Introduction
The skin is largest organ of the human body and is
responsible for protecting organisms against external physical,
chemical and biological insults, including ultraviolet (UV)
radiation and microorganisms. Chronic exposure of the skin to solar
UV radiation is a predominant factor, which contributes to the
development of various skin diseases, although a number of other
environmental and genetic factors are also involved. Overexposure
of the skin to UV radiation causes a number of biological
conditions, including sunburn, hyperpigmentation, solar keratosis,
solar elastosis, skin cancer, immunosuppression and an acute
inflammatory response (1,2). UVB (290–320 nm) radiation induces the
apoptotic cell death of keratinocytes, which manifest within the
epidermis as sunburn cells. The formation of sunburn cells in
UVB-exposed skin reflects the severity of the damage to the DNA.
Absorption of UV produces two predominant types of DNA damage:
Formation of cis-syn cyclobutane pyrimidine dimers (CPD) and
pyrimidone photoproducts. However, the repair of DNA damage in
UVB-exposed skin cells prevents the accumulation of damaged cells
(3). UV-induced DNA damage is also
an important molecular trigger for UV-induced inflammation and
various forms of skin cancer (4).
Sunscreens are typically used to prevent or
ameliorate the harmful effects of UV radiation on the skin
(5). However, sufficient
protection against skin photodamage may not be afforded by
sunscreen alone (6). As a
consequence, non-sunscreen compounds are of interest for a large
proportion of the population in preventative skin care (7). Active compounds, which exert
protective mechanisms against skin damage, or which inhibit
pathological processes in photo-damaged skin, are currently under
investigation. Several plant extracts have been reported to protect
the skin against various UV-induced damage models (8) and there has been considerable
interest in applying plant polyphenols to the skin to prevent
UV-induced skin photodamage (9).
In the present study, 80% ethanol extracts from
>50 plants were screened for inhibitors of UVB-induced
cytotoxicity, using cultured normal human epidermal keratinocytes
(NHEK). Among the plant extracts investigated, extract from the
fruit of rose myrtle (Rhodomyrtus tomentosa) was identified
as the most marked inhibitor of cell death. Rose myrtle, which is a
shrub of the Myrtaceae family and originates from Southeast Asia,
grows under different conditions and is an invasive species in
areas where it was introduced as an ornamental plant. The leaves,
roots, buds and fruits of this plant have long been used in
traditional Vietnamese, Chinese and Malay medicine. In particular,
the fruits have been used to treat diarrhea and dysentery, and to
boost the immune system (10).
Rose myrtle fruit has an astringent taste and exhibits a deep
purple color at maturity. All these properties may, at least in
part, be explained by the presence of polyphenols.
In the present study, the polyphenolic inhibitors
from rose myrtle fruit were isolated, and whether the extract and
its isolated compounds inhibited UVB-induced cell damage and
suppressed the inflammatory mediator prostaglandin E2
(PGE2) was investigated in NHEK. The photoprotective
potential of polyphenols from rose myrtle fruit extract was
subsequently assessed.
Materials and methods
Materials
The fruits of rose myrtle were obtained from Mae Chu
Co., Ltd. (Nara, Japan) and Shinwa Bussan Co., Ltd. (Osaka, Japan).
The NHEK and serum-free keratinocyte growth medium (KGM; trade
names, EpiLife-KG2 and HuMedia-KG2), containing insulin,
hydrocortisone, gentamycin/amphotericin B and growth additives,
including bovine pituitary extract and human epidermal growth
factor, were purchased from Kurabo Industries, Ltd. (Osaka, Japan).
A chemically synthesized DNA template, poly(dA), and a customized
oligo(dT)18 DNA primer were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The radioactive nucleotide,
3H-labeled 2′-deoxythymidine-5′-triphosphate (dTTP; 43
Ci/mmol) was obtained from Moravek Biochemicals, Inc. (Brea, CA,
USA). All other reagents were of analytical grade and were
purchased from Nacalai Tesque, Inc. (Kyoto, Japan).
Cell culture
The NHEK were seeded at a density of
3×105 cells/cm2 into 75 cm2 cell
culture flasks, and were cultured in KGM at 37°C under an
atmosphere of 5% CO2. The test compounds were dissolved
in dimethyl sulfoxide (DMSO) and were diluted with medium to the
appropriate concentrations, prior to adjusting the final volume to
0.05% (v/v) DMSO.
Measurement of cell viability
The NHEK were grown to subconfluence in KGM in
48-well plates (2×104 cells/0.2 ml). The cultures were
washed with Hank's buffer, irradiated with UVB (50
mJ/cm2), and were subsequently treated with the test
compound for 24 h in KGM. Following treatment, the cell viability
was determined using a
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, according to the manufacturer's instructions (11). Absorbance was measured at λ 570 nm
using the µQuant plate reader (BioTek Instruments, Inc.
Winooski, Vermont, USA), and simultaneously, the absorbance at λ
650 nm was measured as turbidity. The difference between these
measurements was regarded as the amount of blue formazan
produced.
Measurement of CPD production
The NHEK were grown to subconfluence using KGM in 60
mm2 culture dishes (2×105 cells/2 ml), and
were treated with the test compound for 24 h in KGM. The cultures
were subsequently washed with Hank's buffer, irradiated with UVB
(80 mJ/cm2), and were treated with the test compound for
6 h in KGM. The cultured cells were collected using a cell scraper
following treatment, and the nuclear DNA was purified using a
QIAamp Blood kit (Qiagen, Tokyo, Japan), according to the
manufacturer's instructions. The CPD levels in the quantified DNA
were measured by an enzyme-linked immunosorbent assay (ELISA) using
a mouse anti-CPD monoclonal antibody (1:1,000; cat. no. NMDND001;
Cosmo Bio Co., Ltd., Tokyo, Japan), according to the manufacturer's
instructions.
Measurement of DNA polymerase (pol)
activity
The NHEK were grown to subconfluence using KGM in 60
mm2 culture dishes (7.5×105 cells/5 ml), and
treated with the test compound for 24 h. The cultures were
subsequently washed with Hank's buffer, irradiated with UVB (100
mJ/cm2) and cultured for 8 h in KGM. Following
treatment, the cultured cells were collected using a cell scraper
and were sonicated for 10 sec in lysis buffer, containing 50 mM
Tris-HCl (pH 7.5), 1 mM EDTA, 5 mM 2-mercaptoethanol, 15% glycerol
and cOmplete mini-protease protease inhibitor cocktail (Roche
Diagnostics, Mannheim, Germany), using a sonicator (model, UR-20P;
TOMY SEIKO CO., LTD., Tokyo, Japan; sonication level, low). The
cell-extract in vitro pol activity was quantified and
analyzed, as described previously (12,13),
with minor modifications.
For pol reactions, poly(dA)/oligo(dT)18
and [3H]-dTTP were used as the DNA template-primer
substrate and nucleotide (2′-deoxynucleotide-5′-triphosphate)
substrate, respectively. The standard reaction mixture for all pol
species contained 50 mM Tris-HCl (pH 7.5), 1 mM dithiothreitol, 1
mM MgCl2, 5 µM poly(dA)/oligo(dT)18
(A/T, 4:1), 10 µM [3H]-dTTP (100 cpm/pmol), 15%
(v/v) glycerol and the prepared cell extract. The standard reaction
mixture for the DNA-repair-associated pol species was identical,
with the exception that it also contained 120 mM KCl. Following
incubation at 37°C for 60 min, the radioactive DNA product was
collected on a DEAE-cellulose paper disc (DE81), as previously
described (14), and the
radioactivity was measured in a scintillation counter (2300TR
TriCarb; PerkinElmer, Downers Grove, IL, USA).
Measurement of PGE2
production
The NHEK were grown to subconfluence using KGM in
48-well plates (2.5×104 cells/0.2 ml). The cells were
cultured in KGM without hydrocortisone for 1 day, irradiated in
Hank's buffer with UVB (50 mJ/cm2), and treated with the
test compound for 24 h in KGM without hydrocortisone. Following
treatment, the culture medium was collected. The level of
PGE2 in the medium was analyzed using PGE2
EIA kits (Cayman Chemical Co., Ann Arbor, MI, USA), according to
the manufacturer's instructions. This assay is based on the
competition between PGE2 and a
PGE2-acetylcholinesterase conjugate (PGE2
Tracer) for a limited amount of PGE2 monoclonal
antibody.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean for at least three independent determinations for each
experiment. The statistical significance between each experimental
group was analyzed using Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Isolation of cell death inhibitors
against UVB-irradiated NHEK from rose myrtle fruit
Screening of UVB-damaged NHEK cytotoxic inhibitors
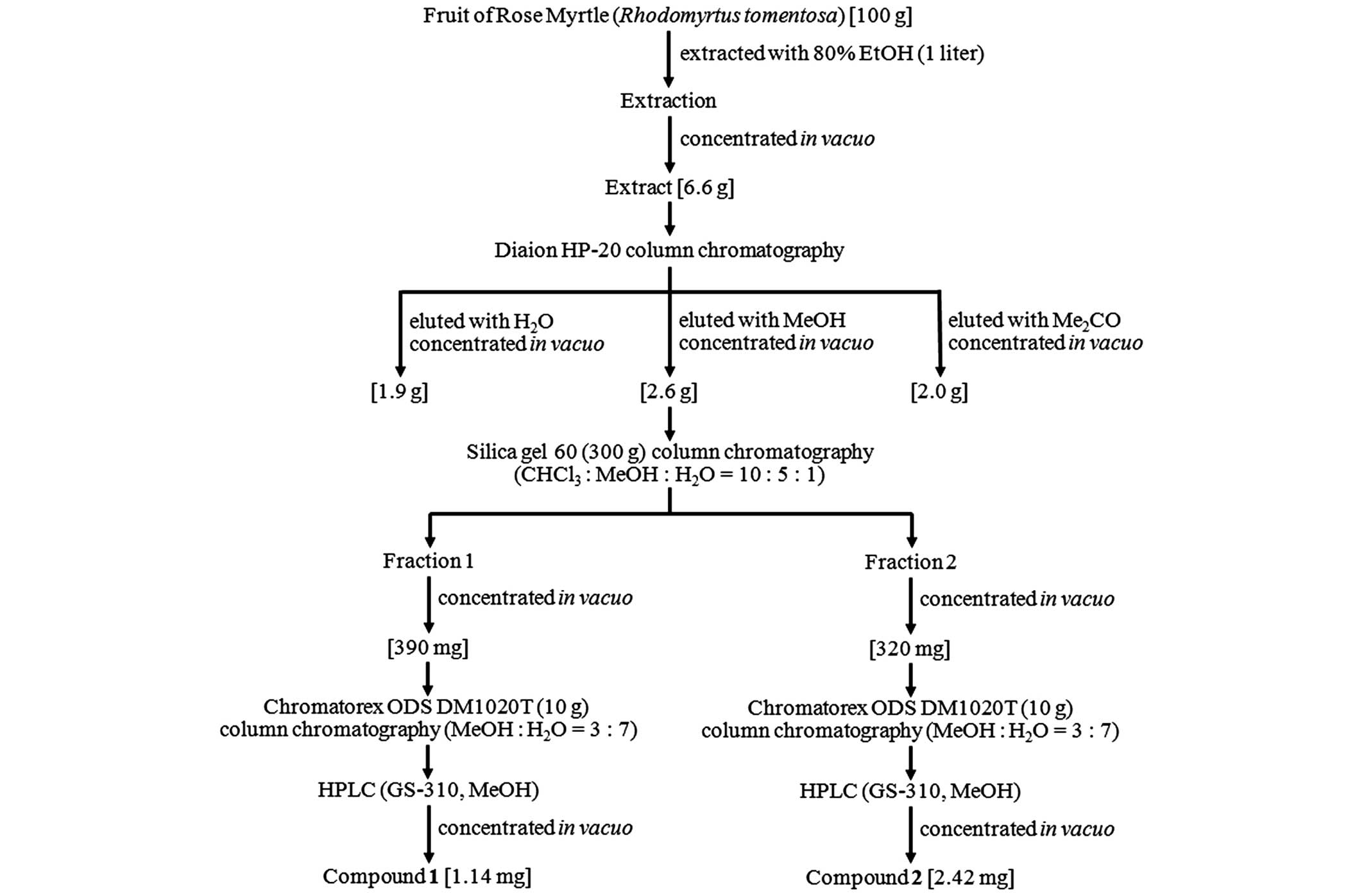
extracted from >50 plants, using 80% ethanol, demonstrated that
the extract from the fruit of rose myrtle exhibited the most marked
protective activity on UVB-induced cytotoxicity. For further
experiments, 100 g rose myrtle fruit was extracted with 1 litre 80%
ethanol. The evaporated extract (6.6 g) was dissolved in distilled
water and subjected to hydrophobic column chromatography (Diaion
HP-20; Sigma-Aldrich; Fig. 1). A
total of three hydrophobic chromatography fractions were collected:
Water, methanol and acetone. The methanol fraction was evaporated
(2.6 g) and subjected to silica gel 60 column chromatography, and
subsequently eluted with chloroform:methanol:water (v:v:v, 10:5:1).
A total of two active fractions were obtained and independently
purified by reverse-phase silica gel column chromatography
(Chromatorex ODS DM1020T; Fuji Silysia Chemical, Ltd., Durham, NC,
USA) and continuous high-performance liquid chromatography. This
process resulted in two white, powdery compounds, 1 and 2 (1.14 and
2.42 mg, respectively; Fig.
1).
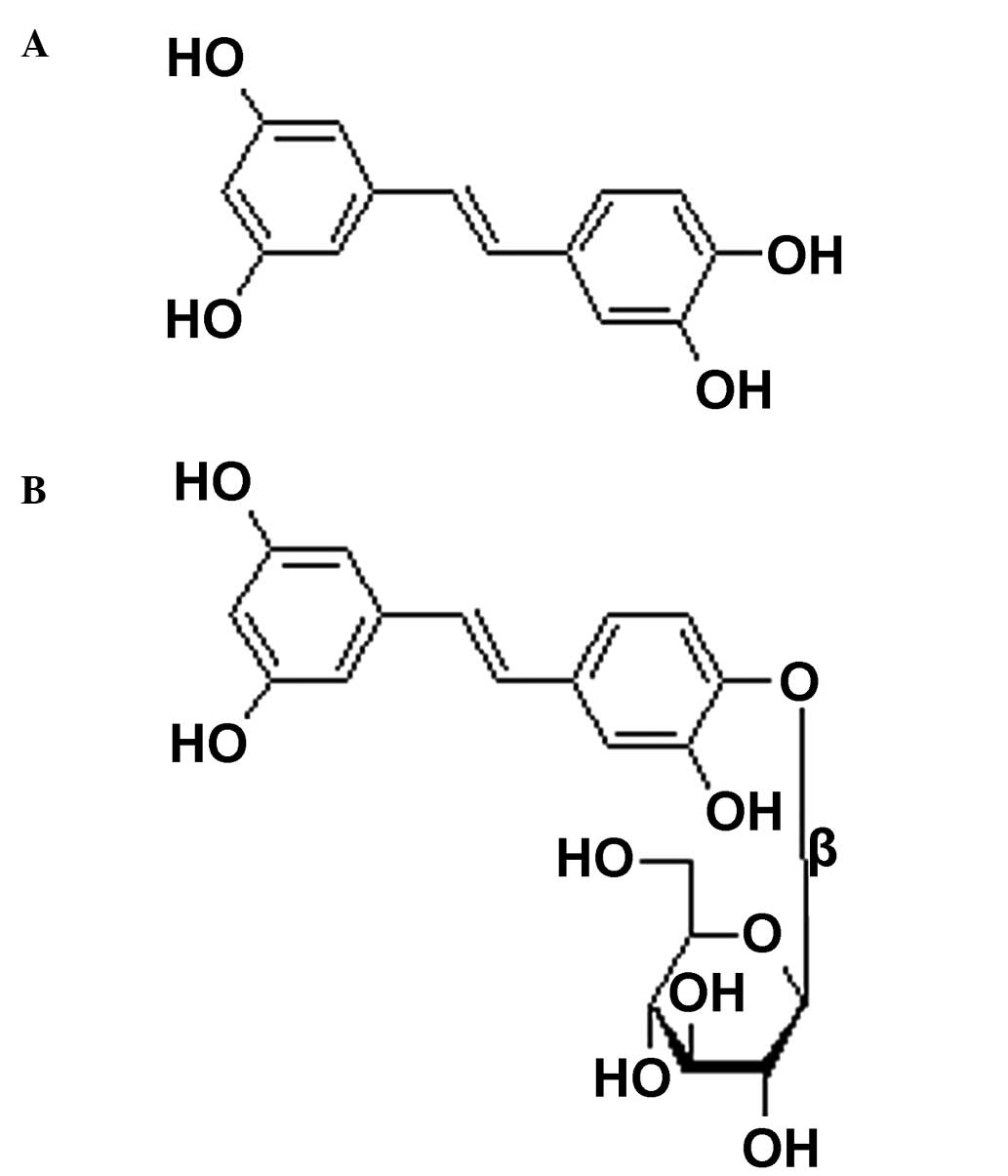
Compounds 1 and 2 were identified as a polyphenol,
piceatannol, and its glucoside, piceatannol-4′-O-β-D-glucop
yranoside, respectively, from the high-resolution mass
spectrometric data and the 1H and 13C nuclear
magnetic resonance (NMR) spectral data (their structures are
demonstrated in Fig. 2). These
spectroscopic data were consistent with a previous report (15). Subsequently, the rose myrtle fruit
extract and these two compounds, purified to >98% as determined
by NMR analysis (data not shown), were used for further
investigation.
Effect of the rose myrtle fruit extract,
piceatannol and pice atannol-4′-O-β-D-glucopyranoside on cell
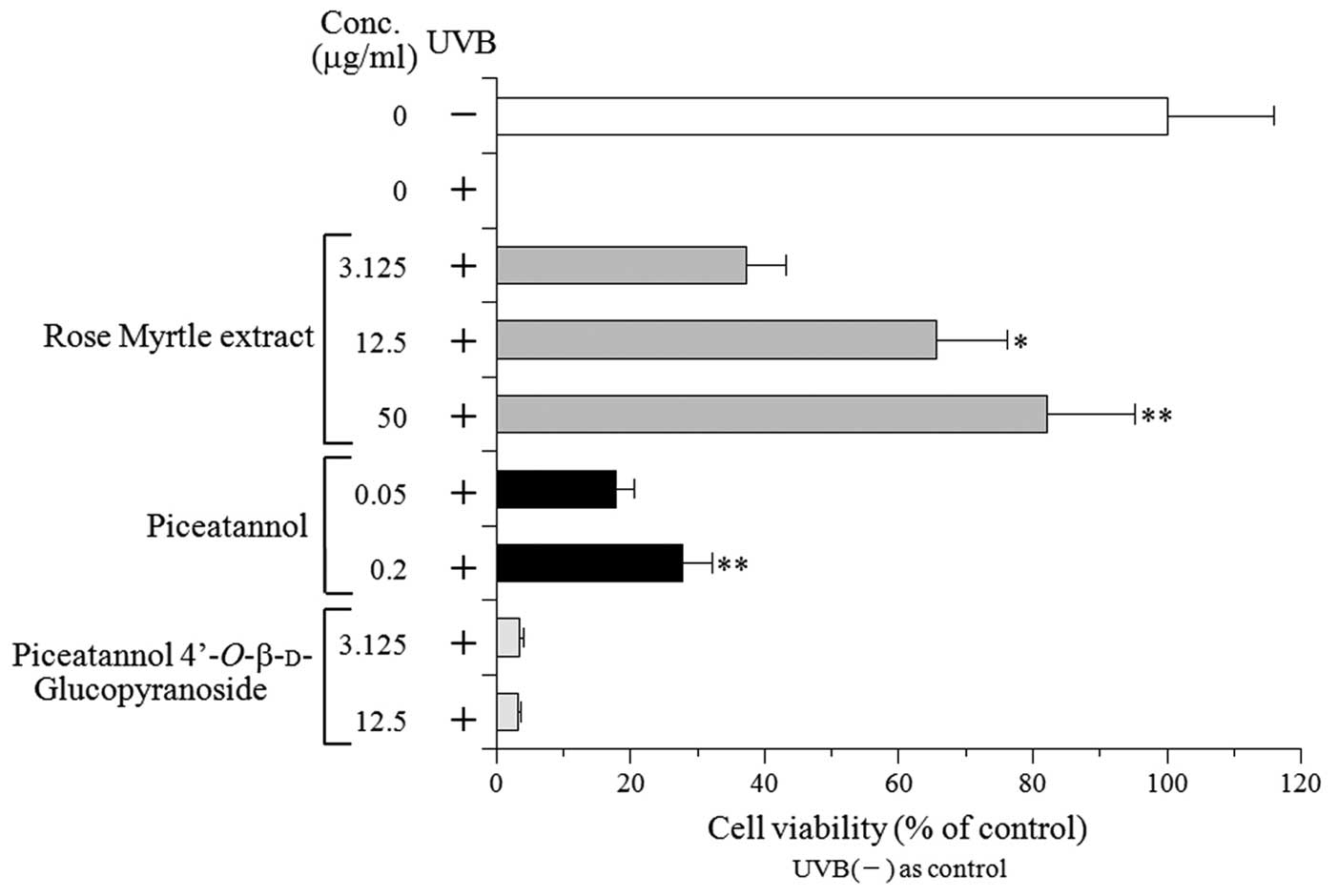
viability in UVB-exposed NHEK
The treatment of cultured NHEK with rose myrtle
fruit extract and its two isolated compounds at concentrations up
to 100 and 20 µg/ml, respectively, failed to induce any
cytotoxic effects (the cell viability was >95% following 24 h
treatment; data not shown). Subsequently, the following experiments
were performed up to the concentration limits mentioned above.
The NHEK were treated following UVB irradiation at a
dose of 50 mJ/cm2. The cell viability was analyzed 24 h
post-irradiation and was compared with non-treated cells. The
extract markedly inhibited UVB-induced NHEK cytotoxicity in a
dose-dependent manner. The cell viability determined using a
concentration of 50 mg/ml extract was improved by >80% compared
with the non-treated cells. Of the two rose myrtle fruit extract
components, piceatannol increased the cell viability of the
UVB-exposed NHEK in a dose-dependent manner; however,
piceatannol-4′-O-β-D-glucopyranoside demonstrated no
protective effect. These results suggested that piceatannol, which
is the aglycone of piceatannol-4′-O-β-D-glucopyranoside, is the
protective component of rose myrtle extract, and that the aglycone
structure is important for this protective activity.
Since rose myrtle fruit extract and piceatannol
protected against UVB-induced cell death, the subsequent
experiments focused on these two compounds.
Effect of rose myrtle fruit extract and
piceatannol on CPD production in UVB-exposed NHEK
CPD formation is one of the most important
characteristics of DNA damage and mutagenesis (16). The subsequent experiments,
therefore, investigated whether rose myrtle extract and its
polyphenolic component, piceatannol, may influence the removal of
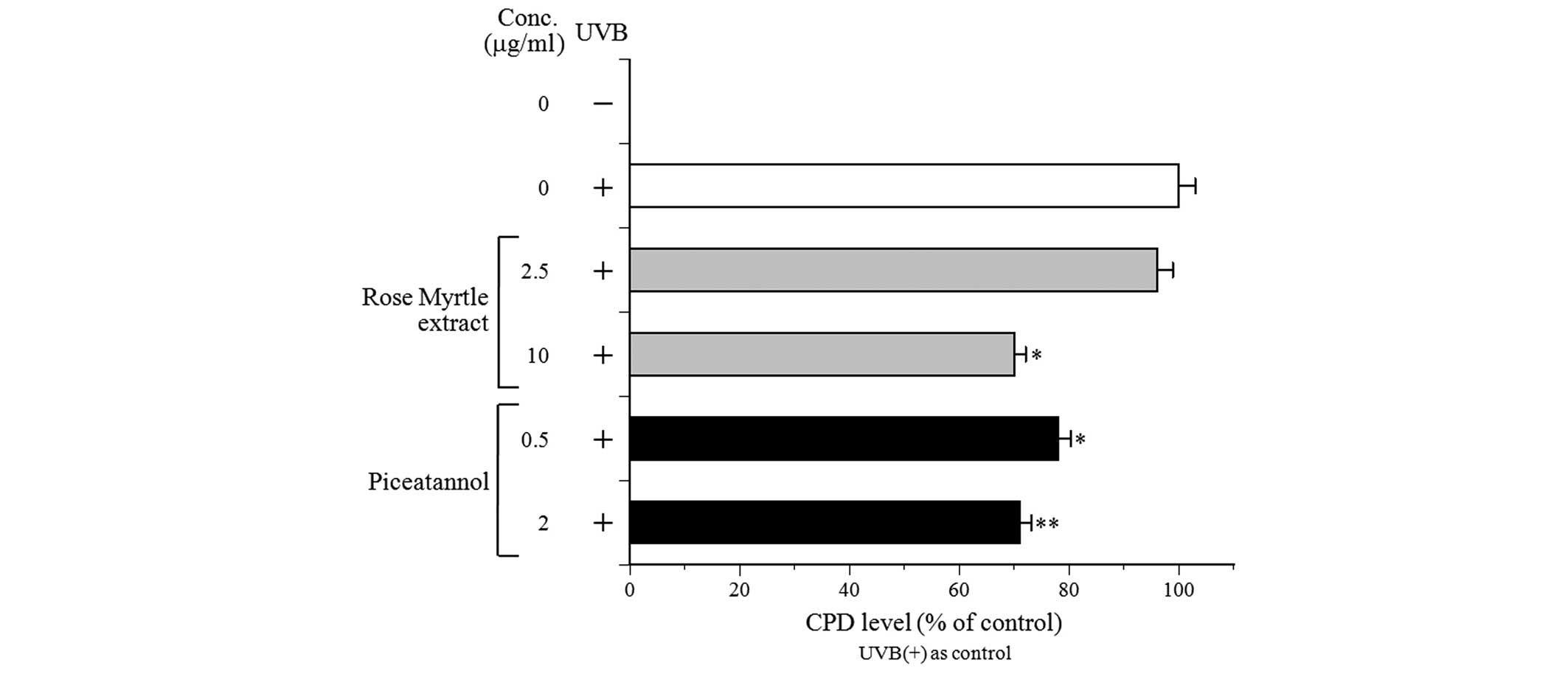
CPD from DNA in UVB-irradiated NHEK. Exposure of NHEK to 80
mJ/cm2 UVB induced CPD formation, as measured
immediately following irradiation, and served as a reference for
DNA damage (Fig. 4). To assess DNA
repair in the irradiated cultures, CPD levels were determined
following UVB exposure and compared with the non-repaired control
cells. Both rose myrtle extract and piceatannol decreased CPD
production in UVB-exposed NHEK in a dose-dependent manner, with 10
µg/ml extract and 0.5 and 2 µg/ml piceatannol
exhibiting a 20% reduction in CPD compared with the non-treated
control cells. These results suggested that rose myrtle extract
and/or piceatannol may stimulate DNA repair activity against
UVB-damaged DNA in NHEK. Consequently, the present study
investigated NHEK cellular pol activity with or without UVB
irradiation.
Effect of rose myrtle fruit extract and
piceatannol on pol activity in UVB-exposed NHEK
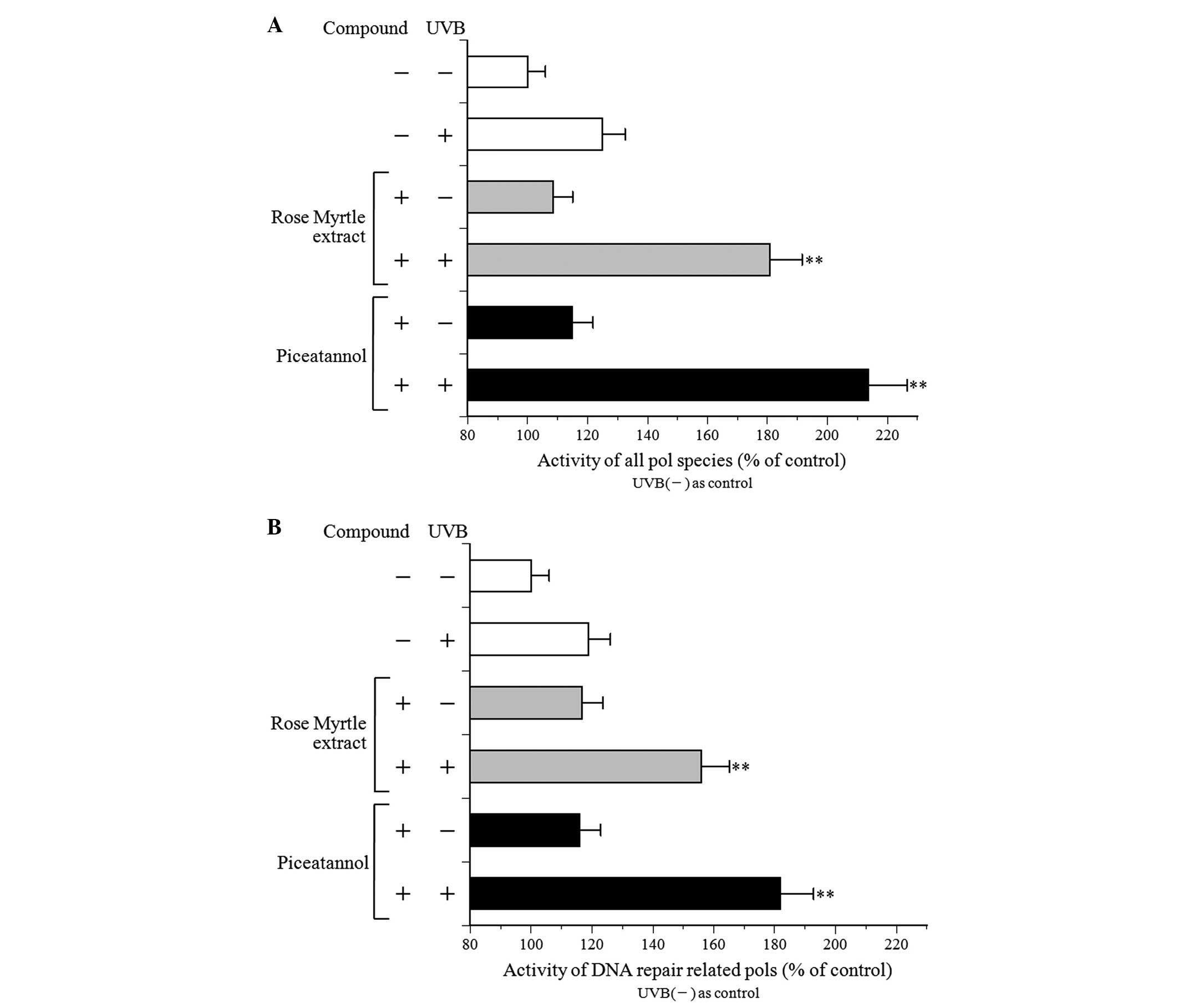
Eukaryotic cells are reported to contain 15 pol
species, which belong to four families: Family A (pols γ, θ, and
ν), family B (pols α, δ, ε, and ζ), family X [pols β, λ, µ
and terminal deoxynucleotidyl transferase (TdT)] and family Y (pols
η, ι, κ and REV1) (17,18). All pol species are active in buffer
with salts, including NaCl and KCl, however B-family pols are
inhibited by salt (19).
Therefore, a standard reaction mixture with or without 120 mM KCl
was used to detect the activity of all pol species (Fig. 5A) or DNA repair-associated pol
species (Fig. 5B). The activity of
the DNA repair-associated pol species, including the X- and
Y-family of pols, was enhanced by salt (120 mM KCl) addition
(19). The activities of the
purified calf pol α and rat pol β, which are B- and X-family pols,
respectively, with 120 mM KCl were 1.5-fold higher and 0.1-fold
lower, respectively, compared with those without KCl (data not
shown). The cellular pol activity of the standard reaction mixture
without salt in NHEK was higher compared with that of the standard
reaction mixture with salt (Fig.
5).
NHEK pol activity with or without UVB irradiation
and treatment with the test compound was similar (Fig. 5). In non-treated compounds, UVB
exposure at 100 mJ/cm2 increased pol enzyme activity by
~1.2-fold. In the non-UVB-irradiated NHEK, the extract and
piceatannol marginally increased the NHEK pol activity. In
addition, the pol activities were increased markedly in the
UVB-exposed NHEK. These results indicated a synergistic effect of
UVB irradiation and rose myrtle extract and/or piceatannol on the
induction of pol enzyme activity, particularly DNA
repair-associated pols, suggesting that activation of these enzymes
reduces CPD production.
Effect of rose myrtle fruit extract and
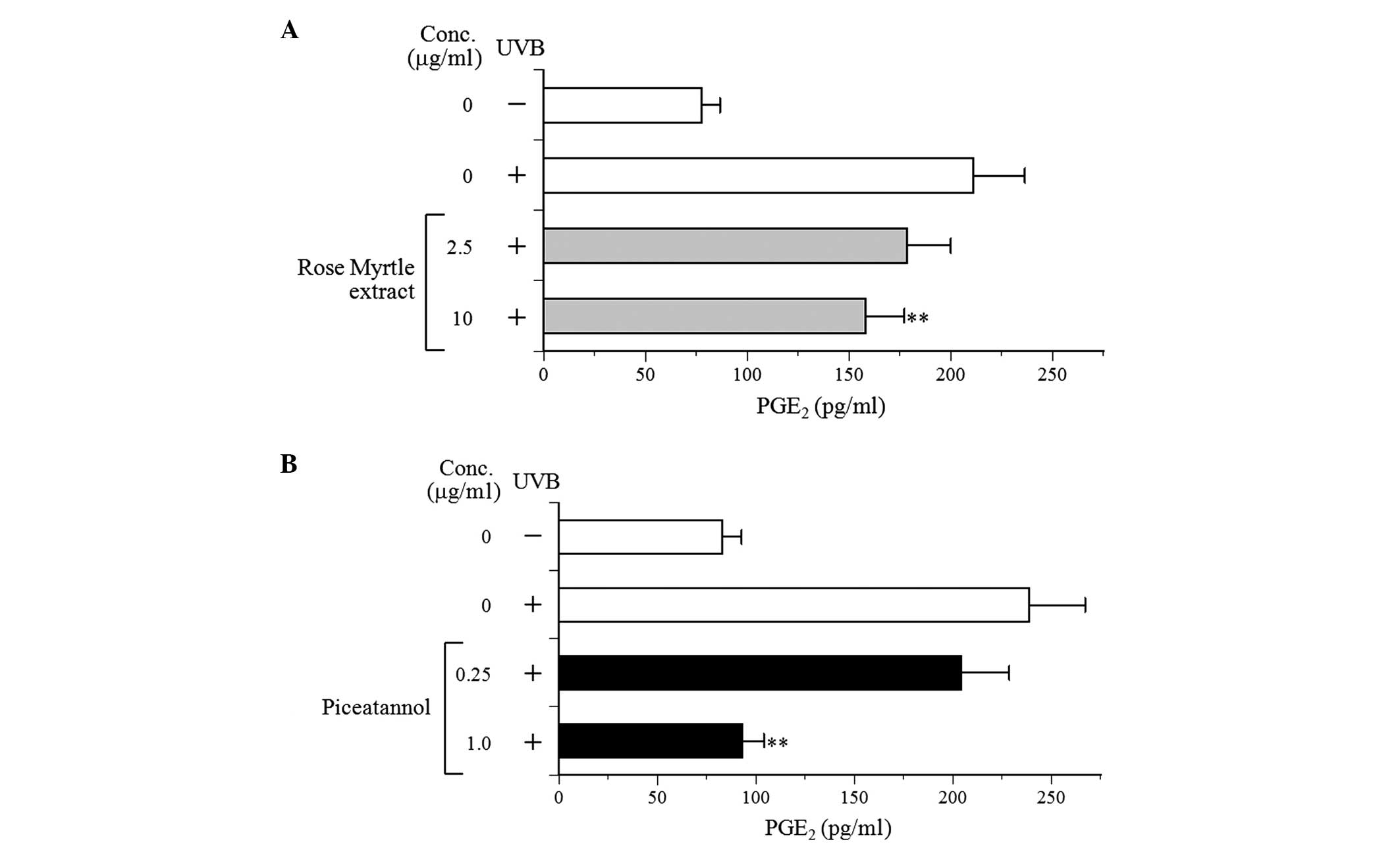
piceatannol on PGE2 production in UVB-exposed NHEK
The potential effects of the extract and piceatannol
on PGE2 production were subsequently investigated to
determine whether they were associated with the anti-inflammatory
properties in NHEK. UVB irradiation increased PGE2
secretion by ~2.9-fold in non-irradiated NHEK to 238.6 pg/ml
PGE2 (Fig. 6). The
extract and piceatannol demonstrated a decreased production of
PGE2 in a dose-dependent manner, suggesting that rose
myrtle extract and/or piceatannol suppressed the inflammation
stimulated by UVB in NHEK.
Discussion
In the present study, the protective effects of 80%
ethanol extracts from the fruit of rose myrtle and its component,
piceatannol (Fig. 2A), against
UVB-induced cytotoxicity in cultured NHEK were investigated. It is
known that ~90% of skin inflammation cases are attributed to solar
UV radiation, particularly its UVB component, which is absorbed
efficiently by cellular DNA (20).
UVB radiation penetrates the skin epidermis, inducing direct and
indirect DNA-damaging effects. Rose myrtle extract and piceatannol
increased cell viability in the UVB-exposed NHEK (Fig. 3), and promoted the removal of CPD
photoproducts (Fig. 4), suggesting
an improvement in DNA damage repair. The formation of CPD and 6-4
pyrimidine-pyrimidone photoproducts are the most predominant DNA
lesions in skin following UVB and UVA exposure (16,21).
The predominant repair mechanism of UVB-induced DNA damage is
nucleotide excision repair (NER). When skin cells are exposed to
excessive UV radiation, the NER capacity is reduced and CPD lesions
remain, which may result in cellular death, senescence, mutagenesis
and carcinogenesis of the skin (21). An enhancement in DNA repair
provides a plausible explanation to account for how extract and
piceatannol exert a protective effect on UVB-irradiated NHEK
viability in culture, and on sun-damaged cell formation in
UVB-irradiated human skin explants.
The effect of rose myrtle extract and piceatannol on
in vitro pol activity in UVB-irradiated cultured NHEK cell
extracts was also analyzed, and the enzyme activity markedly
increased (Fig. 5). DNA-dependent
pol catalyzes the addition of deoxyribonucleotides to the
3′-hydroxy terminus of primed double-stranded DNA molecules
(19). The human genome encodes at
least 15 pols, which function in cellular DNA synthesis (22,23).
Eukaryotic cells contain three replicative pols (α, δ and ε), one
mitochondrial pol (γ) and at least 11 non-replicative pols (β, ζ,
η, θ, ι, κ, λ, µ, γ, TdT and REV1) (17,18).
Pols have a highly conserved structure, with their overall
catalytic subunits exhibiting little variation among species.
Conserved enzyme structures, which are preserved over time,
normally perform important cellular functions, which confer
evolutionary advantages. Based on sequence homology, eukaryotic
pols are divided into four predominant families: A, B, X and Y
(17). Family A includes
mitochondrial pol γ and pols θ and ν; family B includes the three
replicative pols, α, δ and ε, and pol ζ; family X comprises pols β,
λ, µ and TdT; family Y includes pols η, ι, κ and REV1
(18). At least seven pols,
comprising two of the A-family pols (pols θ and ν), one B-family
pol (pol ζ) and four Y-family pols (pols η, ι, κ and REV1), are
capable of substantial translesion DNA synthesis (TLS) activity
(18). The most notable TLS pol,
which can bypass UV radiation-induced DNA damage, is pol η, which
bypasses thymine-thymine (TT)-CPD with high efficiency and
fidelity. Purified human pol η correctly inserts A
deoxy-nucleotides opposite to linked bases of a TT-CPD (24). Pol β, the base excision repair pol,
enhances UV-induced genetic instability, and facilitates
translesion replication of CPD in a UV lesion bypass (25). Consequently, the activation of the
DNA repair-associated pols, β and η, is likely to be important for
maintaining UVB-induced DNA damage.
The present study demonstrated that rose myrtle
extract and piceatannol decreased UVB-induced secretion of the
inflammatory mediator, PGE2 (Fig. 6). NHEK-derived inflammatory
mediators exert an important role in the development of the
inflammatory reaction in UVB-exposed skin (26). Numerous studies have demonstrated
that PGE2 mediates the signals involved in the induction
of erythema, angiogenesis, vasodilatation and vascular permeability
(27), and PGE2
signaling pathways promote photoaging and the development of
UVB-induced skin carcinogenesis (28). Taken together, the present data on
the inhibitory effects of rose myrtle extract and piceatannol
against UVB-induced PGE2 expression in NHEK demonstrated
the anti-inflammatory properties of these compounds. These results
supported the hypothesis that these compounds have
anti-inflammatory capability, not only against UVB-induced
inflammation, but also against inflammatory reactions caused by
other irritants. Since rose myrtle extract and piceatannol do not
absorb UVB, the data in the present study suggested that they act
in a non-sunscreen manner to protect against UVB-induced
inflammatory induction.
On analysis of >50 plants, rose myrtle fruit
extracts were identified as being the most effective at increasing
cell viability in UVB-irradiated NHEK. Rose myrtle fruit extract
and its isolated polyphenolic component, piceatannol, decreased the
production of CPD and PGE2, which are a DNA damage
photoproduct and an inflammatory mediator, respectively. These
results suggested that rose myrtle piceatannol protects the skin
from UVB-induced damage via the enhancement of DNA
repair-associated pol enzyme activity, and suppressed inflammation.
Consequently, the extract and/or piceatannol may be beneficial in
the photoprotection of skin; however, further studies are required
to elucidate the mechanism by which piceatannol confers this
protective activity.
Acknowledgments
This study was supported, in part, by the Ministry
of Education, Culture, Sports, Science and Technology, Japan
(MEXT)-Supported Program for the Strategic Research Foundation at
Private Universities, 2012–2016. Dr Yoshiyuki Mizushina received a
Grants-in-Aids for the 25th (2014) Cosmetology Research Foundation
(Japan).
Abbreviations:
|
NHEK
|
normal human epidermal
keratinocytes
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
CPD
|
cyclobutane pyrimidine dimers
|
|
PGE2
|
prostaglandin E2
|
|
pol
|
DNA polymerase
|
|
UV
|
ultraviolet
|
|
KGM
|
keratinocyte growth medium
|
|
dTTP
|
2′-deoxythymidine-5′-triphosphate
|
|
DMSO
|
dimethyl sulfoxide
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
NMR
|
nuclear magnetic resonance
|
|
NER
|
nucleotide excision repair
|
|
TLS
|
translesion DNA synthesis
|
References
|
1
|
Berneburg M, Plettenberg H and Krutmann J:
Photoaging of human skin. Photodermatol Photoimmunol Photomed.
16:239–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verschooten L, Claerhout S, Van Laethem A,
Agostinis P and Garmyn M: New strategies of photoprotection.
Photochem Photobiol. 82:1016–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moriwaki S and Takahashi Y: Photoaging and
DNA repair. J Dermatol Sci. 50:169–176. 2008. View Article : Google Scholar
|
|
4
|
Kripke ML, Cox PA, Alas LG and Yarosh DB:
Pyrimidine dimers in DNA initiate systemic immunosuppression in
UV-irradiated mice. Proc Natl Acad Sci USA. 89:7516–7520. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Autier P: Sunscreen abuse for intentional
sun exposure. Br J Dermatol. 161(Suppl 3): 40–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schroeder P and Krutmann J: What is needed
for a sunscreen to provide complete protection. Skin Therapy Lett.
15:4–5. 2010.PubMed/NCBI
|
|
7
|
Matsui MS, Hsia A, Miller JD, Hanneman K,
Scull H, Cooper KD and Baron E: Non-sunscreen photoprotection:
Antioxidants add value to a sunscreen. J Investig Dermatol Symp
Proc. 14:56–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yaar M and Gilchrest BA: Photoageing:
Mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010. View Article : Google Scholar
|
|
10
|
Do TL: SIM. Medicine plants and remedies
of Vietnam. 16th edition. Thoi Dai Publication House; Hanoi,
Vietnam: pp. 434–435. 2011
|
|
11
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizushina Y, Yagi H, Tanaka N, Kurosawa T,
Seto H, Katsumi K, Onoue M, Ishida H, Iseki A, Nara T, et al:
Screening of inhibitor of eukaryotic DNA polymerases produced by
microorganisms. J Antibiot (Tokyo). 49:491–492. 1996. View Article : Google Scholar
|
|
13
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lindell TJ, Weinberg F, Morris PW, Roeder
RG and Rutter WJ: Specific inhibition of nuclear RNA polymerase II
by α-amanitin. Science. 170:447–449. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kashiwada Y, Nonaka G, Nishioka I,
Nishizawa M and Yamagishi T: Studies on rhubarb (Rhei rhizoma). XIV
Stilbene glucosides. Chem Pharm Bull. 36:1545–1549. 1988.
View Article : Google Scholar
|
|
16
|
Marrot L and Meunier JR: Skin DNA
photodamage and its biological consequences. J Am Acad Dermatol.
(Suppl 2)58:S139–S148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nat Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lange SS, Takata K and Wood RD: DNA
polymerases and cancer. Nat Rev Cancer. 11:96–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kornberg A and Baker TA: Eukaryotic DNA
replication. DNA replication. 2nd edition. Freeman WD; Co.:
University Science Books; California, Chapter: Chapter 6; pp.
197–225. 1990
|
|
20
|
Gailani MR, Leffell DJ, Ziegler A, Gross
EG, Brash DE and Bale AE: Relationship between sunlight exposure
and a key genetic alteration in basal cell carcinoma. J Natl Cancer
Inst. 88:349–354. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pagès V and Fuchs RP: How DNA lesions are
turned into mutations within cells? Oncogene. 21:8957–8966. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bebenek K and Kunkel TA: DNA Repair and
Replication. Advances in Protein Chemistry. Yang W: Elsevier; San
Diego, CA: pp. 137–165. 2004, View Article : Google Scholar
|
|
23
|
Hübscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Annu Rev Biochem. 71:133–163. 2002.
View Article : Google Scholar
|
|
24
|
Johnson RE, Washington MT, Prakash S and
Prakash L: Fidelity of human DNA polymerase eta. J Biol Chem.
275:7447–7450. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Servant L, Cazaux C, Bieth A, Iwai S,
Hanaoka F and Hoffmann JS: A role for DNA polymerase β in mutagenic
UV lesion bypass. J Biol Chem. 277:50046–50053. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kabashima K, Nagamachi M, Honda T,
Nishigori C, Miyachi Y, Tokura Y and Narumiya S: Prostaglandin
E2 is required for ultraviolet B-induced skin
inflammation via EP2 and EP4 receptors. Lab Invest. 87:49–55. 2007.
View Article : Google Scholar
|
|
27
|
Trompezinski S, Pernet I, Schmitt D and
Viac J: UV radiation and prostaglandin E2 up-regulate
vascular endothelial growth factor (VEGF) in cultured human
fibroblasts. Inflamm Res. 50:422–427. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rundhaug JE, Mikulec C, Pavone A and
Fischer SM: A role for cyclooxygenase-2 in ultraviolet
light-induced skin carcinogenesis. Mol Carcinog. 46:692–698. 2007.
View Article : Google Scholar : PubMed/NCBI
|