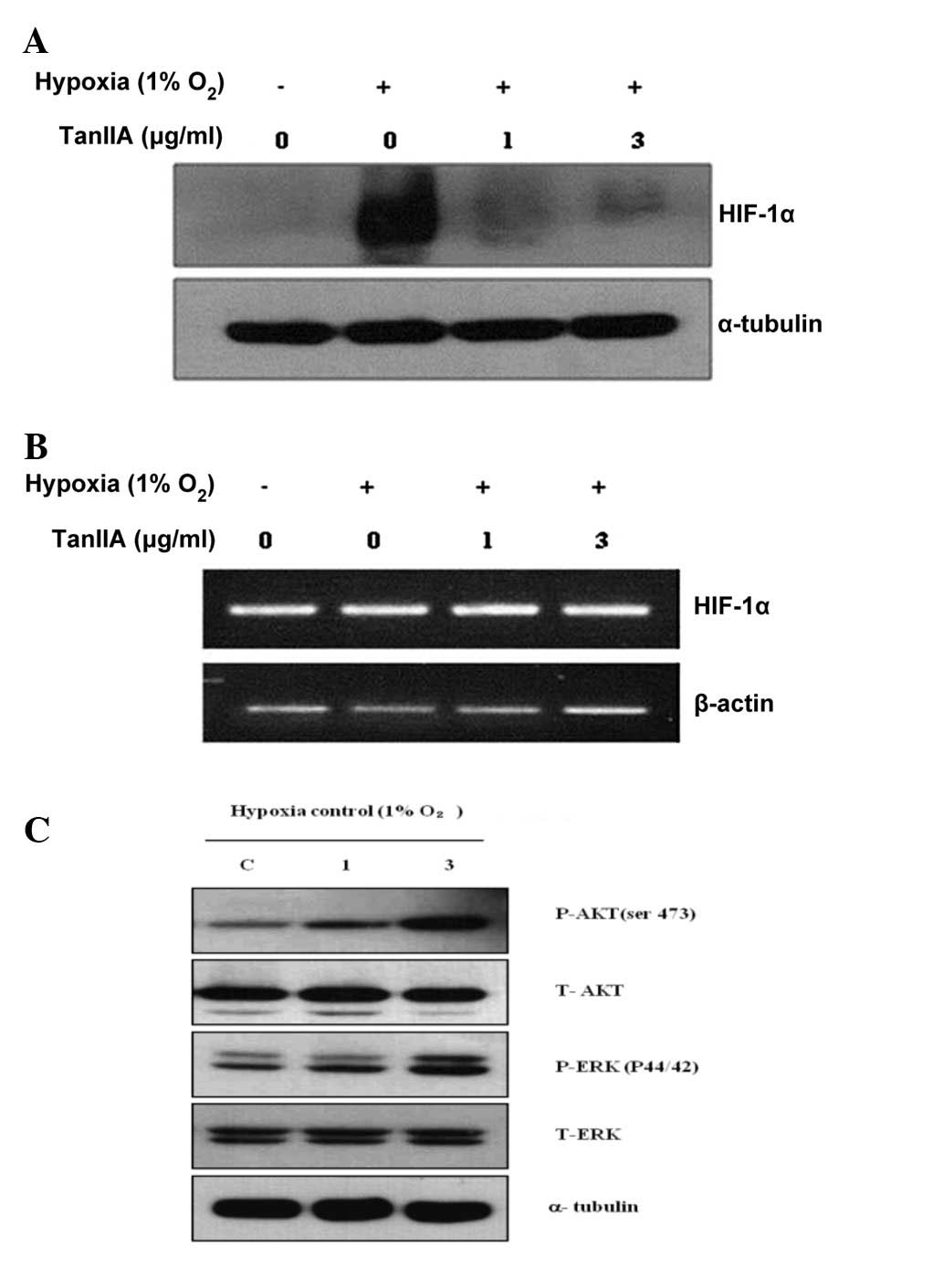
Tan IIA attenuates the protein expression
of HIF-1α in schwannoma cells under hypoxia
The effect of Tan IIA on the expression of HIF-1α in
the HEI-193 cells was assessed. The HIF-1α protein was not
expressed in the HEI-193 cells under normoxic conditions and
following a 4-h exposure to hypoxia, the expression of HIF-1α was
markedly increased in the HEI-193 cells. Tan IIA decreased the
hypoxia-induced protein expression of HIF-1α in the HEI-193 cells
(Fig. 3A). However, Tan IIA
treatment did not alter the mRNA expression of HIF-1α in the
HEI-193 cells under normoxic and hypoxic conditions (Fig. 3B). AKT and ERK (upstream modulators
of HIF-1α) signaling was not altered by treatment with Tan IIA
(Fig. 3C).
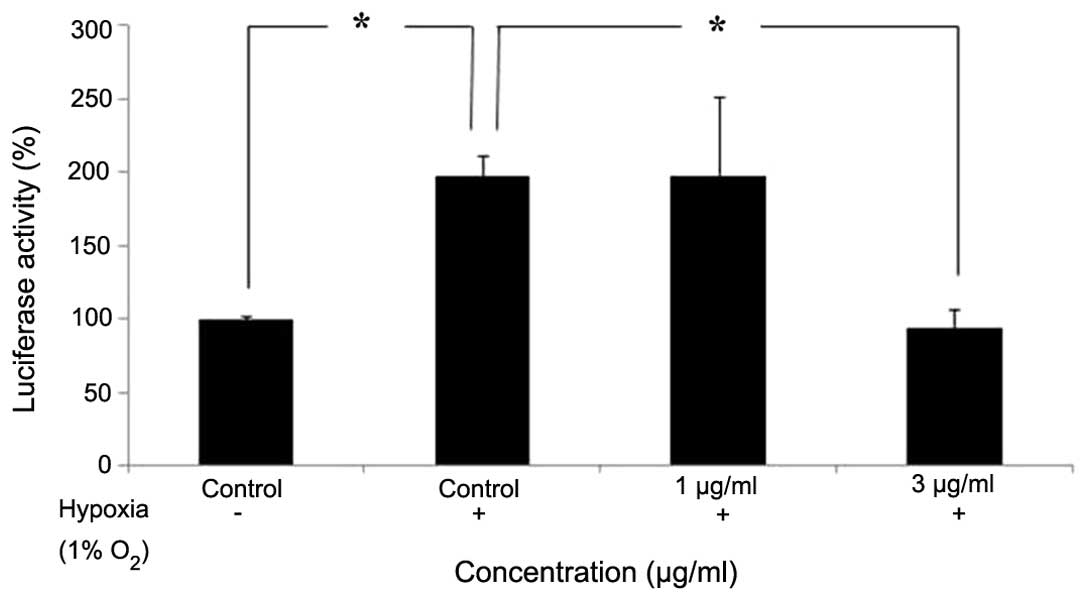
Tan IIA attenuates HIF-1α promoter
activity in schwannoma cells under hypoxia
The HEI-193 cells were treated with 1 or 3
µg/ml Tan IIA under hypoxic conditions for 4 h. The hypoxia
response element (HRE) promoter activity in HEI-193 cells following
a 24-h incubation period was assessed using a luciferase reporter
assay with the luc2P/HRE/Hygro construct. The expression of
HRE-regulated luciferase reporter gene was reduced following
treatment with Tan IIA, suggesting that Tan IIA was able to inhibit
the translational activity fo HIF-1α (Fig. 4).
Discussion
Although there is currently no approved medical
therapeutic strategy for VS, various chemotherapeutic agents have
been developed based on the underlying biology of schwannomas.
Various previous studies have attempted to inhibit the growth of
schwannomas by suppressing various receptor tyrosine kinases,
including ErbB family receptors, vascular endothelial growth
factors and their downstream mediators, including the MAPK/ERK and
PI3K/AKT signaling pathways (10).
However, the emerging medications for treating schwannomas are the
chemotherapeutic agents that are administered for treating cancer,
and the cost and toxicity associated with these agents for treating
a VS benign tumor complicates their clinical use.
Numerous natural products appear to protect against
cancer by interfering with multiple cell signaling pathways
(11). Danshen, a herbal drug
derived from the dried root or rhizome of Salvia
miltiorrhiza Bunge, has been administered clinically in China
and certain other Asian countries to prevent or manage
cardiovascular disease (2). Tan
IIA is a lipid-soluble phenolic compound and the most abundant
component of Danshen. Previous studies have revealed that Tan IIA
inhibits the growth and induces the apoptosis of various human
cancer cell models, including breast cancer (12), leukemia cells (13), human lung cancer cells (14), colon cancer cells (15) and hepatocellular carcinoma cells
(16). The present study
demonstrates for the first time, to the best of our knowledge, that
Tan IIA inhibits the growth of VS cells. Using flow cytometry and
caspase-3 activity to detect apoptosis, it was revealed that Tan
IIA induces apoptosis in HEI-193 cells.
As the master regulator of the hypoxic
transcriptional response, HIF-1α is central in tumor growth and
angiogenesis (17). HIF-1α is
overexpressed in various types of human cancer, including brain,
breast, colon, lung, ovary and prostate (18). Although little is known about the
impact of HIF-1α in benign tumors, the present study revealed that
HIF-1α is expressed in VS cells under hypoxic conditions.
Therapeutic agents that target HIF-1a have the potential to target
multiple cancer processes, and numerous chemical inhibitors of
HIF-1α have been developed. In addition, certain natural products
inhibit the activity of HIF-1α in various types of cancer cell
(19). Although it was reported
that Tan IIA reduced lipopolysaccharide-induced lung injury by
inhibiting the HIF-1α signaling pathway (20), the molecular mechanism of its
antitumor activity under hypoxia remains to be elucidated. The
present study demonstrates that Tan IIA decreases the
hypoxia-induced expression of HIF-1α in HEI-193 cells.
The protein expression of HIF-1α is tightly
regulated via protein degradation and synthesis. The present study
revealed that Tan IIA does not alter the mRNA expression of HIF-1α
in HEI-193 cells under hypoxic conditions. The results indicated
that Tan IIA inhibits the expression of HIF-1α at the translational
level. It was also demonstrated that Tan IIA inhibits the activity
of HIF-1α under hypoxic conditions, as assessed using a luciferase
reporter assay. However, the potential mechanisms, including
protein degradation and DNA binding activity are currently being
investigated.
Previous studies have reported that various
signaling pathways regulate HIF-1α, including the PI3K/AKT/mTOR and
MAPK signaling pathways (21–23).
Certain compounds inhibit the expression of HIF-1α via the
PI3K/AKT/mTOR signaling pathways. In addition, ERK and c-Jun
N-terminal kinases signaling are involved in the regulation of
HIF-1α. However, the present study demonstrated that Tan IIA
exerted a marginal effect on inhibiting these signaling
pathways.
Although the present study is limited by its in
vitro nature, further investigation, including clinical trials
of human schwan-noma, may be considered since Tan IIA has been
administered safely for the treatment of various human diseases,
such as chronic renal failure and coronary heart disease.
In conclusion, the present study provides evidence
that HIF-1α is expressed in hypoxic VS cells and that Tan IIA
inhibits VS cells by suppressing the activity of HIF-1α. These
findings indicated that Tan IIA may be considered as a
chemotherapeutic agent for the treatment of VS.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant
no. 2013R1A1A2010381) and the Soonchunhyang University Research
Fund. The authors would like to thank Dr M. Giovannini (UCLA, Los
Angeles, CA, USA) for providing the HEI-193 and SC4 cells.
References
|
1
|
Theodosopoulos PV and Pensak ML:
Contemporary management of acoustic neuromas. Laryngoscope.
121:1133–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations attenuate
aminogly-coside-induced free radical formation in vitro and
ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong Y, Morris-Natschke SL and Lee KH:
Biosynthesis, total syntheses, and antitumor activity of
tanshinones and their analogs as potential therapeutic agents. Nat
Prod Rep. 28:529–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
7
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
8
|
Maxwell PH, Dachs GU, Gleadle JM, Nicholls
LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW and Ratcliffe PJ:
Hypoxia-inducible factor-1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poon E, Harris AL and Ashcroft M:
Targeting the hypoxia-inducible factor (HIF) pathway in cancer.
Expert Rev Mol Med. 11:e262009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sughrue ME, Yeung AH, Rutkowski MJ, Cheung
SW and Parsa AT: Molecular biology of familial and sporadic
vestibular schwannomas: Implications for novel therapeutics. J
Neurosurg. 114:359–366. 2011. View Article : Google Scholar
|
|
11
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang
J and Wang W: Potential anticancer activity of tanshinone IIA
against human breast cancer. Int J Cancer. 116:799–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu JJ, Lin DJ, Liu PQ, Huang M, Li XD and
Huang RW: Induction of apoptosis and inhibition of cell adhesive
and invasive effects by tanshinone IIA in acute promyelocytic
leukemia cells in vitro. J Biomed Sci. 13:813–823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
15
|
Su CC, Chen GW and Lin JG: Growth
inhibition and apoptosis induction by tanshinone I in human colon
cancer Colo 205 cells. Int J Mol Med. 22:613–618. 2008.PubMed/NCBI
|
|
16
|
Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF
and Zhang J: Growth inhibition and apoptosis induction of
tanshinone II-A on human hepatocellular carcinoma cells. World J
Gastroenterol. 10:2024–2028. 2004.PubMed/NCBI
|
|
17
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8(4 Suppl):
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagle DG and Zhou YD: Natural
product-based inhibitors of hypoxia-inducible factor 1 (HIF-1).
Curr Drug Targets. 7:355–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu M, Cao F, Liu L, Zhang B, Wang Y, Dong
H, Cui Y, Dong M, Xu D, Liu Y, et al: Tanshinone IIA-induced
attenuation of lung injury in endotoxemic mice is associated with
reduction of hypoxia-inducible factor 1vα expression. Am J Respir
Cell Mol Biol. 45:1028–1035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minet E, Michel G, Mottet D, Raes M and
Michiels C: Transduction pathways involved in Hypoxia-Inducible
Factor-1 phosphorylation and activation. Free Radic Biol Med.
31:847–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia Y, Choi HK and Lee K: Recent advances
in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem.
49:24–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melillo G: Targeting hypoxia cell
signaling for cancer therapy. Cancer metastasis Rev. 26:341–352.
2007. View Article : Google Scholar : PubMed/NCBI
|