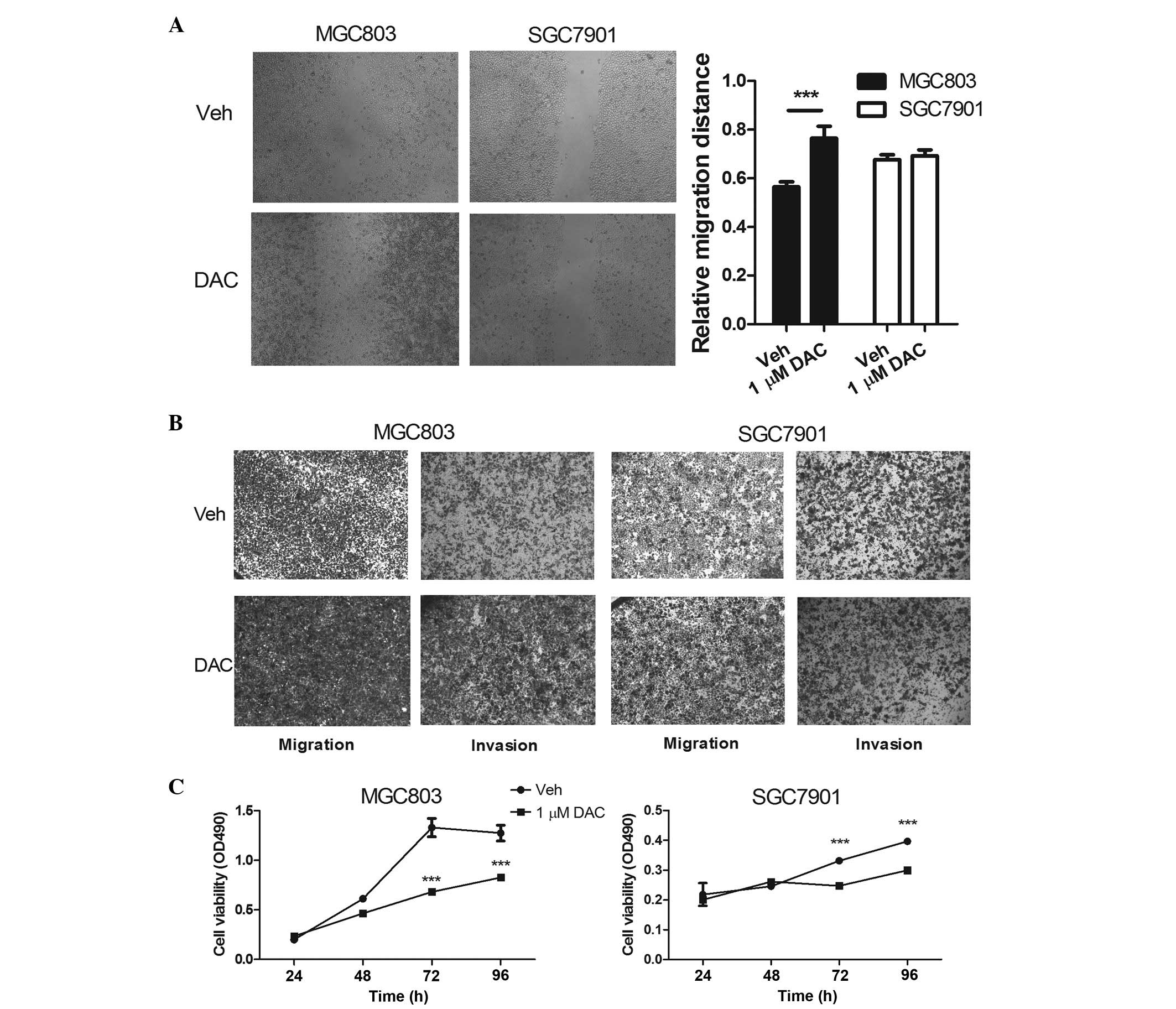
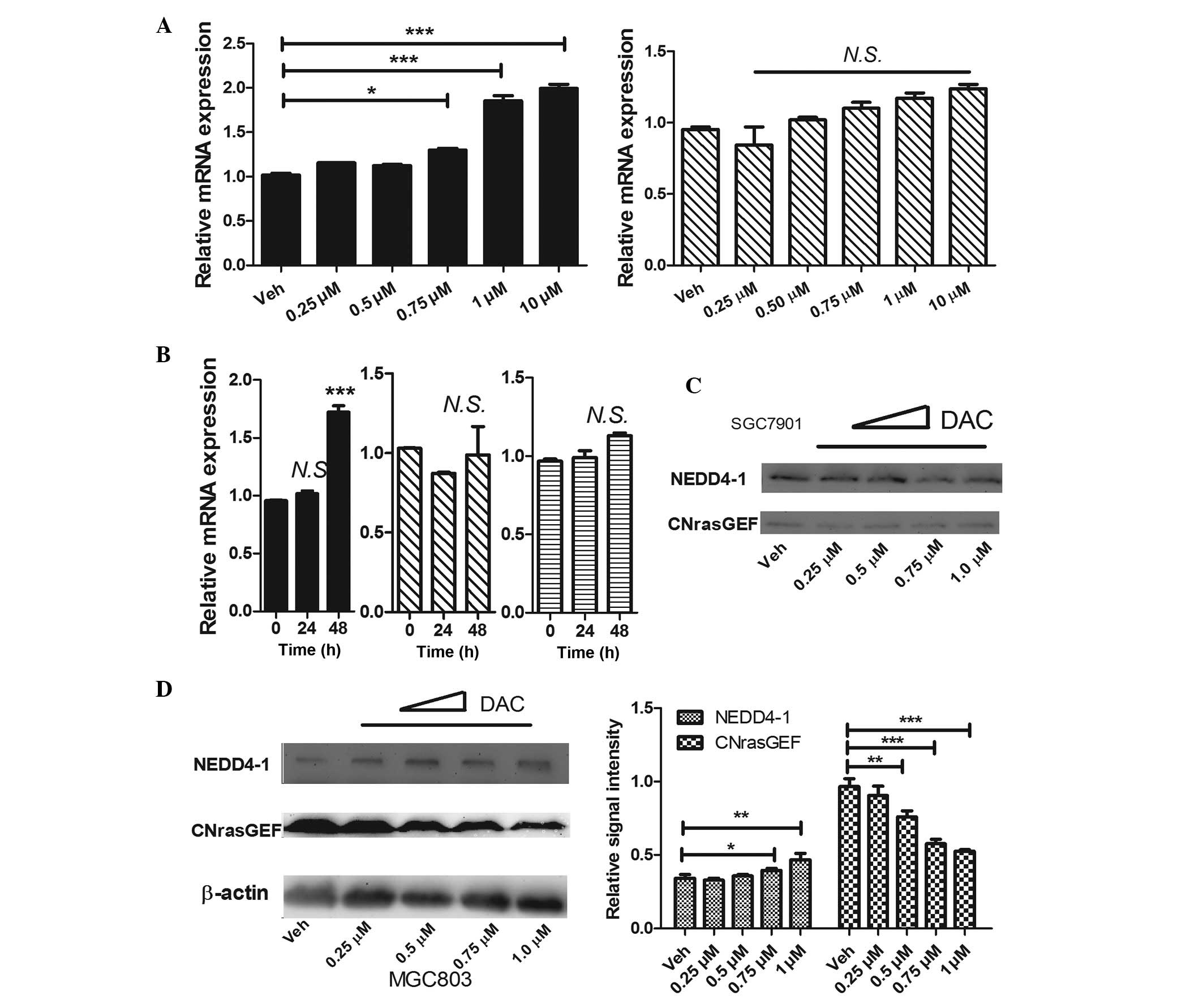
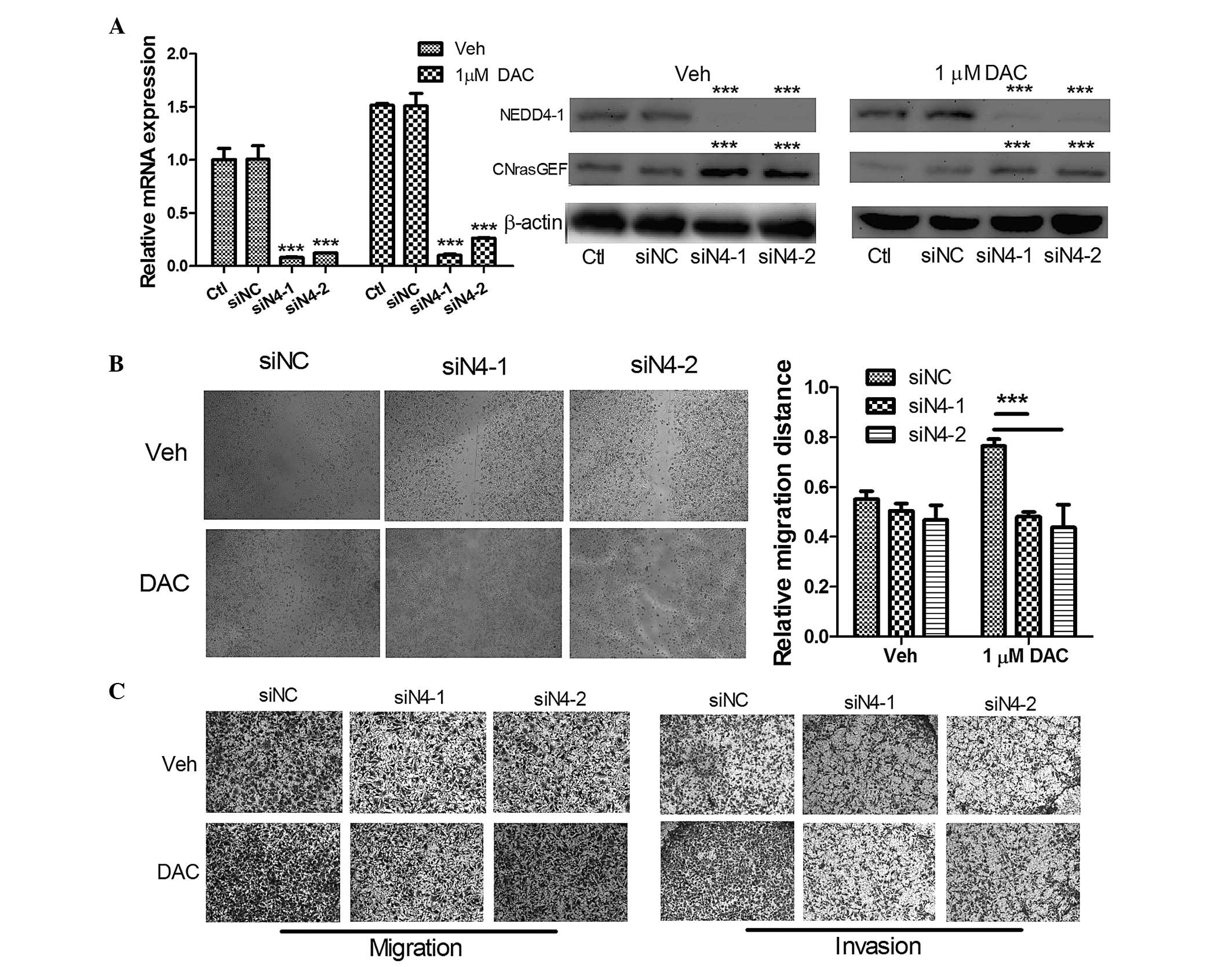
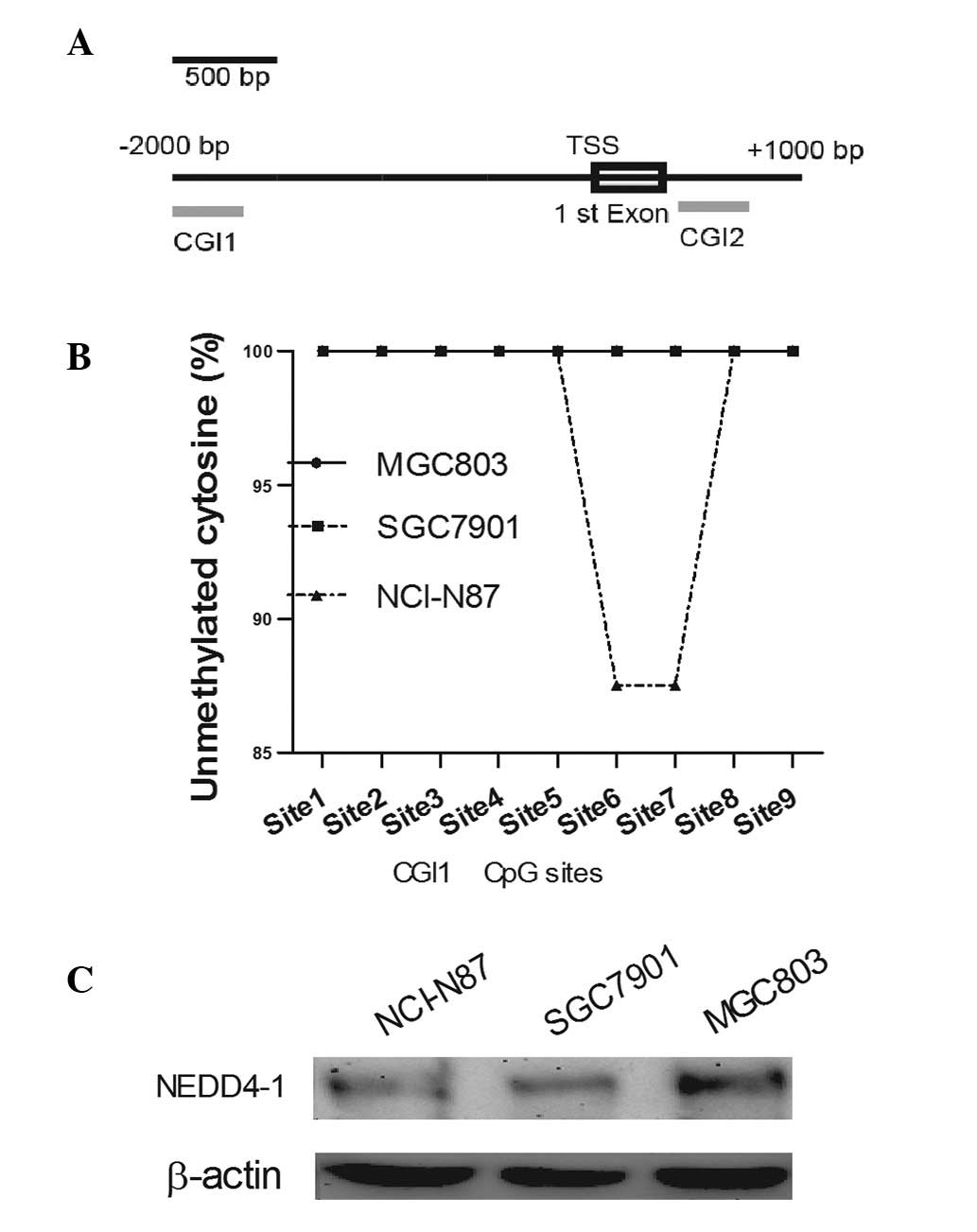
|
1
|
Melton SD, Genta RM and Souza RF:
Biomarkers and molecular diagnosis of gastrointestinal and
pancreatic neoplasms. Nat Rev Gastroenterol Hepatol. 7:620–628.
2010.PubMed/NCBI
|
|
2
|
Correa P and Houghton J: Carcinogenesis of
Helicobacter pylori. Gastroenterology. 133:659–672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
5
|
Robertson KD: DNA methylation,
methyltransferases and cancer. Oncogene. 20:3139–3155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo M and Yan W: Epigenetics of gastric
cancer. Methods Mol Biol. 1238:783–799. 2015. View Article : Google Scholar
|
|
7
|
Illingworth RS, Gruenewald-Schneider U,
Webb S, Kerr AR, James KD, Turner DJ, Smith C, Harrison DJ, Andrews
R and Bird AP: Orphan CpG islands identify numerous conserved
promoters in the mammalian genome. PLoS Genet. 6:e10011342010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nie J, Liu L, Li X and Han W: Decitabine,
a new star in epigenetic therapy: The clinical application and
biological mechanism in solid tumors. Cancer Lett. 354:12–20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kantarjian H, Issa JP, Rosenfeld CS,
Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C,
Ravandi F, et al: Decitabine improves patient outcomes in
myelodysplastic syndromes: Results of a phase III randomized study.
Cancer. 106:1794–1803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Issa JP, Garcia-Manero G, Giles FJ,
Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS,
Cortes J and Kantarjian HM: Phase 1 study of low-dose prolonged
exposure schedules of the hypomethylating agent
5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies.
Blood. 103:1635–1640. 2004. View Article : Google Scholar
|
|
11
|
Sacchi S, Kantarjian HM, O'Brien S, Cortes
J, Rios MB, Giles FJ, Beran M, Koller CA, Keating MJ and Talpaz M:
Chronic myelogenous leukemia in nonlymphoid blastic phase: Analysis
of the results of first salvage therapy with three different
treatment approaches for 162 patients. Cancer. 86:2632–2641. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kantarjian HM, O'Brien S, Cortes J, Giles
FJ, Faderl S, Issa JP, Garcia-Manero G, Rios MB, Shan J, Andreeff
M, et al: Results of decitabine (5-aza-2′deoxycytidine) therapy in
130 patients with chronic myelogenous leukemia. Cancer. 98:522–528.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lubbert M, Suciu S, Baila L, Rüter BH,
Platzbecker U, Giagounidis A, Selleslag D, Labar B, Germing U,
Salih HR, et al: Low-dose decitabine versus best supportive care in
elderly patients with intermediate- or high-risk myelodysplastic
syndrome (MDS) ineligible for intensive chemotherapy: Final results
of the randomized phaseIII study of the European organisation for
research and treatment of cancer Leukemia group and the German MDS
study group. J Clin Oncol. 29:1987–1996. 2011. View Article : Google Scholar
|
|
14
|
Shin DY, Kim GY, Kim CG, Kim WJ, Kang HS
and Choi YH: Anti-invasive effects of decitabine, a DNA
methyltransferase inhibitor, through tightening of tight junctions
and inhibition of matrix metalloproteinase activities in AGS human
gastric carcinoma cells. Oncol Rep. 28:1043–1050. 2012.PubMed/NCBI
|
|
15
|
Liu WH, Sang MX, Hou SY, Zhang C and Shan
BE: Low-dose decitabine induces MAGE-A expression and inhibits
invasion via suppression of NF-κB2 and MMP2 in Eca109 cells. Biomed
Pharmacother. 68:745–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao F, Bai SY, Ma Y, Yan ZH, Yue Z, Yu Y,
Wang X and Wang J: DNA methylation of heparanase promoter
influences its expression and associated with the progression of
human breast cancer. PLoS One. 9:e921902014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borges S, Döppler HR and Storz P: A
combination treatment with DNA methyltransferase inhibitors and
suramin decreases invasiveness of breast cancer cells. Breast
Cancer Res Treat. 144:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rougier JS, Albesa M, Abriel H and Viard
P: Neuronal precursor cell-expressed developmentally downregulated
4-1 (NEDD4-1) controls the sorting of newly synthesized Ca (V) 1.2
calcium channels. J Biol Chem. 286:8829–8838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwak YD, Wang B, Pan W, Xu H, Jiang X and
Liao FF: Functional interaction of phosphatase and tensin homologue
(PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc.
J Biol Chem. 285:9847–9857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Oppenheim RW, Sugiura Y and Lin W:
Abnormal development of the neuromuscular junction in
Nedd4-deficient mice. Dev Biol. 330:153–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawabe H, Neeb A, Dimova K, Young SM Jr,
Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang DE,
Umikawa M, et al: Regulation of Rap2A by the ubiquitin ligase
Nedd4-1 controls neurite development. Neuron. 65:358–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawabe H and Brose N: The ubiquitin E3
ligase Nedd4-1 controls neurite development. Cell Cycle.
9:2477–2478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lackovic J, Howitt J, Callaway JK, Silke
J, Bartlett P and Tan SS: Differential regulation of Nedd4
ubiquitin ligases and their adaptor protein Ndfip1 in a rat model
of ischemic stroke. Exp Neurol. 235:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwak YD, Wang B, Li JJ, Wang R, Deng Q,
Diao S, Chen Y, Xu R, Masliah E, Xu H, et al: Upregulation of the
E3 ligase NEDD4-1 by oxidative stress degrades IGF-1 receptor
protein in neurodegeneration. J Neurosci. 32:10971–10981. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Trotman LC, Koppie T, Alimonti A,
Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo
C, et al: NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN.
Cell. 128:129–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amodio N, Scrima M, Palaia L, Salman AN,
Quintiero A, Franco R, Botti G, Pirozzi P, Rocco G, De Rosa N and
Viglietto G: Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a
PTEN negative regulator, in non-small-cell lung carcinomas. Am J
Pathol. 177:2622–2634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SS, Yoo NJ, Jeong EG, Kim MS and Lee
SH: Expression of NEDD4-1, a PTEN regulator, in gastric and
colorectal carcinomas. APMIS. 116:779–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Nie W, Zhang X, Zhang G, Li Z, Wu
H, Shi Q, Chen Y, Ding Z, Zhou X and Yu R: NEDD4-1 regulates
migration and invasion of glioma cells through CNrasGEF
ubiquitination in vitro. PLoS One. 8:e827892013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun A, Yu G, Dou X, Yan X, Yang W and Lin
Q: Nedd4-1 is an exceptional prognostic biomarker for gastric
cardia adenocarcinoma and functionally associated with metastasis.
Mol Cancer. 13:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin F, Lin P, Zhao D, Chen Y, Xiao L, Qin
W, Li D, Chen H, Zhao B, Zou H, et al: Sox2 targets cyclinE, p27
and survivin to regulate androgen-independent human prostate cancer
cell proliferation and apoptosis. Cell Prolif. 45:207–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Wang H, Jiang N, Lu W, Zhang XF
and Fang JY: Effect of inhibition of MEK pathway on
5-aza-deoxycytidine-suppressed pancreatic cancer cell
proliferation. Genet Mol Res. 12:5560–5573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Li H, Yang R, Zhou S and Zou S:
Decitabine inhibits the cell growth of cholangiocarcinoma in
cultured cell lines and mouse xenografts. Oncol Lett. 8:1919–1924.
2014.PubMed/NCBI
|
|
33
|
Li LC and Dahiya R: MethPrimer: Designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Everson RG, Antonios JP, Lisiero DN, Soto
H, Scharnweber R, Garrett MC, Yong WH, Li N, Li G and Kruse CA: et
al Efficacy of systemic adoptive transfer immunotherapy targeting
NY-ESO-1 for glioblastoma. Neuro Oncol. Sep 1–2015.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan W, Herman JG and Guo M:
Epigenome-based personalized medicine in human cancer. Epigenomics.
Sep 7–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|