Introduction
Multiple myeloma (MM) is a malignant blood cancer
type with the characteristic of plasma-cell clonal proliferation,
which accounts for ~10% of all hematological malignancies and has a
yearly increasing incidence rate (1). In recent years, with the application
of novel chemotherapeutic drugs and improvements in treatment
methods, as well as progress in the development and optimization of
supportive treatments, 50–70% of patients receive effective
chemotherapy; however, multiple cycles of chemotherapeutic
treatments cause drug resistance, leading to refractory MM
(2).
Studies on Drosophila (D.)
melanogaster have led to the discovery of the Toll gene,
which mainly determines the developmental direction of the front
and lateral body axes in D. melanogaster as well as
the non-specific immune response. Toll genes encode Toll-like
receptors (TLR); the first TLR identified on the human cell surface
displaying homology with D. melanogaster TLRs was
TLR4 (3). Studies have shown that
signaling pathways induced by TLRs, including TLR4 and TLR9, are
important in tumor formation, and that the upregulation of TLRs may
be closely associated with the development of cancer types,
including gastric and colon cancer (4).
Nuclear factor (NF)-κB is a key nuclear
transcription factor which, under normal conditions, exists in the
inactive forms of homologous or heterodimers in the cytoplasm of
almost all types of cells, and which is associated with multiple
cellular activities, including the activation of immune cells,
development of T- and B-lymphocytes, stress response and cell
apoptosis (5). Recent studies have
shown that NF-κB is closely associated with the occurrence of
hematopoietic malignancies, including leukemia, lymphoma and MM
(6).
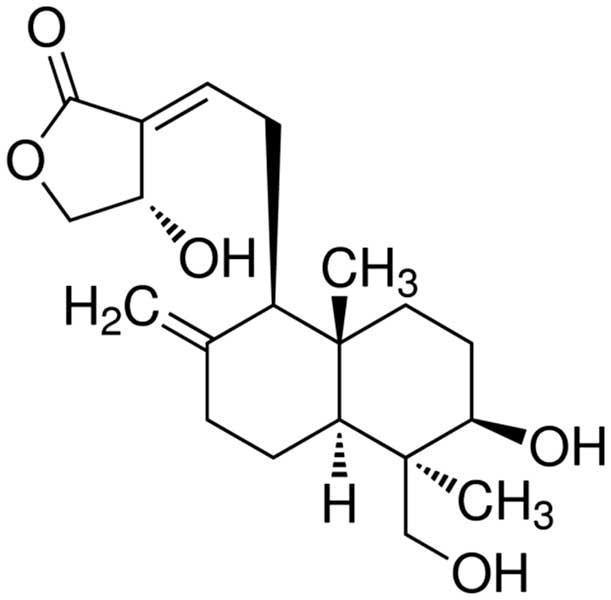
Andrographolide (Fig.
1) is a diterpene lactone compound extracted from
Andrographis paniculata [(Burm.f) Nees], a medicinal plant
from the Acanthaceae family, and is one of the major active
components of the traditional Chinese medicine Andrographis with a
content of up to 1.8% in the leaves (7). In China, Andrographis is being
mass-produced as a raw material for the isolation of
andrographolide used as an anti-inflammatory drug in formulations
including Kalii Dehydrographolidi Succinas and Andrographis
injection (8). Pharmacological
studies have shown that andrographolide has anti-inflammatory,
anti-bacterial, anti-viral, anti-tumor, immunoregulatory and
hepato- and gallbladder-protective effects, as well as beneficial
effects on cardiovascular diseases, with characteristics of low
toxicity and low cost (9–12). However, to date, the potential of
andrographolide to be used in the treatment of human MM has not
been studied. The present study provided experimental evidence for
the anti-cancer efficacy of andrographolide on MM cells; in
addition, the mechanism of action and potential regulatory
molecules involved, including TLR4 and NF-κB, were assessed.
Materials and methods
Reagents
Dulbecco's modified Eagle' medium (DMEM) was
obtained from Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
Fetal bovine serum (FBS) was purchased from Thermo Fisher
Scientific.
3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was provided by Invitrogen (Thermo Fisher Scientific).
Annexin V/propidium iodide (PI) was purchased from eBioscience (San
Diego, CA, USA). Caspase-9/3 activation ELISA colorimetric assay
kits were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The bicinchoninic acid (BCA) Protein Assay kit was purchased
from Beyotime Institute of Biotechnology (Jiangsu, China).
Cells and cell culture
The OPM1 human myeloma cell line was purchased from
Shanghai Cell Bank (Shanghai, China) and cultured in complete DMEM
with 10% heat-inactivated FBS, 100 U/ml penicillin and streptomycin
(100 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
Cell viability assay
OPM1 cells were seeded into 96-well plates at
1×104/well and allowed to attach overnight, following
which they were treated with 1.0, 5.0 or 10.0 µM
andrographolide (Sigma-Aldrich; purity, >98%) for 24, 48 or 72 h
according to the procedure of a previous study (13). Subsequently, 20 µl MTT (5
mg/ml) was added to each well and plates were cultured for an
additional 4 h, followed by aspiration of the media, addition of
150 µl dimethylsulfoxide (Invitrogen; Thermo Fisher
Scientific) to each well and agitation for 20 min. The absorbance
values were determined at 550 nm using an automatic microplate
reader (Wallac Victor 1420; PerkinElmer, Inc., Waltham, MA,
USA).
Flow cytometric analysis
OPM1 cells were inoculated into six-well plates at
2×106/well and treated with 1.0, 5.0 and 10.0 µM
andrographolide for 24 h. Each well was washed twice with
phosphate-buffered saline (PBS) and following trypsinization
(Beyotime Institute of Biotechnology), cells were suspended in 1 ml
binding buffer. Annexin V (5 µl) was added and cells were
incubated for 15 min in the dark. Subsequently, 5 µl PI was
added and cells were incubated for 30 min in the dark on ice. The
apoptotic rate of OPM1 cells was then assessed by flow cytometry
(FACSCalibur; BD Biosciences) with 1×106 events
recorded.
Caspase-9/3 activation
OPM1 cells were seeded into 96-well plates at
1×104/well and treated with 1.0, 5.0 or 10.0 µM
andrographolide for 24 h. Caspase-9/3 activation in OPM1 cells was
determined using ELISA colorimetric assay kits. Caspase-9 inhibitor
LEHD-pNA and caspase-3 inhibitor Ac-DEVD-pNA were added to each
well, and caspase-9/3 activation-associated fluorescence was
detected at the wavelength of 405 nm using an automatic microplate
reader (Wallac Victor 1420).
Western blot analysis
OPM1 cells were inoculated into six-well plates at
2×106/well and treated with 1.0, 5.0 or 10.0 µM
andrographolide for 24 h. Each well was washed twice with PBS and
incubated with ice-cold lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. The protein contents were
determined using the BCA Protein Assay kit. Following loading of 10
µg protein per lane, total protein was fractionated by 10%
SDS-PAGE and transfer onto a polyvinylidene difluoride membrane at
4°C over 2 h. Membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline (TBS) containing 0.05% Tween-20 prior to
incubation with anti-TLR4 (cat. no. sc-293072; 1:1,000; Santa Cruz
Biotechnology, Inc.); anti-NF-κB (cat. no. sc-56735; 1:1,000; Santa
Cruz Biotechnology, Inc.) and β-actin (cat. no. AC106; 1:1,000;
Beyotime Institute of Biotechnology, Inc.) overnight at 4°C with
agitation. After extensive washing, membranes were incubated with
secondary antibody (1:3,000; Tiangen, Beijing, China) for enhanced
chemiluminescence (ECL) detection using Pierce ECL Western Blotting
substrate (cat. no. 32109; Thermo Fisher Scientific).
Transfection of TLR4 small interfering
(si)RNA and NF-κB siRNA
TLR4 siRNA and NF-κB siRNA were chemically
synthesized by BeastBio Co., Ltd. (Shanghai, China). The siRNA
sequences were as follows: T LR4 5′- GATCCCGACT
TACAGTTTCTACGTTTCAAGAGAACGTAGAAACTGTAAGTCGTTA-3′ and 5′-AG
CTTAACGACTTACAGTTTCTACGTTCTCTTGAAACGTAGAAACTGTAAGTCGG-3′; and
NF-κB: 5′-CCCCTTCCA AGTTCCTATA-3′ and 5′-GGACATATGAGACCTTCAA-3′.
OPM1 cells were seeded into six-well plates at
2×106/well. 100 pmol TLR4 siRNA or NF-κB siRNA were
transfected into OPM1 cells with Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. All quantitative values were
obtained from experiments performed at least three times. Values
are expressed as the mean ± standard deviation. Statistical
significance was analyzed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
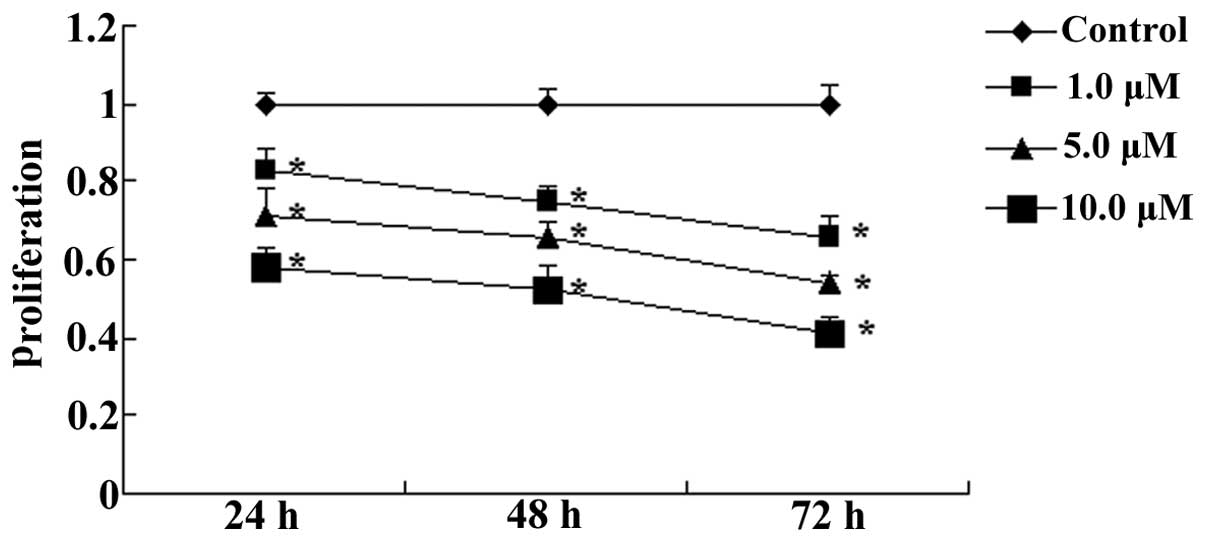
Andrographolide inhibits the
proliferation of MM cells
To investigate whether andrographolide inhibited the
proliferation of MM cells, OPM1 cells were treated with
andrographolide (1, 5 or 10 µM) for 24, 48 or 72 h and
subjected to the MTT assay. As shown in Fig. 2, andrographolide significantly
inhibited the proliferation of MM cells in vitro in a dose-
and time-dependent manner (Fig.
2).
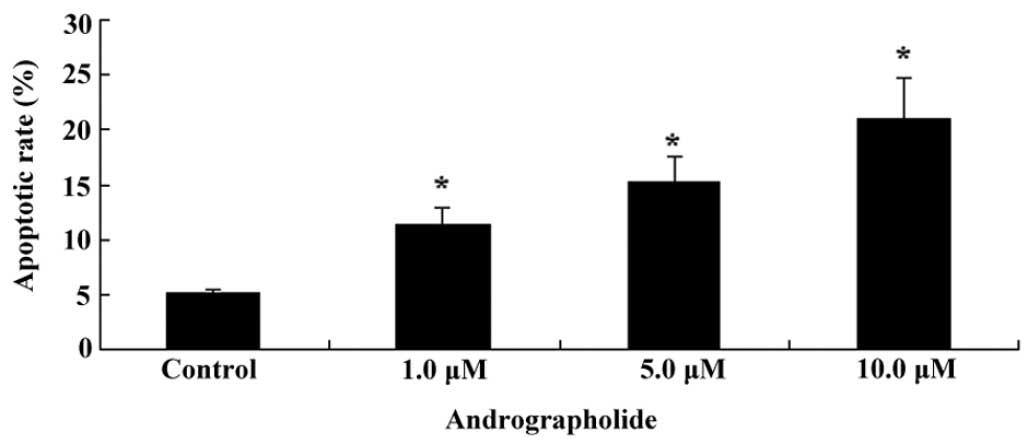
Andrographolide induces apoptosis of MM
cells
To detect whether andrographolide induced apoptosis
of MM cells, OPM1 cells were treated with andrographolide (1, 5 or
10 µM) for 24 h and subjected to Annexin V/PI double
staining followed by flow-cytometric evaluation. As shown in
Fig. 3, andrographolide
significantly induced apoptosis of MM cells in vitro in a
dose-dependent manner.
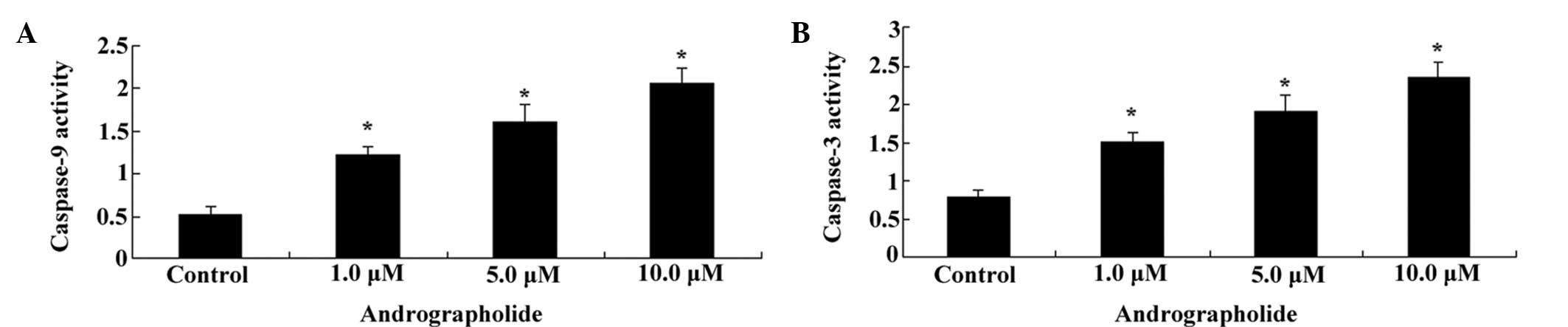
Andrographolide induces caspase-9/3
activation of MM cells
To evaluate whether andrographolide induced
caspase-9/3 activation in MM cells, OPM1 cells were treated with
andrographolide (1, 5 or 10 µM) for 24 h and subjected to a
colorimetric ELISA assay. As shown in Fig. 4A and B, andrographolide effectively
increased caspase-9/3 activation in MM cells in vitro in a
dose-dependent manner (Fig. 4A and
B).
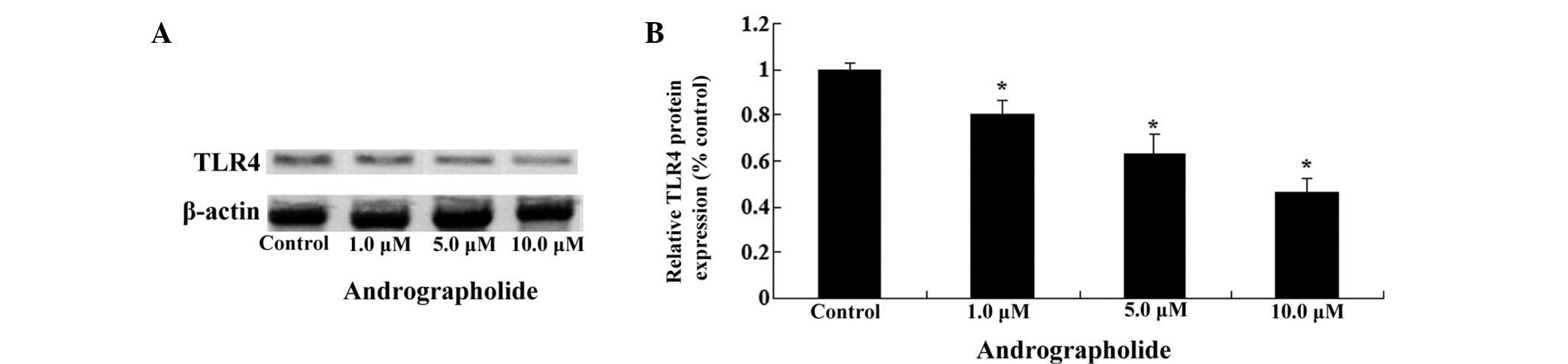
Andrographolide inhibits TLR4 protein
expression in MM cells
To further investigate the potential regulatory
mechanisms of the effects exerted by andrographolide, the TLR4
protein expression of MM cells was determined using western blots
analysis. As shown in Fig. 5A and
B, andrographolide effectively reduced the levels of TLR4
protein expression in OPM1 cells in a dose-dependent manner
(Fig. 5A and B).
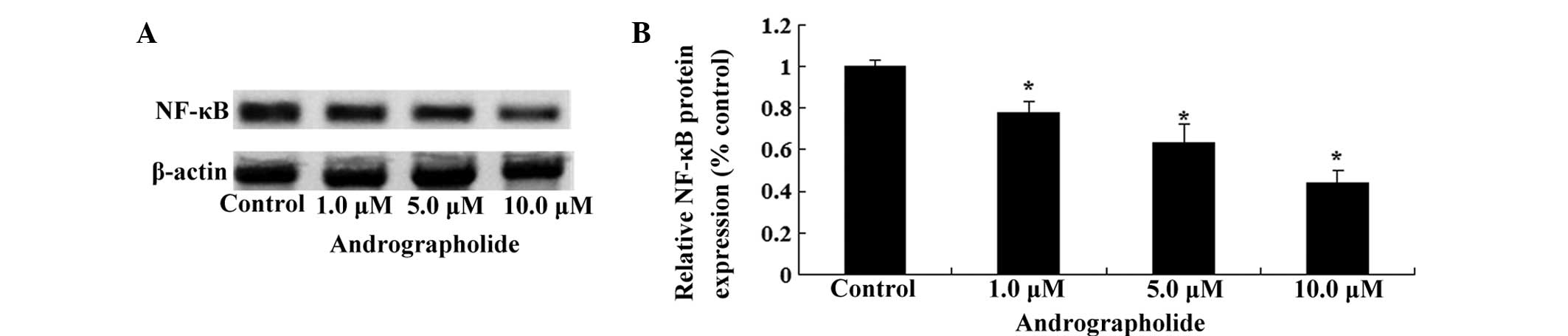
Andrographolide inhibits NF-κB protein
expression in MM cells
To further elucidate the potential regulatory
mechanism of andrographolide on the growth of MM cells, NF-κB
protein expression in MM cells was detected using western blot
analysis. As shown in Fig. 6A and
B, andrographolide effectively reduced the level of NF-κB
protein expression in OPM1 cells in a dose-dependent manner
(Fig. 6A and B).
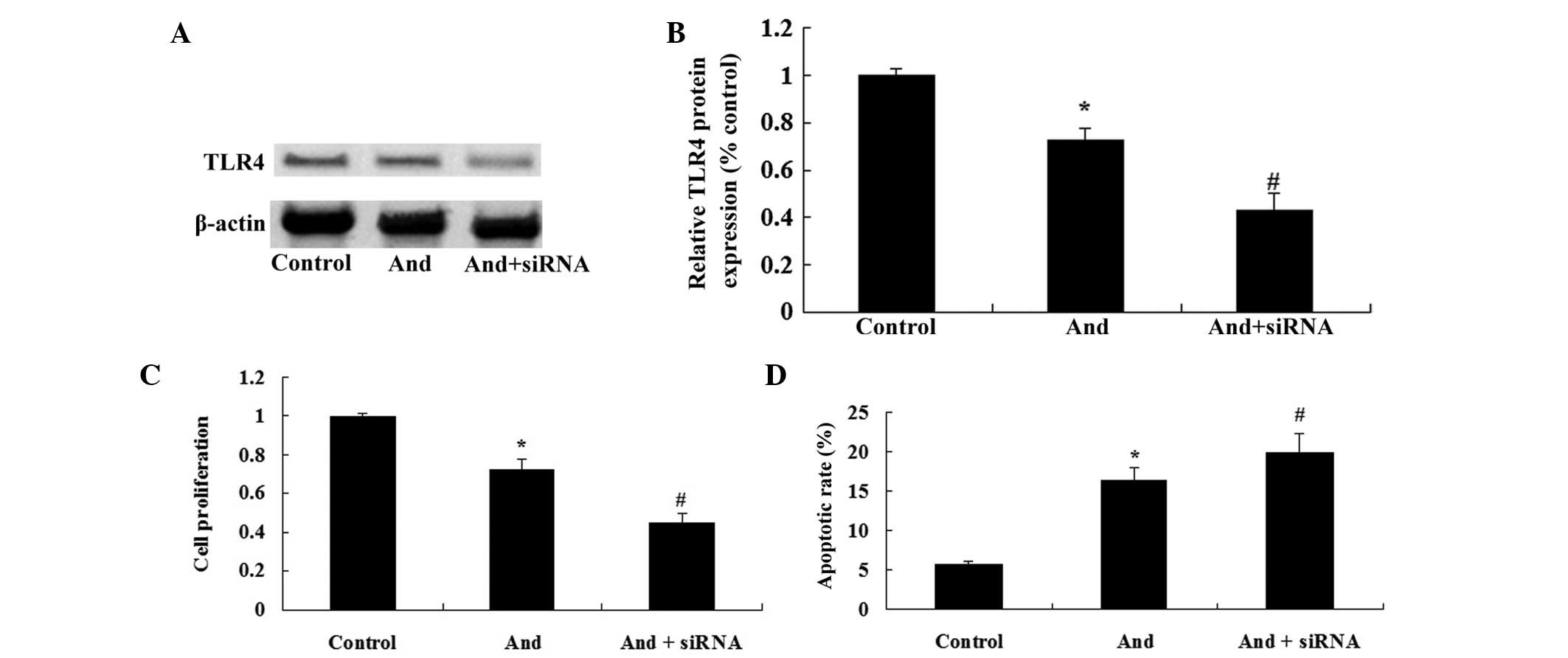
TLR4 siRNA enhances
andrographolide-mediated inhibition of cell proliferation and
induction of apoptosis of MM cells
To further confirm whether the TLR4/NF-κB signaling
pathway was the functional target of andrographolide, TLR4 siRNA
was transfected into MM cells. As shown in Fig. 7A and B, TLR4 siRNA inhibited the
TLR4 protein expression in OPM1 cells. Of note, TLR4 siRNA
efficiently enhanced the andrographolide-mediated inhibition of the
cell proliferation and induction of apoptosis of MM cells (Fig. 7C and D). These results indicated
that downregulation of TLR4 expression significantly enhanced the
anti-cancer effects of andrographolide on MM cells.
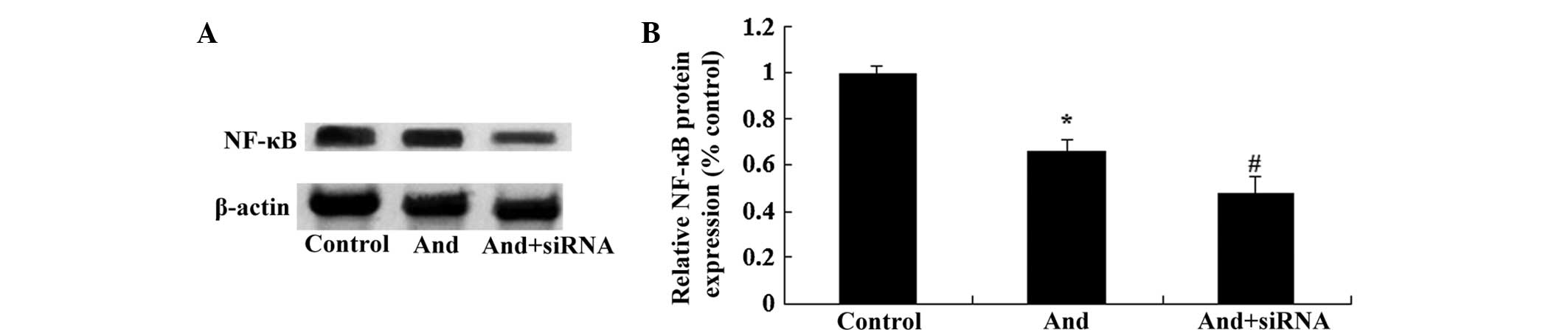
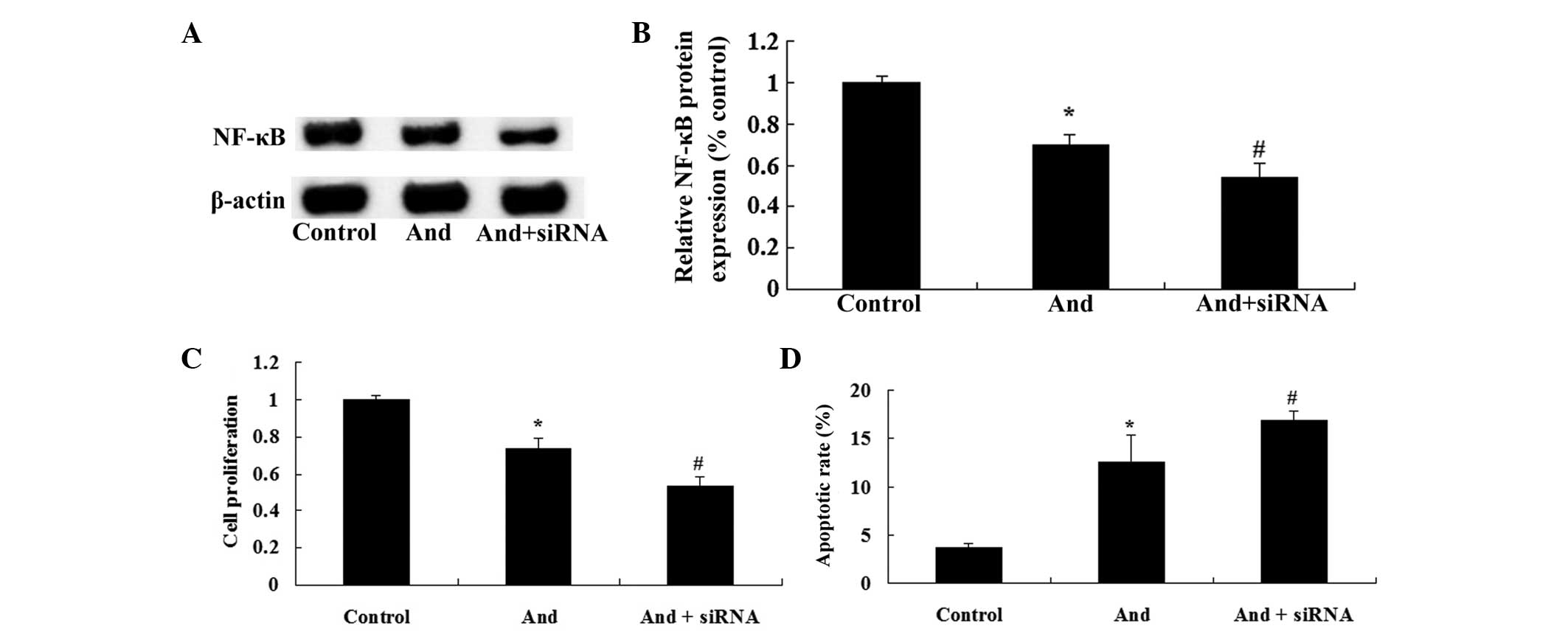
TLR4 siRNA regulates NF-κB protein
expression in MM cells
To further confirm whether the TLR4/NF-κB signaling
pathway was the functional target of andrographolide, the NF-κB
protein expression of MM cells was detected using western blot
analysis. As shown in Fig. 8A and
B, TLR4 siRNA significantly inhibited the expression of NF-κB
protein in MM cells. The results indicated that downregulation of
TLR4 expression significantly enhanced the inhibitory effects of
andrographolide on NF-κB expression in the MM cells.
NF-κB siRNA enhances
andrographolide-mediated reduction of cell proliferation and
induction of apoptosis of MM cells
To further assess whether the TLR4/NF-κB signaling
pathway is a functional target of andrographolide, NF-κB siRNA was
transfected into MM cells. As shown in Fig. 9A and B, NF-κB siRNA inhibited the
protein expression of NF-κB in MM cells. Of note, NF-κB siRNA
markedly enhanced andrographolide-mediated inhibition of cell
proliferation and induction of apoptosis of MM cells (Fig. 9C and D). These results indicated
that downregulation of NF-κB expression significantly enhanced the
anti-cancer effects of andrographolide on MM cells.
Discussion
MM is a neoplasm of the blood, which is common in
the elderly; its major cause is the proliferation of malignant
plasma cells in the blood. With the increasing mean age of the
Chinese population, the incidence of MM has been increasing
(14). Autologous hematopoietic
stem-cell transplantation can improve the number of normal blood
plasma cells in cancer patients to prolong the survival time to a
certain extent; however, as the age of patients with MM is
generally high, stem-cell transplantation is not suitable for most
of the patients (15). In the
present study, andrographolide restrained the proliferation of MM
cells in a dose- and time-dependent manner. Furthermore,
andrographolide induced cellular apoptosis and caspase-9/3
activation in MM cells in a dose-dependent manner. Yang et
al (16) reported that
andrographolide induced apoptosis in glioma cells through the
extracellular signal-regulated kinase/p53/caspase 7/poly(adenosine
triphosphatase ribose) polymerase signaling pathway. Furthermore,
andrographolide was shown to inhibit tumor angiogenesis through
downregulation of vascular endothelial growth factor (VEGF)A/VEGF
receptor 2/mitogen-activated protein kinase pathway (17).
In humans, 11 types of TLRs have been identified,
among which TLR4 was the first TLR found in mammals (3). Lipopolysaccharide (LPS) is the
exogenous ligand of TLR4 and an in vivo study has shown that
LPS stimulates the growth and metastasis of tumor cells (18). TLR4 is expressed in a variety of
murine tumor-cell lines, and LPS-activated TLR4 signaling is
conducive to tumor cells escaping from the microenvironment of
immune surveillance; in addition, following siRNA-mediated TLR4
silencing, the inhibition of tumor cell growth was enhanced, which
thus prolonged the survival time of mouse models with tumors
(19). TLR4 is expressed on the
surface of human ovarian cancer epithelial cells and can induce
proliferation as well as enhance the production of cell cytokines
following activation by LPS; therefore, it can be speculated that
tumor cells regulate the tumor microenvironment via TLR4 and
influence the activity of immune cells (20–22).
The exogenous ligand of TLR4, LPS, promotes the proliferation of MM
cells. Studies have revealed that andrographolide inhibits the
growth of melanoma (23) and
insulinoma (24) through
inhibition of the TLR4/NF-κB signaling pathway. The present study
demonstrated that andrographolide suppressed the protein expression
levels of TLR4 in a dose-dependent manner. Furthermore, TLR4 siRNA
enhanced andrographolide-mediated inhibition of cell proliferation
and induction of apoptosis, while restraining the protein
expression of NF-κB in MM cells.
NF-κB is a protein which can specifically combine
with κB sites in a variety of gene promoters or enhancers to
promote transcription (25). It
regulates the expression of numerous genes, including cytokines,
adhesion molecules, chemokines, immune factors, oxidative
stress-associated enzymes and transcription factors, and therefore
has a variety of biological functions, including the participation
in inflammatory immune responses, the regulation of cell apoptosis,
self-transcription, cell cycle regulation, tumorigenesis and drug
resistance (26,27). As a transcription factor with a
designated DNA-binding sequence, NF-κB has an important role in
solid tumor-cell proliferation and transformation as well as tumor
development; furthermore, it is closely associated with neoplasms
of the blood system (28). Luo
et al (13) showed that
andrographolide inhibited the activation of NF-κB as well as matrix
metalloproteinase-9 activity in H3255 lung cancer cells.
Furthermore, Wang et al (29) demonstrated that andrographolide
inhibited the proliferation of oral squamous cell carcinogenesis
through inhibition of NF-κB inactivation. In the present study,
andrographolide decreased the protein expression NF-κB in MM cells.
Of note, NF-κB siRNA significantly enhanced
andrographolide-mediated inhibition of cell proliferation and
induction of apoptosis of MM cells.
In conclusion, to the best of our knowledge, the
present study was the first to show that andrographolide suppressed
the proliferation and promoted apoptosis and caspase-9/3 activation
in MM cells. The underlying mechanism may involve the suppression
of the TLR4/NF-κB signaling pathway. It is thus suggested that
andrographolide may a promising candidate anti-cancer drug for the
clinical treatment of MM through the TLR4/NF-κB signaling
pathway.
References
|
1
|
Auner HW, Szydlo R, Hoek J, Goldschmidt H,
Stoppa AM, Morgan GJ, Moreau P, Attal M, Marit G, Russell N, et al:
Trends in autologous hematopoietic cell transplantation for
multiple myeloma in Europe: increased use and improved outcomes in
elderly patients in recent years. Bone Marrow Transplant.
50:209–215. 2015. View Article : Google Scholar
|
|
2
|
Peng J, Chen Y, Lin J, Zhuang Q, Xu W,
Hong Z and Sferra TJ: Patrinia scabiosaefolia extract suppresses
proliferation and promotes apoptosis by inhibiting the STAT3
pathway in human multiple myeloma cells. Mol Med Rep. 4:313–318.
2011.PubMed/NCBI
|
|
3
|
Li H, Xu H and Sun B: Lipopolysaccharide
regulates MMP-9 expression through TLR4/NF-κB signaling in human
arterial smooth muscle cells. Mol Med Rep. 6:774–778.
2012.PubMed/NCBI
|
|
4
|
Wang AC, Ma YB, Wu FX, Ma ZF, Liu NF, Gao
R, Gao YS and Sheng XG: TLR4 induces tumor growth and inhibits
paclitaxel activity in MyD88-positive human ovarian carcinoma in
vitro. Oncol Lett. 7:871–877. 2014.PubMed/NCBI
|
|
5
|
Murray MY, Zaitseva L, Auger MJ, Craig JI,
MacEwan DJ, Rushworth SA and Bowles KM: Ibrutinib inhibits
BTK-driven NF-κB p65 activity to overcome bortezomib-resistance in
multiple myeloma. Cell Cycle. 14:2367–2375. 2015. View Article : Google Scholar
|
|
6
|
Dou A, Wang Z, Zhao J, Liu J and Zheng C:
Identification of therapeutic target genes with DNA microarray in
multiple myeloma cell line treated by IKKβ/NF-κB inhibitor. Acta
Cir Bras. 29:696–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Serrano FG, Tapia-Rojas C, Carvajal FJ,
Hancke J, Cerpa W and Inestrosa NC: Andrographolide reduces
cognitive impairment in young and mature AβPPswe/PS-1 mice. Mol
Neurodegener. 9:612014. View Article : Google Scholar
|
|
8
|
Chua LS: Review on liver inflammation and
antiinflammatory activity of Andrographis paniculata for
hepatoprotection. Phytother Res. 28:1589–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang D, Zhang W, Song L and Guo F:
Andrographolide protects against cigarette smoke-induced lung
inflammation through activation of heme oxygenase-1. J Biochem Mol
Toxicol. 27:259–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng X, Liu X, Bian J, Pei G, Dai H,
Polyak SW, Song F, Ma L, Wang Y and Zhang L: Synergistic effect of
14-alpha-lipoyl andrographolide and various antibiotics on the
formation of biofilms and production of exopolysaccharide and
pyocyanin by Pseudomonas aeruginosa. Antimicrob Agents Chemother.
55:3015–3017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JC, Tseng CK, Young KC, Sun HY, Wang
SW, Chen WC, Lin CK and Wu YH: Andrographolide exerts
anti-hepatitis C virus activity by upregulating haeme oxygenase-1
via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br J
Pharmacol. 171:237–252. 2014. View Article : Google Scholar :
|
|
12
|
Lee C, Oh JI, Park J, Choi JH, Bae EK, Lee
HJ, Jung WJ, Lee DS, Ahn KS and Yoon SS: TNF α mediated IL-6
secretion is regulated by JAK/STAT pathway but not by MEK
phosphorylation and AKT phosphorylation in U266 multiple myeloma
cells. Biomed Res Int. 2013:5801352013. View Article : Google Scholar
|
|
13
|
Luo W, Liu Y, Zhang J, Luo X, Lin C and
Guo J: Andrographolide inhibits the activation of NF-κB and MMP-9
activity in H3255 lung cancer cells. Exp Ther Med. 6:743–746.
2013.PubMed/NCBI
|
|
14
|
Yang M, Huang J, Ma QL, Xu GX and Jin J:
Antitumor activity of CDA-, a urinary preparation, on human
multiple myeloma cell lines via the mitochondrial pathway. Mol Med
Rep. 9:1025–1031. 2014.PubMed/NCBI
|
|
15
|
Corso A, Mangiacavalli S, Barbarano L,
Montalbetti L, Mazzone A, Fava S, Varettoni M, Zappasodi P, Morra E
and Lazzarino M: Low efficacy of thalidomide in improving response
after induction in multiple myeloma patients who are candidates for
high-dose therapy. Leuk Res. 32:1085–1090. 2008. View Article : Google Scholar
|
|
16
|
Yang SH, Wang SM, Syu JP, Chen Y, Wang SD,
Peng YS, Kuo MF and Kung HN: Andrographolide induces apoptosis of
C6 glioma cells via the ERK-p53-caspase 7-PARP pathway. Biomed Res
Int. 2014:3128472014.PubMed/NCBI
|
|
17
|
Shen K, Ji L, Lu B, Xu C, Gong C, Morahan
G and Wang Z: Andrographolide inhibits tumor angiogenesis via
blocking VEGFA/VEGFR2-MAPKs signaling cascade. Chem Biol Interact.
218:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, He H, Li D, Zhu W, Duan K, Le Y,
Liao Y and Ou Y: The role of the TLR4 signaling pathway in
cognitive deficits following surgery in aged rats. Mol Med Rep.
7:1137–1142. 2013.PubMed/NCBI
|
|
19
|
Bao H, Lu P, Li Y, Wang L, Li H, He D,
Yang Y, Zhao Y, Yang L, Wang M, et al: Triggering of toll-like
receptor-4 in human multiple myeloma cells promotes proliferation
and alters cell responses to immune and chemotherapy drug attack.
Cancer Biol Ther. 11:58–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang AC, Su QB, Wu FX, Zhang XL and Liu
PS: Role of TLR4 for paclitaxel chemotherapy in human epithelial
ovarian cancer cells. Eur J Clin Invest. 39:157–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y,
Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, Cheng J and Deng BF:
Atractylenolide-I sensitizes human ovarian cancer cells to
paclitaxel by blocking activation of TLR4/MyD88-dependent pathway.
Sci Rep. 4:38402014.PubMed/NCBI
|
|
22
|
Klink M, Nowak M, Kielbik M, Bednarska K,
Blus E, Szpakowski M, Szyllo K and Sulowska Z: The interaction of
HspA1A with TLR2 and TLR4 in the response of neutrophils induced by
ovarian cancer cells in vitro. Cell Stress Chaperones. 17:661–674.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang QQ, Zhou DL, Ding Y, Liu HY, Lei Y,
Fang HY, Gu QL, He XD, Qi CL, Yang Y, et al: Andrographolide
inhibits melanoma tumor growth by inactivating the TLR4/NF-κB
signaling pathway. Melanoma Res. 24:545–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang QQ, Ding Y, Lei Y, Qi CL, He XD, Lan
T, Li JC, Gong P, Yang X, Geng JG and Wang LJ: Andrographolide
suppress tumor growth by inhibiting TLR4/NF-κB signaling activation
in insulinoma. Int J Biol Sci. 10:404–414. 2014. View Article : Google Scholar
|
|
25
|
Suzuki E, Daniels TR, Helguera G, Penichet
ML, Umezawa K and Bonavida B: Inhibition of NF-kappaB and Akt
pathways by an antibody-avidin fusion protein sensitizes malignant
B-cells to cisplatin-induced apoptosis. Int J Oncol. 36:1299–1307.
2010.PubMed/NCBI
|
|
26
|
Calabrese C, Berman SH, Babish JG, Ma X,
Shinto L, Dorr M, Wells K, Wenner CA and Standish LJ: A phase I
trial of andrographolide in HIV positive patients and normal
volunteers. Phytother Res. 14:333–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manni S, Brancalion A, Mandato E, Tubi LQ,
Colpo A, Pizzi M, Cappellesso R, Zaffino F, Di Maggio SA, Cabrelle
A, et al: Protein kinase CK2 inhibition down modulates the NF-κB
and STAT3 survival pathways, enhances the cellular proteotoxic
stress and synergistically boosts the cytotoxic effect of
bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS
One. 8:e752802013. View Article : Google Scholar
|
|
28
|
Siveen KS, Mustafa N, Li F, Kannaiyan R,
Ahn KS, Kumar AP, Chng WJ and Sethi G: Thymoquinone overcomes
chemoresistance and enhances the anticancer effects of bortezomib
through abrogation of NF-κB regulated gene products in multiple
myeloma xenograft mouse model. Oncotarget. 5:634–648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang LJ, Zhou X, Wang W, Tang F, Qi CL,
Yang X, Wu S, Lin YQ, Wang JT and Geng JG: Andrographolide inhibits
oral squamous cell carcinogenesis through NF-κB inactivation. J
Dent Res. 90:1246–1252. 2011. View Article : Google Scholar : PubMed/NCBI
|