Introduction
Reactive oxygen species (ROS) are produced following
single electron reduction of molecular oxygen. One to 2% of the
oxygen consumed by cells escapes from the mitochondrial respiratory
chain and is converted into highly reactive species (1). Although physiological concentrations
of ROS in aerobic organisms are beneficial as they are involved in
cell signaling pathways and survival from invading pathogens,
accumulation of such species, known as oxidative stress (OS), can
contribute to the development of various diseases (1), including pregnancy-associated ones
(2). Diseases are caused by
incurring indiscriminate damage causing alteration of cellular
macromolecules such as membrane lipids, DNA, proteins and
alteration in gene expression, including apoptosis-related genes
(3,4). In addition, ROS affect cell function
through changes in intracellular calcium or pH which can eventually
lead to cell death (5).
Under physiological conditions the most common ROS
are the superoxide anions (SOA) created from molecular oxygen by
the addition of an electron. SOA lack the ability to penetrate
lipid membranes and are thus confined to the compartment in which
they are generated (6). The
formation of SOA occurs spontaneously especially in an
electron-rich environment in the vicinity of the inner
mitochondrial membrane and the respiratory chain (6) as a result of the leakage of 1–3% of
electrons being transported along the chain enzymes. Similarly, SOA
are generated through electron leakage from the shorter electron
transport chain within the endoplasmic reticulum (7). The formation of disulphide bonds
during protein folding is an oxidative process and ~25% of SOA are
generated within cells in the endoplasmic reticulum. Other sources
of SOA include the enzyme nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase which constitutes a major source of the
anions in neutrophils, vascular endothelial cells and vascular
smooth muscle (8,9). This oxidase is known to produce
substantial quantities of SOA throughout pregnancy and particularly
in early pregnancy (10).
Additional SOA sources include cytochrome P450, xanthine oxidase
and other oxidoreductases (11).
SOA constitute the initial step in the formation and
propagation of ROS intra- and extracellularly, and is the precursor
of most ROS, and is therefore a mediator in oxidation reactions.
The anti-oxidant defense of aerobic cells begins by dismutation of
SOA into hydrogen peroxide by the action of superoxide dismutase
(SOD) which is considered to play a fundamental role against
oxidant generation. Three distinct forms of SOD have been
identified in mammals. Two of these forms, SOD1 and SOD3, have
copper (Cu) and zinc (Zn) in their catalytic center and are
localized in the intracellular cytoplasmic compartments including
the cytosol, nuclear compartments and lysosomes, SOD1, or
extracellular SOD3 (12). A third
SOD has manganese (Mn) as a cofactor, SOD2 and is located in the
mitochondria (13).
Recurrent miscarriage (RM), is strictly defined as
the occurrence of ≥3 consecutive pregnancy losses prior to 24 weeks
of gestation (14), and affects
1–3% of pregnancies (15). The
etiologies of RM include parental chromosomal abnormalities,
uterine anomalies, autoimmune diseases, thyroid pathologies, blood
clotting disorders, infectious diseases, endocrinopathies and
environmental factors (14–17).
RM causes are identified in 50% of patients, and the remaining 50%
is idiopathic (16). However,
mounting evidence suggests that OS and loss of antioxidant capacity
in utero-placental tissues are important in the development of
placental and pregnancy-related diseases including RM, preeclampsia
and intrauterine growth restriction (2,14,18–20).
To this end, it has been shown that OS causes impaired placental
development and apoptosis of syncytotrophoblast leading to RM
(2,21).
Recent reports (27) have demonstrated significantly lower
activity levels of enzymatic and non-enzymatic antioxidants in
plasma, whole blood and/or placental tissue of RM patients
including glutathione peroxidase, catalase, glutathione reductase,
reduced glutathione and selenium (22–26).
The reports also indicated concurrent significant increases in
oxidant levels including hydrogen peroxide, oxidized glutathione
and malondialdehyde. However, studies associating SOD activity in
RM patients are scarce. Only two studies (24,28)
demonstrated a significant decrease in plasma SOD activities of
such patients following a comparison with healthy pregnant (HP)
women. Furthermore, there are no studies associated with the
corresponding levels of SOA in such patients. Thus, the present
study was undertaken to examine SOD1 and SOD2 activities and the
related Zn, Cu and Mn micronutrients levels, as well as SOA
generation rates in plasma, whole blood and placental tissue of RM
patients and their comparison with non-pregnant (NP) and HP women.
In addition, the present study examined the mRNA expression levels
of SOD1 in the placental tissue of RM patients compared to those of
HP women.
Materials and methods
Subjects
The current study population was the same one used
in our previous study (27), and
comprised three groups of women: Those who were NP (n=15), those
who were HP (n=25) and those who suffered from RM (n=25). NP and HP
subjects did not have a history of any reproductive disease and
subjects of all three groups were age-matched. Details regarding
age means, gestational age of RM patients at the time of abortion,
average number of abortions and hemoglobin values of all the
subjects were identical to those recorded in our previous study
(27). Blood and placental tissue
samples were collected shortly after delivery for HP women, or at
the time of abortion for RM patients. Ethics approval was obtained
from the Institutional Review Board of the College of Medicine,
King Saud University (project≠E-13-920, approval
letter≠14/4001/IRB, June 2014). Written informed consent was
obtained from each of the study subjects.
Sample collection and preparation
Venous blood sample were obtained from all the
subjects and placed into EDTA-chilled tubes for the assays used to
assess SOD1 and SOD2 activities and SOA generation, or into
polyethylene terephthalate tubes for Zn, Cu and Mn determination.
Plasma, whole blood suspensions and hemolysates and placental
tissue supernatants were prepared as previously described (27). Samples used for Zn, Cu, and Mn
analysis required preparation as described below.
SOD1 and SOD2 assays
SOD1 and SOD2 activities were assayed in all the
samples according to de Haan et al (29) and as previously modified by us
(30). Total SOD activity was
measured by the addition of previously 1:10 diluted plasma (250
µl), blood hemolysate (100 µl) or placental tissue
supernatant (50 µl) to xanthine (25 µl, 1.142 mg/ml),
hydroxyl ammonium chloride (25 µl), water (125 µl)
and xanthine oxidase (0.1 U/ml, 75 µl). The mixture was then
incubated at 25°C for 20 min, and sulphonilic acid (0.5 ml, 3.3
mg/ml) and α-naphthylamine (0.5 ml, 1 ng/ml) were added and further
incubated at a room temperature of 25°C for 20 min and absorbance
was read at 530 nm using an Ultrospec 2100 pro UV/VIS
Spectrophotometer (Amersham Biosciences, Piscataway, NJ, USA). The
addition of potassium cyanide (125 µl, 4 mM) instead of
water specifically inhibited SOD1 activity. Thus, subtraction of
the activity remaining after the addition of potassium cyanide
(SOD2) from total SOD activity yielded SOD1 activity.
SOA assay
SOA generation in all the samples was determined by
modification of the method of Johnston et al (31). Plasma (50 µl), blood
hemolysate (100 µl) or placental tissue supernatant (50
µl) were incubated for 5 min at 37°C with phosphate-buffered
saline (potassium phosphate 10 mM and sodium chloride 150 mM, pH
7.4, 1 ml) containing glucose (2 g/l) and fatty acid-free bovine
serum albumin (2 g/l) with or without SOD (30 µg, 50
µl). To initiate the reaction, ferricytochrome C solution
(1.2 mM, 100 µl) was added to the incubation mixture, and
the increase in absorbance was monitored at 550 nm in a recording
thermostated spectrophotometer (Shimatzu model UV-2401 PC, Dubai,
United Arab Emirates). SOA generation was determined by calculating
the difference between the sample without SOD and that with added
SOD. Tubes containing only buffer and ferricytochrome c were
incubated as mentioned above and run as blanks. The results were
expressed as the amount of reduced ferricytochrome c in nmol/min/ml
for plasma and whole blood or nmol/min/mg for placental tissue.
Zn, Cu and Mn assays
Plasma, blood hemolysate and placental supernatant
samples were freezedried at −45°C and pulverized. The samples were
than digested with ultra-pure nitric acid (0.5 ml, 68%) and
hydrogen peroxide (0.2 ml, 35%). Clear and colorless digests were
appropriately diluted with ultra-pure water and assayed for Zn, Cu
and Mn using inductively coupled plasma mass spectrometry (HP 4500;
Yokokawa Electric Co., Kanazawa, Japan) according to Osada et
al (32). Analytical accuracy
was checked using standards obtained from the National Institute of
Standards and Technology (Gaithersburg, MD, USA).
Gene expression profiling of hsSOD1 using
reverse transcription quantitative polymerase chain reaction
(RT-qPCR)
Placental tissue (100 mg), which had been freshly
collected and stored in RNAlater® RNA Stabilization
Solution (Qiagen, Hilden, Germany) at −80°C, was homogenized using
a TissueLyser LT (Qiagen) in 1.0 ml TRIzol® Reagent
(Invitrogen Life Technologies, Paisley, UK) and total RNA was
extracted according to standard procedures. The quality and
quantity of the purified RNA was determined by a Qubit®
2.0 flurometer using the Qubit RNA assay kit. To eliminate any
contaminating genomic DNA, a gDNA wipeout reaction was undertaken
in a wipeout buffer at 42°C for 2 min. cDNA was synthesized from
RNA (1 µg) in a final reaction volume (20 µl) using
the QuantiTect Reverse Transcriptase kit (QuantiTect®;
Qiagen), according to the manufacturer's instructions. The
resultant diluted cDNA (1:10, 5 µl), was used to perform
RT-qPCR by the QuantiTect SYBR-Green PCR kit (Qiagen) and the SOD
Qiagen Primer Assay (Hs_SOD_1_SG QuantiTect Primer assay,
QTO1664327) in a final reaction volume (25 µl) containing
the diluted cDNA sample (5 µl), 2X SYBR-Green PCR Master Mix
(12.5 µl), forward and reverse primer (10 µM stock,
2.5 µl) and RNAase-free water (2.5 µl).
Following an initial polymerase activation at 95°C
for 10 min, the samples were subjected to 40 cycles of denaturation
at 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. Following
amplification, the specificity of the product was assessed from a
melting curve program in a three-segment cycle of 95°C for 0 sec,
65°C for 15 sec and 95°C for 0 sec at the continuous acquisition
mode. Each placental tissue sample was represented by biological
replicas and three technical replicas, with the inclusion of a
no-template control. Raw data were analyzed using the Rotor-Gene
cycler software version 2.3 to calculate the threshold cycle using
the second derivative maximum. The fold change in each gene was
determined after normalization to the expression levels of 18 S as
a housekeeping gene as previously described (33).
Other assays and statistical
analysis
Total protein content of samples (20 µl), was
assayed according to Bradford (34). Drabkin's reagent was used to
measure hemoglobin concentration in blood hemolysates as previously
described (27).
Statistical analysis was performed using the
statistical package for social sciences software (SPSS version
17.0; SPSS, Inc., Chicago, IL, USA). Sample analysis was run in
duplicate for all the investigated parameters and results were
presented as means ± standard deviation. Values of the activities
and levels of individual parameters were compared between groups of
the study using one-way analysis of variance followed by the post
hoc Tukey-HSD test. P<0.05 was considered to indicate a
statistically significant difference.
Results
SOD1 and SOD2 activities and SOA
generation in plasma, whole blood and placental tissue of NP, HP
and RM women
Table I data
clearly indicate that SOD1 plasma and whole blood activities in HP
women were slightly decreased (6.11±0.73 nmol/min/ml and 542±74.7
U/g Hb, respectively), and much more significantly decreased in RM
patients (5.43±0.69 nmol/min/ml and 333±45.9 U/g Hb, respectively),
when both were compared to the enzyme's activities identified for
NP women (6.69±0.84 nmol/min/ml and 602±84.1 U/g Hb, respectively;
P<0.05 and P<0.0001). In addition, whereas SOD1 plasma
activity was significantly decreased in RM patients (5.43±0.69
nmol/min/ml) when compared to that in HP women (6.11±0,73
mol/min/ml; P<0.001), it was much more significantly lower in
whole blood of the same patients when compared to HP women
(333±45.9 against 542±74.7 U/g Hb; P<0.0001). A similar
reduction in the enzyme's placental tissue activity in RM patients
was observed following a comparison with that obtained in HP women
(1.81±0.23 against 3.42±0.44 µmol/min/mg protein;
P<0.0001). Similar to SOD1, plasma and whole blood SOD2
activities in HP women were moderately lower in HP women (5.12±0.66
nmol/min/ml and 460±60.2 U/g Hb, respectively), and much more
significantly lower in RM patients (4.50±0.55 nmol/min/ml and
278±37.7 U/g Hb, respectively), when the results were compared to
the enzyme's activities recorded for NP women (5.59±0.72
nmol/min/ml and 504±62.5 U/g Hb, respectively; P<0.05 and
P<0.0001).
 | Table ISuperoxide dismutase (SOD1 and SOD2)
activities and superoxide anion (SOA) generation in plasma, whole
blood and placental tissue of non-pregnant (NP), healthy pregnant
(HP) women and recurrent miscarriage (RM) patients. |
Table I
Superoxide dismutase (SOD1 and SOD2)
activities and superoxide anion (SOA) generation in plasma, whole
blood and placental tissue of non-pregnant (NP), healthy pregnant
(HP) women and recurrent miscarriage (RM) patients.
| Samples assayed and
units | Subjects
|
|---|
| NP women, n=15 | HP women, n=25 | RM patients,
n=25 |
|---|
| Plasma SOD1,
nmol/min/ml | 6.69±0.84 | 6.11±0.73a | 5.43±0.69b,c |
| Whole blood SOD1,
U/g Hb | 602±84.1 | 542±74.7a | 333±45.9b,d |
| Placental SOD1,
µmol/min/mg protein | – | 3.42±0.44 | 1.81±0.23d |
| Plasma SOD2,
nmol/min/mg | 5.59±0.72 | 5.12±0.66a | 4.50±0.55b,c |
| Whole blood SOD2,
U/g Hb | 504±62.5 | 460±60.2a | 278±37.7b,d |
| Placental SOD2,
μmol/min/mg protein | – | 2.86±0.37 | 1.51±0.20d |
| Plasma SOA,
nmol/ml | 178±22.2 | 197±24.7a | 223±27.80b,c |
| Whole blood SOA,
nmol/ml | 241±30.9 | 263±34.2a | 407±50.7b,d |
| Placental SOA,
nmol/min/mg | – | 28.1±3.58 | 47.7±5.96d |
Furthermore, although SOD2 plasma activity was
significantly decreased in RM patients (4.50±0.55 nmol/min/ml) when
compared to that in HP women (5.12±0.66 nmol/min/ml; P<0.001),
it was much more significantly decreased in whole blood of the same
patients when compared to HP women (278±37.7 against 460±60.2 U/g
Hb; P<0.0001). Similarly, SOD2 placental tissue activity in RM
patients was highly significantly lower than that identified in HP
women (1.51±0.20 against 2.86±0.37 µmol/min/mg protein;
P<0.0001).
Concurrent with the above decrease in SOD1 and SOD2
activities, Table I data indicate
that SOA generation levels under-went slight but significant
increases in the plasma and whole blood of HP women (197±24.7 and
263±34.2 nmol/ml, respectively), and much more significant
increases in RM patients (223±27.8 and 407±50.7 nmol/ml,
respectively) when both were compared to those obtained for NP
women (178±22.2 and 241±30.9 nmol/ml, respectively) (P<0.05 and
P<0.0001). In addition, although plasma SOA levels were
significantly increased in RM patients (223±27.8 nmol/min) when
compared with those generated in HP women (197±24.7 nmol/min;
P<0.001), the whole blood SOA levels of the same patients were
much more significantly increased (407±50.7 nmol/ml) when compared
to those of HP women (263±34.2 nmol/ml; P<0.0001). Furthermore,
the placental tissue SOA levels of RM patients (47.7±5.96
nmol/min/mg) were significantly increased when compared to those
documented for HP women (28.1±3.58 nmol/min/mg; P<0.0001).
Zn, Cu and Mn levels in plasma, whole
blood and placental tissue of NP, HP and RM women
Parallel to the reduced SOD1 and SOD2 levels
identified above, Table II data
clearly show that, whereas plasma and whole blood Zn levels of HP
women were slightly decreased (4.11±0.49 and 5.39±0.70
µmol/l, respectively), these levels were much more
significantly reduced in RM patients (3.67±0.39 and 3.50±0.46
µmol/l, respectively) when compared to those documented for
NP women (4.47±0.58 and 5.89±0.77 µmol/l, respectively;
P<0.05 and P<0.0001). In addition, although plasma Zn levels
were significantly reduced in RM patients (3.67±0.39 µmol/l)
when compared to those in HP women (4.11±0.49 µmol/l;
P<0.001), the whole blood Zn levels of the same patients
(3.50±0.46 µmol/l) were much more significantly decreased
than those recorded for HP women (5.39±0.70 µmol/l;
P<0.0001). Similarly the placental Zn levels of RM patients were
very significantly reduced (466±60.5 nmol/g) when compared to those
obtained for HP women (685±85.8 nmol/g; P<0.0001).
 | Table IIZinc (Zn), Copper (Cu) and Manganese
(Mn) levels in plasma, whole blood and placental tissue of
non-pregnant (NP), healthy pregnant (HP) women and recurrent
miscarriage (RM) patients. |
Table II
Zinc (Zn), Copper (Cu) and Manganese
(Mn) levels in plasma, whole blood and placental tissue of
non-pregnant (NP), healthy pregnant (HP) women and recurrent
miscarriage (RM) patients.
| Samples assayed and
units | Subjects
|
|---|
| NP women, n=15 | HP women, n=25 | RM patients,
n=25 |
|---|
| Plasma Zn,
µmol/l | 4.47±0.58 | 4.11±0.49a | 3.67±0.39b,c |
| Whole blood Zn,
µmol/l | 5.89±0.77 | 5.39±0.70a | 3.50±0.46b,d |
| Placental tissue
ZnSOD1, nmol/g | – | 685±85.8 | 466±60.5d |
| Plasma Cu,
µmol/l | 31.6±3.79 | 28.8±3.42a | 25.60±3.25b,c |
| Whole blood Cu,
µmol/l | 41.3±5.16 | 37.8±4.54a | 24.50±2.93b,d |
| Placental tissue
Cu, nmol/g | – | 108±13.70 | 67.40±8.09d |
| Plasma Mn,
nmol/l | 53.9±6.47 | 48.9±5.63a | 43.70±5.10b,c |
| Whole blood Mn,
nmol/l | 69.3±9.00 | 63.1±8.07a | 40.50±5.09b,d |
| Placental tissue
Mn, nmol/g | – | 7.49±0.89 | 5.42±0.68d |
Cu level variations in the analyzed samples
exhibited similar patterns and statistical magnitudes as those
observed for Zn (Table II).
Plasma and whole blood Cu levels of HP women were slightly reduced
(28.8±3.42 and 37.8±4.54 µmol/l, respectively), and much
more significantly lowered in RM patients (25.60±3.25 and
24.50±2.93 µmol/l, respectively) when compared to NP women
(31.6±3.79 and 41.3±5.16 µmol/l, P<0.05 and P<0.0001,
respectively). Furthermore, although plasma Cu levels were
significantly reduced in RM patients (25.60±3.25 µmol/l)
when compared to HP women (28.8±3.42 µmol/l; P<0.001),
the whole blood Cu levels of RM patients (24.50±2.93 µmol/l)
were much more significantly lower than those observed for HP women
(37.8±4.54 µmol/l; P<0.0001). Similarly, the placental
tissue Cu levels of RM patients were significantly lower
(67.40±8.09 nmol/g) when compared to those for HP women (108±13.70
nmol/g; P<0.0001).
Results shown in Table
II also indicate that plasma and whole blood Mn levels in HP
women were slightly lower (48.9±5.63 and 63.1±8.07 nmol/l,
respectively), and much more significantly decreased in RM patients
(43.70±5.10 and 40.50±5.09 nmol/l, respectively) following a
comparison of both those observed for NP women (53.9±6.47 and
69.3±9.00 nmol/l, respectively; P<0.05 and P<0.0001). In
addition, although plasma Mn levels were significantly lower in RM
patients (43.70±5.10 nmol/l) when compared to those observed in HP
women (48.9±5.63 nmol/l; P<0.001), the whole blood Mn levels of
the same patients (40.5±5.09 nmol/l) were much more significantly
decreased than those recorded for HP women (63.1±8.07 nmol/l;
P<0.0001). Similarly, the placental tissue Mn levels of RM
patients were significantly decreased (5.42±0.68 nmol/g) when
compared to those of HP women (7.49±0.89 nmol/g; P<0.0001).
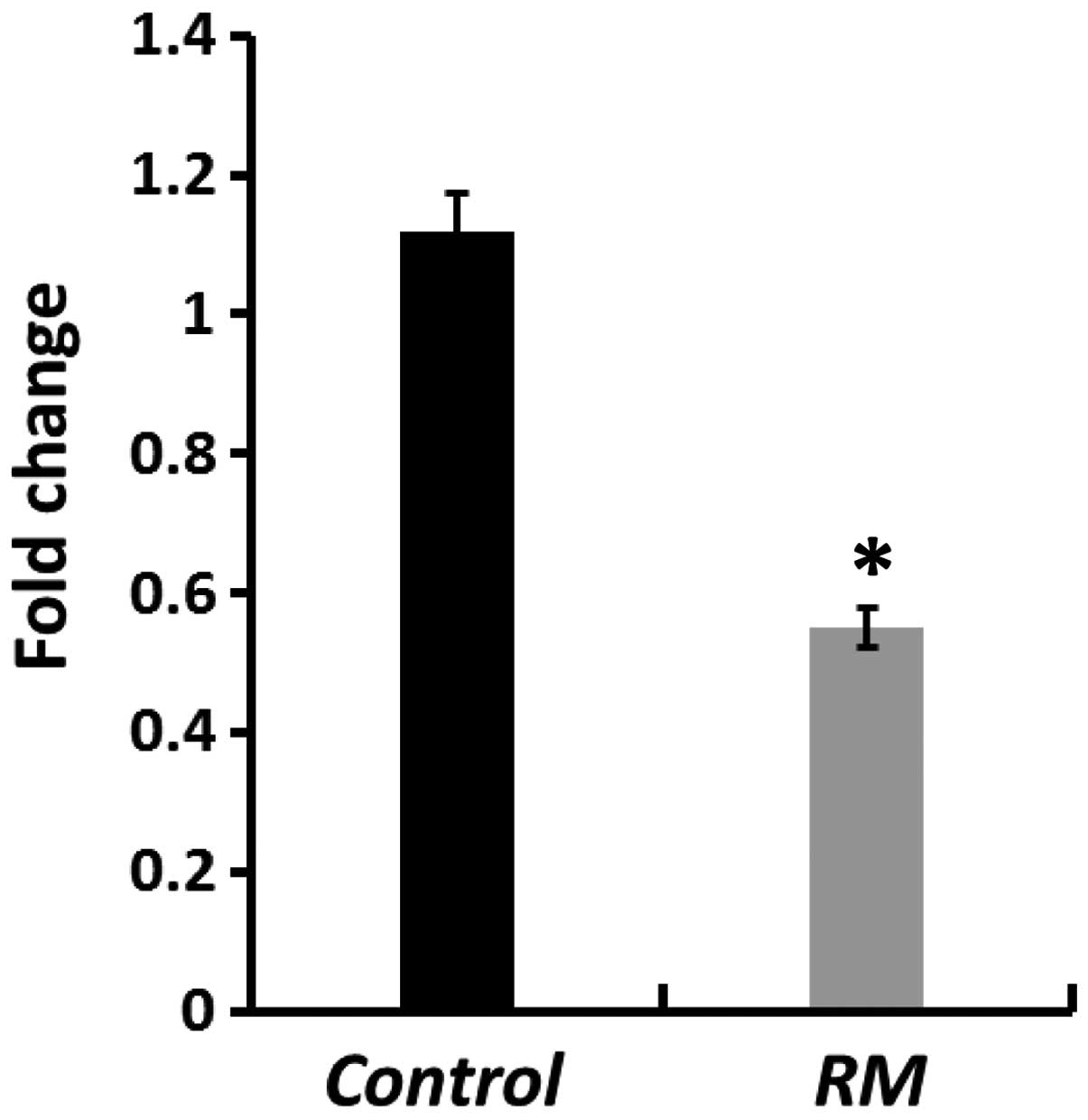
Gene expression of hsSOD1 in placental
tissue of HP and RM women
Fig. 1 shows that,
the intracellular hsSOD1 transcripts were found significantly
downregulated by almost 54% in placental tissues of RM patients as
compared to HP women (P<0.0001).
Discussion
Pregnancy presents a condition of metabolic
challenge for the mother and the developing fetus, and has been
associated with increased OS in HP women compared to the NP state
(27,35). The placenta is regarded as a key
source of this OS (36), as it has
been shown to exhibit low antioxidant enzyme activities especially
during the first trimester, making the syncytotrophoblast
vulnerable to oxygen-mediated damage and increased apoptosis
(22). At commencement of the
second trimester, oxygen tension increases 3-fold in the
intervillous space with the onset of maternal arterial flow thus
causing an increase in placental OS (36). This oxidative injury alters
placental remodeling and function and affects the course of
gestation. Abnormal placentation and the resultant endothelial
dysfunction (37) have been shown
to cause pregnancy disorders including RM (2,14,18–22).
In healthy pregnancy, however, ROS generation is controlled by
enzymatic and non-enzymatic antioxidants and OS is minimized
(27), ensuring healthy placental
function and normal development, growth and delivery of the fetus.
By contrast, recent findings (27), have shown significantly decreased
activities and levels of enzymatic and non-enzymatic antioxidants
including SOD, glutathione peroxidase, catalase, glutathione
reductase, reduced glutathione, selenium, vitamins A and E and β
carotene, which was coupled with an increased generation of
oxidants including hydrogen peroxide, lipoperoxides and oxidized
glutathione in plasma and/or whole blood and placental tissue of RM
patients in comparison with HP women (23–27).
Antioxidant and oxidant status have been suggested
as useful tools in the assessment of oxidative damage, and may be
used in the design of strategies for the management of OS-related
pregnancy disorders. To this end, the present study was undertaken
to examine SOD1 and SOD2 activities and SOA levels in plasma, whole
blood and placental tissue of RM patients in comparison with those
obtained in NP and HP women. Antioxidant defenses are initiated by
quenching and removal of SOA through SOD activity, which catalyzes
the dismutation of the anion into hydrogen peroxide, followed by
the action of catalase and glutathione peroxidase which further
degrade hydrogen peroxide into water. Thus, SOD activity is
regarded as a first line of defense and has a fundamental role in
combating OS. Although hydrogen peroxide is not a free radical, it
is able to penetrate cell membranes and interact with SOA leading
to the generation of the more toxic and reactive hydroxyl radicals
via the Haber-Weiss and Fenton reactions (38,39).
The latter reacts with purines and pyrimidines causing DNA damage
(40). Results of the present
study indicated significant increases in SOA generation in the
plasma of RM patients, and much more significant ones in whole
blood and placental tissue of the same patients compared to HP
women (P<0.001 and P<0.0001, respectively). This result was
likely caused by the parallel equally significant decreases
identified in SOD1 and SOD2 activities. In a previous study
(27), we identified equally
significant increases in hydrogen peroxide generation rates in
whole blood and placental tissue of the same RM patients
investigated in concurrence with significantly lowered glutathione
peroxide and catalase activities. These increases in the SOA and
hydrogen peroxide concentrations may have resulted in the
generation of large amounts of hydroxyl ions, causing subsequent
reduced gene expression of the antioxidant enzymes, excessive OS
and miscarriage in these patients. Although many studies, including
our previous study, have demonstrated significantly decreased
glutathione peroxidase and catalase activities in plasma, whole
blood and/or placental tissue of RM patients in comparison with HP
women (23–27,41),
only two studies have examined total SOD activity in such patients
(24,28), and none were related to SOA levels,
thereby marking the importance of the findings of the present
study.
Pregnancy is regarded as an exceptional condition of
enhanced demand for various micronutrients and vitamins including
selenium, zinc, copper, manganese and vitamins C, E and B12
(42). Physiological and metabolic
changes of pregnant women as well as increased demands for these
micronutrients by the developing fetus, causes a decrease in their
bioavailability (42), and may
lead to pregnancy complications. Cu, Zn and Mn are important
cofactors of many enzymes including Cu/Zn-SOD (SOD1) and Mn-SOD
(SOD2), which protect the placenta from SOA generation and
initiation of OS (42). In the
current study, we were able to observe significant decreases in
plasma Zn and Cu levels, and much more significant ones in whole
blood and placental tissue of RM patients compared to HP women
(P<0.001 and <0.0001, respectively). In addition, the plasma
levels of these micronutrients underwent moderate but significant
decreases in HP women compared to NP women (P<0.05). These
findings are in agreement with those reported by other authors
(25,26,43,44).
In this context many authors have reported a decrease in plasma Zn
levels as pregnancy progresses especially since Zn is used for the
development of fetal brain (45,46).
By contrast, those authors reported significant increases in plasma
Cu concentration during pregnancy before returning to normal NP
values post-delivery. This result may be partly associated with the
fact that almost 96% of plasma Cu during pregnancy is bound to its
carrier protein ceruloplasmin which is synthesized in large
quantities in response to elevated estrogen levels or to counteract
anemia since this protein has ferroxidase properties (45–48).
In the current study, free cationic Zn and Cu levels underwent
significant decreases in whole blood and the placental tissue of RM
patients, which may have constituted a major causative factor for
the observed significant reduction in SOD1 activity, and the
simultaneous increase in SOA concentrations and OS, thus leading to
miscarriage. This total SOD activity was shown to decrease in
Cu-deficient embryos as compared to controls (49). Similar to Zn and Cu, Mn levels in
plasma, whole blood and placental tissue exhibited decreases of
equal magnitude in RM patients compared to HP women. We suggest
that these lowered levels may have resulted in decreased SOD2
activity and subsequent increases in SOA levels thereby inflicting
oxidative damage and miscarriage. However, these results could not
be compared owing to the absence of such reports in the literature.
However, whole blood Mn concentrations have been shown to be
reduced in women with fetal growth restriction, indicating that the
element may be important in maintaining fetal growth (50). In addition, findings of a previous
study showed lowered umbilical cord whole blood Mn levels in
neonates of pre-eclamptic women compared to controls (51).
Results of the present study revealed that the
expression level of the hsSOD1 gene encoding an antioxidant
enzymatic marker in the placental tissue of RM women relative to HP
women was significantly downregulated by almost 54%. The currently
observed depletion of SOD1 activity, and its lowered gene
expression levels may have been caused by a direct damaging effect
of the enzyme molecules or DNA damage incurred by the uncontrolled
generation of ROS in RM patients, thereby leading to the
accumulation of enzyme substrates and downregulation of
transcription and translation processes. To this end, the increased
generation of SOA to biologically dangerous levels, has been shown
to activate key cell hall-mark events including mitochondrial
alterations and DNA damage (4,41),
and may have in turn, triggered a programmed cell death (apoptotic)
process. In this context, SOD1 loss has been previously reported to
induce the phosphorylation of a DNA damage marker (γ-H2AX), and
upregulated p21, a target gene of p53, in fibroblasts (52). Notably, the SOD1-ablated
fibroblasts exhibited loss of mitochondrial membrane potential and
enhanced mitochondrial ROS generation (52).
In conclusion, the present study provided data that
revealed significant decreases in blood and placental tissue of
SOD1 and SOD2 activation, as well as markedly reduced placental
hsSOD1 gene expression levels in RM patients relative to HP
women. The resultant increased generation of SOA and subsequent OS
may have been a major causative factor of miscarriage. Although
many studies have reported on the importance of the bioavailability
of sufficient amounts of Cu, Zn and Mn as micronutrients necessary
for antioxidant function, placental development, cell division and
differentiation, embryogenesis, fetal growth and development, and a
healthy course and outcome of pregnancy, clinical trial studies
related to their supplemental use in preventing RM and other
pregnancy-related disorders are scarce, if not absent altogether.
In this context, the dietary intake of Cu in 19 to 24-year-old
women has been documented as generally below recommended levels and
may cause problems during pregnancy when requirements increase
(53). Furthermore, Mn is one of
the least investigated micronutrients and clinical trials regarding
its supplementary use are not available (53). In light of the results of the
present study, the supplementary use of Zn, Cu and Mn may be of
beneficial use in patients of RM prior to and throughout
pregnancy.
Acknowledgments
This study was financially supported by King Saud
University, Vice Deanship of Research Chairs.
References
|
1
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
2
|
Al-Gubory KH, Fowler PA and Garrel C: The
roles of cellular reactive oxygen species, oxidative stress and
antioxidants in pregnancy outcomes. Int J Biochem Cell Biol.
42:1634–1650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marnett LJ: Oxyradicals and DNA damage.
Carcinogenesis. 21:361–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aboul-Soud MA, Al-Othman AM, El-Desoky GE,
Al-Othman ZA, Yusuf K, Ahmad J and Al-Khedhairy AA:
Hepatoprotective effects of vitamin E/selenium against
malathion-induced injuries on the antioxidant status and
apoptosis-related gene expression in rats. J Toxicol Sci.
36:285–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pham-Huy LA, He H and Pham-Huy C: Free
radicals, antioxidants in disease and health. Int J Biomed Sci.
4:89–96. 2008.PubMed/NCBI
|
|
6
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tu BP and Weissman JS: Oxidative protein
folding in eukaryotes: Mechanisms and consequences. J Cell Biol.
164:341–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griendling KK, Sorescu D and Ushio-Fukai
M: NAD(P)H oxidase: Role in cardiovascular biology and disease.
Circ Res. 86:494–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Touyz RM, Chen X, Tabet F, Yao G, He G,
Quinn MT, Pagano PJ and Schiffrin EL: Expression of a functionally
active gp91phox-containing neutrophil-type NAD(P)H oxidase in
smooth muscle cells from human resistance arteries: Regulation by
angiotensin II. Circ Res. 90:1205–1213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raijmakers MT, Burton GJ, Jauniaux E, Seed
PT, Peters WH, Steegers EA and Poston L: Placental NAD(P)H oxidase
mediated superoxide generation in early pregnancy. Placenta.
27:158–163. 2006. View Article : Google Scholar
|
|
11
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liou W, Chang LY, Geuze HJ, Strous GJ,
Crapo JD and Slot JW: Distribution of CuZn superoxide dismutase in
rat liver. Free Radic Biol Med. 14:201–207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weisiger RA and Fridovich I: Mitochondrial
superoxide simutase. Site of synthesis and intramitochondrial
localization. J Biol Chem. 248:4793–4796. 1973.PubMed/NCBI
|
|
14
|
Branch DW, Gibson M and Silver RM:
Clinical practice. Recurrent miscarriage. N Engl J Med.
363:1740–1747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carrington B, Sacks G and Regan L:
Recurrent miscarriage: Pathophysiology and outcome. Curr Opin
Obstet Gynecol. 17:591–597. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ford HB and Schust DJ: Recurrent pregnancy
loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol.
2:76–83. 2009.PubMed/NCBI
|
|
17
|
Arredondo F and Noble LS: Endocrinology of
recurrent pregnancy loss. Semin Reprod Med. 24:33–39. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta S, Agarwal A, Banerjee J and Alvarez
JG: The role of oxidative stress in spontaneous abortion and
recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv.
62:335–347; quiz 353–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal A, Gupta S and Sikka S: The role
of free radicals and antioxidants in reproduction. Curr Opin Obstet
Gynecol. 18:325–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biri A, Kavutcu M, Bozkurt N, Devrim E,
Nurlu N and Durak I: Investigation of free radical scavenging
enzyme activities and lipid peroxidation in human placental tissues
with miscarriage. J Soc Gynecol Investig. 13:384–388. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burton GJ, Hempstock J and Jauniaux E:
Oxygen, early embryonic metabolism and free radical-mediated
embryopathies. Reprod Biomed Online. 6:84–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poston L and Raijmakers MT: Trophoblast
oxidative stress, antioxidants and pregnancy outcome - a review.
Placenta. 25(Suppl A): S72–S78. 2004. View Article : Google Scholar
|
|
23
|
Sane AS, Chokshi SA, Mishra VV, Barad DP,
Shah VC and Nagpal S: Serum lipoperoxides in induced and
spontaneous abortions. Gynecol Obstet Invest. 31:172–175. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Far M, El-Sayed IH, El-Motwally AE,
Hashem IA and Bakry N: Serum levels of TNF-alpha and antioxidant
enzymes and placental TNF-alpha expression in unexplained recurrent
spontaneous miscarriage. J Physiol Biochem. 65:175–181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Talat TK: The relationship between serum
copper, zinc and glutathione peroxidase with malondialdehyde in
women with unexplaianed recurrent miscarriage. Kufa Med J.
12:29–37. 2009.
|
|
26
|
Abdul-Barry J, Al-Rubai SA and Qasim QA:
Study of oxidant - antioxidant status in recurrent spontaneous
abortion. Thi-Qar Med J. 5:35–46. 2011.
|
|
27
|
Ghneim HK and Alshebly MM: Biochemical
markers of oxidative stress in Saudi women with recurrent
miscarriage. J Korean Med Sci. 31:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jenkins C, Wilson R, Roberts J, Miller H,
McKillop JH and Walker JJ: Antioxidants: their role in pregnancy
and miscarriage. Antioxid Redox Signal. 2:623–628. 2000. View Article : Google Scholar
|
|
29
|
de Haan JB, Cristiano F, Iannello R,
Bladier C, Kelner MJ and Kola I: Elevation in the ratio of
Cu/Zn-superoxide dismutase to glutathione peroxidase activity
induces features of cellular senescence and this effect is mediated
by hydrogen peroxide. Hum Mol Genet. 5:283–292. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Sheikh YA and Ghneim HK: The effect of
micronutrients on superoxide dismutase in senescent fibroblasts.
Cell Biochem Funct. 29:384–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnston RB Jr, Keele BB Jr, Misra HP,
Lehmeyer JE, Webb LS, Baehner RL and RaJagopalan KV: The role of
superoxide anion generation in phagocytic bactericidal activity.
Studies with normal and chronic granulomatous disease leukocytes. J
Clin Invest. 55:1357–1372. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osada H, Watanabe Y, Nishimura Y, Yukawa
M, Seki K and Sekiya S: Profile of trace element concentrations in
the feto-placental unit in relation to fetal growth. Acta Obstet
Gynecol Scand. 81:931–937. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saquib Q, Attia SM, Siddiqui MA,
Aboul-Soud MA, Al-Khedhairy AA, Giesy JP and Musarrat J:
Phorate-induced oxidative stress, DNA damage and transcriptional
activation of p53 and caspase genes in male Wistar rats. Toxicol
Appl Pharmacol. 259:54–65. 2012. View Article : Google Scholar
|
|
34
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morris JM, Gopaul NK, Endresen MJ, Knight
M, Linton EA, Dhir S, Anggård EE and Redman CW: Circulating markers
of oxidative stress are raised in normal pregnancy and
pre-eclampsia. Br J Obstet Gynaecol. 105:1195–1199. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Myatt L and Cui X: Oxidative stress in the
placenta. Histochem Cell Biol. 122:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cindrova-Davies T: Gabor Than Award
Lecture 2008: pre-eclampsia - from placental oxidative stress to
maternal endothelial dysfunction. Placenta. 30(Suppl A): S55–S65.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kehrer JP: The Haber-Weiss reaction and
mechanisms of toxicity. Toxicology. 149:43–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liochev SI: The mechanism of 'Fenton-like'
reactions and their importance for biological systems. A
biologist's view. Met Ions Biol Syst. 36:1–39. 1999.
|
|
40
|
Agarwal A: oxidants and antioxidants in
human fertility. Middle East Soc Fertil J. 9:187–197. 2004.
|
|
41
|
Yiyenoğlu ÖB, Uğur MG, Özcan HÇ, Can G,
Öztürk E, Balat Ö and Erel Ö: Assessment of oxidative stress
markers in recurrent pregnancy loss: A prospective study. Arch
Gynecol Obstet. 289:1337–1340. 2014. View Article : Google Scholar
|
|
42
|
Black RE: Micronutrients in pregnancy. Br
J Nutr. 85(Suppl 2): S193–S197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jameson S: Zinc status in pregnancy: the
effect of zinc therapy on perinatal mortality, prematurity, and
placental ablation. Ann N Y Acad Sci. 678:178–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pathak P, Kapoor SK, Kapil U, Joshi YK and
Dwivedi SN: Copper nutriture amongst pregnant women in a rural area
of India. Eastern J Med. 8:15–17. 2003.
|
|
45
|
Izquierdo Alvarez S, Castañón SG, Ruata
ML, Aragüés EF, Terraz PB, Irazabal YG, González EG and Rodríguez
BG: Updating of normal levels of copper, zinc and selenium in serum
of pregnant women. J Trace Elem Med Biol. 21(Suppl 1): 49–52. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu J, Yang H, Shi H, Shen C, Zhou W, Dai
Q and Jiang Y: Blood copper, zinc, calcium, and magnesium levels
during different duration of pregnancy in Chinese. Biol Trace Elem
Res. 135:31–37. 2010. View Article : Google Scholar
|
|
47
|
Alebic-Juretic A and Frkovic A: Plasma
copper concentrations in pathological pregnancies. J Trace Elem Med
Biol. 19:191–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shakour-Shahabi L, Abbasali-Zadeh S and
Rashtchi-Zadeh N: Serum level and antioxidant activity of
ceruloplasmin in preeclampsia. Pak J Biol Sci. 13:621–627. 2010.
View Article : Google Scholar
|
|
49
|
Keen CL, Uriu-Hare JY, Hawk SN, Jankowski
MA, Daston GP, Kwik-Uribe CL and Rucker RB: Effect of copper
deficiency on prenatal development and pregnancy outcome. Am J Clin
Nutr. 67(Suppl 5): S1003–S1011. 1998.
|
|
50
|
Vigeh M, Yokoyama K, Ramezanzadeh F,
Dahaghin M, Fakhriazad E, Seyedaghamiri Z and Araki S: Blood
manganese concentrations and intrauterine growth restriction.
Reprod Toxicol. 25:219–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jones EA, Wright JM, Rice G, Buckley BT,
Magsumbol MS, Barr DB and Williams BL: Metal exposures in an
inner-city neonatal population. Environ Int. 36:649–654. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lei XG, Zhu JH, McClung JP, Aregullin M
and Roneker CA: Mice deficient in Cu,Zn-superoxide dismutase are
resistant to acetaminophen toxicity. Biochem J. 399:455–461. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mistry HD and Williams PJ: The importance
of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev.
2011:8417492011. View Article : Google Scholar : PubMed/NCBI
|