Introduction
Cervical cancer is one of the most common types of
cancer among women, and is a major contributor to morbidity and
mortality rates in women worldwide (1,2).
Inducing cancer cell apoptosis has been a critical strategy in
cancer therapy, and has been the aim of several research groups.
Apoptosis is triggered predominantly through the extrinsic or
intrinsic caspase-dependent pathways, specifically caspase-8 and
-9, respectively (3,4). Mitochondria are the most important
sensors for apoptosis in the intrinsic caspase-dependent pathways
(5). There is also crosstalk
between the extrinsic and intrinsic caspase-dependent pathways, and
activation of caspase-8 transforms BH3 interacting-domain death
agonist (Bid) into truncated Bid, thereby promoting
mitochondrial-mediated caspase-9-dependent apoptosis (6,7).
Reactive oxygen species (ROS) also induce intrinsic apoptosis by
triggering DNA damage (8).
Synthetic double-stranded (ds)RNA, including
polyinosinic acid:polycytidylic acid, or poly (I:C), is a mimic of
viral dsRNA and is, therefore, a promising immune stimulant
candidate for vaccines directed against intracellular pathogens.
Previous investigation revealed that poly (I:C) suppresses the
growth of murine melanoma B16F10 cells (9). Studies have also reported that
combining poly (I:C) with the Toll-like receptor 9 agonist, CpG
oligodeoxynucleotide (ODN), results in a more marked pro-apoptotic
effect on human hepatocellular carcinoma cells, compared with using
either CpG ODN or poly (I:C) alone (10). Poly (I:C)-containing liposome
transfection promotes cell apoptosis in human hepatic carcinoma,
which correlates with the upregulation of retinoic acid-inducible
gene-I-like receptors (11).
However, the effect of poly (I:C) on apoptosis in cervical cancer
remains to be fully elucidated.
Interferons (IFNs) are multifunctional cytokines,
which regulate cellular and immune responses as well as antiviral
and antitumor activity (12). In
addition, IFNs have been generally considered to be
anti-proliferative proteins (13,14).
IFNs are divided into two groups: Type I IFNs and type II IFNs.
Type I IFNs (IFN-α and IFN-β) markedly inhibit tumor cell growth
and induce apoptosis in vitro and in vivo (15,16).
It has been reported that IFN-β inhibits glioma angiogenesis
through the downregulation of vascular endothelial growth factor
and the upregulation of IFN-inducible protein 10 (17). Low levels of constitutively
produced endogenous IFN-β are sufficient to restrict tumor
angiogenesis (18). Previous
investigation has shown that poly (I:C) transfection induces the
endogenous expression of IFN-β, which results in cell cycle arrest
in human renal carcinoma cells (19). However, whether IFN-β is involved
in poly (I:C) transfection-induced apoptosis in cervical cancer
remains to be elucidated.
In the present study, the effect and underlying
mechanisms of poly (I:C) transfection on the HeLa human cervical
cancer cell line were investigated. The present study aimed to
provide evidence supporting the potential use of poly (I:C) for the
treatment of cervical cancer.
Materials and methods
Cell line and cell culture
The HeLa human cervical cancer cell line was
purchased from American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and 2.5
µg/ml amphotericin B (Sangon Biotech Co., Ltd., Shanghai,
China) at 37°C in a 5% CO2 incubator. The medium was
replaced every 2 days.
Poly (I:C) transfection
For poly (I:C) transfection, 1×105 HeLa
cells were seeded in a 12-well plate and maintained at 37°C in a 5%
CO2 incubator for 15 h. A dose of 2 µl
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
was added to 100 µl of serum-free medium, following which
the mixture was incubated at room temperature for 5 min. At the
same time, 10 µl poly (I:C), purchased from Sigma-Aldrich
(St. Louis, MO, USA) was added to the 100 µl of serum-free
medium. Subsequently, the Lipofectamine™ 2000 and poly (I:C) were
mixed gently and incubated at room temperature for 20 min.
Following incubation, the cell culture medium was replaced with
serum-free medium, which was added to each well containing the
Lipofectamine™ 2000 and poly (I:C) mixture, and incubated at 37°C
for 4 h. Finally, the medium in each well was replaced with a fresh
serum-containing medium.
Analysis of cell apoptosis
Cell apoptosis was measured by flow cytometry using
an Annexin V-propidium iodide (PI) kit (cat. no. 556420; BD
Pharmingen, San Diego, CA, USA), according to the manufacturer's
protocol. Briefly, the cells were harvested and washed three times
with phosphate-buffered saline (PBS). Following centrifugation at
300 × g for 10 min at 4°C, the cells were resuspended in 500
µl binding buffer (0.1 M HEPES/NaOH, pH 7.4; 1.4 M NaCl; 25
mM CaCl2) containing 5 µl fluorescein
isothiocyanate-conjugated Annexin V, the mixture was incubated at
25°C in the dark for 10 min, following which 5 µl PI was
added. Finally, cell apoptosis was analyzed using flow cytometry
(FACSCalibur; BD Biosciences, San Jose, CA, USA) with CellQuest
software (BD Biosciences), with the results are expressed as a
percentage of the total cells counted.
Western blot analysis
The proteins were extracted from the cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Nantong, China). Western blot analyses were
performed, as previously reported (17). Briefly, total protein was
quantified using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology) and 40 µg protein per lane was separated by
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis prior
to electroblotting onto a nitrocellulose membrane (GE Healthcare,
Munich, Germany). Non-specific binding was blocked by incubating
the membrane with 5% non-fat milk in Tris-buffered-saline with
Tween (TBST; 10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.05% Tween-20)
at room temperature for 1 h. After blocking, the membrane was
incubated with various primary antibodies overnight at 4°C. The
antibodies used included the following: Mouse monoclonal
anti-cytochrome c (1:1,000; cat. no. ab13575; Abcam,
Cambridge, UK), rabbit polyclonal anti-cleaved caspase-9 (1:500;
cat. no. ab2325; Abcam) and -3 (1:500; cat. no. ab13847; Abcam),
rabbit polyclonal anti-IFN-β (1:400; cat. no. sc-83256; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal
anti-phosphorylated (p)-H2A.X (cat. no. 07-627; 1:1,500; EMD
Millipore, Billerica, MA, USA) and rabbit polyclonal anti-β-actin
(1:2,000; cat. no. ab59381; Abcam). The membrane was then incubated
at room temperature for 2 h with anti-mouse horseradish
peroxidase-conjugated (1:5,000) secondary antibodies (cat. no.
sc-2497), obtained from Santa Cruz Biotechnology Inc. The blots
were visualized by enhanced chemiluminescence (Amersham Pharmacia
Biotech Inc., Piscataway, NJ, USA) and normalized to β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was isolated from the HeLa cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total
RNA (1–2 µg) was reverse transcribed using
SuperScript® IV Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.). The RT-qPCR reactions were
performed on a Rotor-Gene RG-3000 Real-Time Thermal Cycler (Corbett
Research, Sydney, Australia) using a SYBR® Premix Ex
Taq™ II kit (Takara Biotechnology Co., Ltd., Dalian, China). PCR
primers specific for IFN-β were designed, as previously reported
(20): sense
5′-TTGAATGGGAGGCTTGAATA-3′ and antisense
5′-CTATGGTCCAGGCACAGTGA-3′. These primers were synthesized by
Takara Biotechnology Co., Ltd. The PCR procedure was as follows:
Polymerase activation for 30 sec at 95°C, 40 cycles of
amplification, each consisting of 95°C for 5 sec and 60°C for 20
sec, and 1 cycle of dissociation consisting of 95°C for 15 sec,
60°C for 30 sec and 95°C for 15 sec. All reactions were performed
in triplicate. Fluorescence data were analyzed using Rotor-Gene 6
software (version 6.0; Corbett Research). The mRNA expression
levels were calculated using the 2−ΔΔCq method (21), and were normalized to β-actin and
reported as arbitrary units.
Measurement of ROS
The generation of intracellular ROS generation in
the HeLa cells was evaluated in the homogenate using
5-(and−6)-carboxy-2′, 7′-dichlorohydrofluorescein diacetate
(carboxy-H2DCFDA; DCFH), which is a specific ROS-detecting
fluorescent dye. DCFH is sensitive to ROS, and can be oxidized to
the highly fluorescent dichlorofluorescein (DCF) (22). The protocol was performed,
according to a previous report (23). Briefly, 1×106 HeLa cells
were incubated with 10 µl DCFH (Sigma-Aldrich) for 30 min at
37°C in the dark. The cells were then washed twice in PBS and
analyzed using flow cytometry (Cytomics FC 500; Beckman Coulter,
Brea, CA, USA) or observed using a fluorescence microscope (BX53;
Olympus Corporation, Tokyo, Japan). The redox state of the samples
can be monitored by detecting increases in fluorescence.
Accumulation of DCF in the cells is measured by an increase in
fluorescence at 530 nm.
Mitochondrial membrane potential (ΔΨm)
assay
The mitochondrial ΔΨm in the cells was determined
according to a previous report (24). The HeLa cells were collected
following the different treatments in 6-well plates. Following
being washed twice with PBS, the cells were incubated with
MitoProbe™ 3, 3′-diethyloxacarbicyanine iodide using a Molecular
Probes DiOC 2 (3) kit (Thermo
Fisher Scientific, Inc.) at a concentration of 8 nM for 30 min at
37°C. These stained cells were examined using a flow cytometer
(FACSCalibur; BD Biosciences).
Caspase activity assay
The cell lysates were prepared following the
different treatments using cell lysis buffer (Beyotime Institute of
Biotechnology). Briefly, 5×106 cells were suspended in
50 µl chilled cell lysis buffer (20 mM Tris-HCl, pH 7.5; 150
mM NaCl; 1 mM Na2EDTA; 1 mM EGTA; 1% Triton; 2.5 mM
sodium pyrophosphate; 1 mM beta-glycerophosphate; 1 mM
Na3VO4; 1 µg/ml leupeptin) and
incubated on ice for 10 min. Following centrifugation at 14,000 × g
and 4°C for 10 min, the supernatant (cytosolic extract) was
transferred to a fresh tube and placed on ice, and 300 µg of
the protein was diluted in 50 µl cell lysis buffer. The
activity of caspase-3 was determined using a Caspase-3 Activity kit
(cat. no. C1116; Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. The assays were performed on 96-well
microtitre plates by incubating 10 µl cell lysate
protein/sample in 80 µl reaction buffer, containing 1%
NP-40, 20 mM Tris-HCl (pH 7.5), 137 mM Nad and 10% glycerol, and 10
µl caspase-3 substrate (acetyl-Asp-Glu-Val-Asp
p-nitroanilide; Ac-DEVDpNA; 2 mM; BioVision, Inc., Milpitas, CA,
USA). The lysates were incubated at 37°C for 4 h, following which
the samples were measured using an ELISA reader (Labsystems,
Helsinki, Finland) at an absorbance of 405 nm (25). Caspase-4 activity was measured
using a commercially available Caspase-4 Assay kit (cat. no. C1122;
Beyotime Institute of Biotechnology). The procedure was performed,
according to the manufacturer's protocol. Briefly, ~300 µg
protein was diluted in 50 µl cell lysis buffer.
Subsequently, 50 µl of 2X reaction buffer, containing 10 mM
dithiothreitol, was added to each sample. Finally, 5 µl of
the 4 mM LEVD-pNA substrate (final concentration, 200 µM;
BioVision, Inc.) was added and incubated at 37°C for 1.5 h. The
absorbance was measured in an ELISA reader (Labsystems, Helsinki,
Finland) at 405 nm. The caspase-9 activity assay was performed
using the caspase-9 Assay kit (cat. no. ab119508; Abcam), according
to the manufacture's protocol, and the samples were prepared using
the same method used for caspase-3, described above. Subsequently,
85 µl reaction buffer and 5 µl
Leu-Glu-His-Asp-p-nitroanilide (LEHD-pNA) were added to each
sample, and incubated at 37°C for 2 h. The absorbance was measured
in an ELISA reader (Labsystems, Helsinki, Finland) at 405 nm.
Statistical analysis
Data are presented as the mean ± standard deviation.
The results were analyzed using a two-tailed t-test or one-way
analysis of variance followed by Duncan's test to evaluate the
differences among groups. Statistical analysis was performed using
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Poly (I:C) transfection induces cervical
cancer cell apoptosis
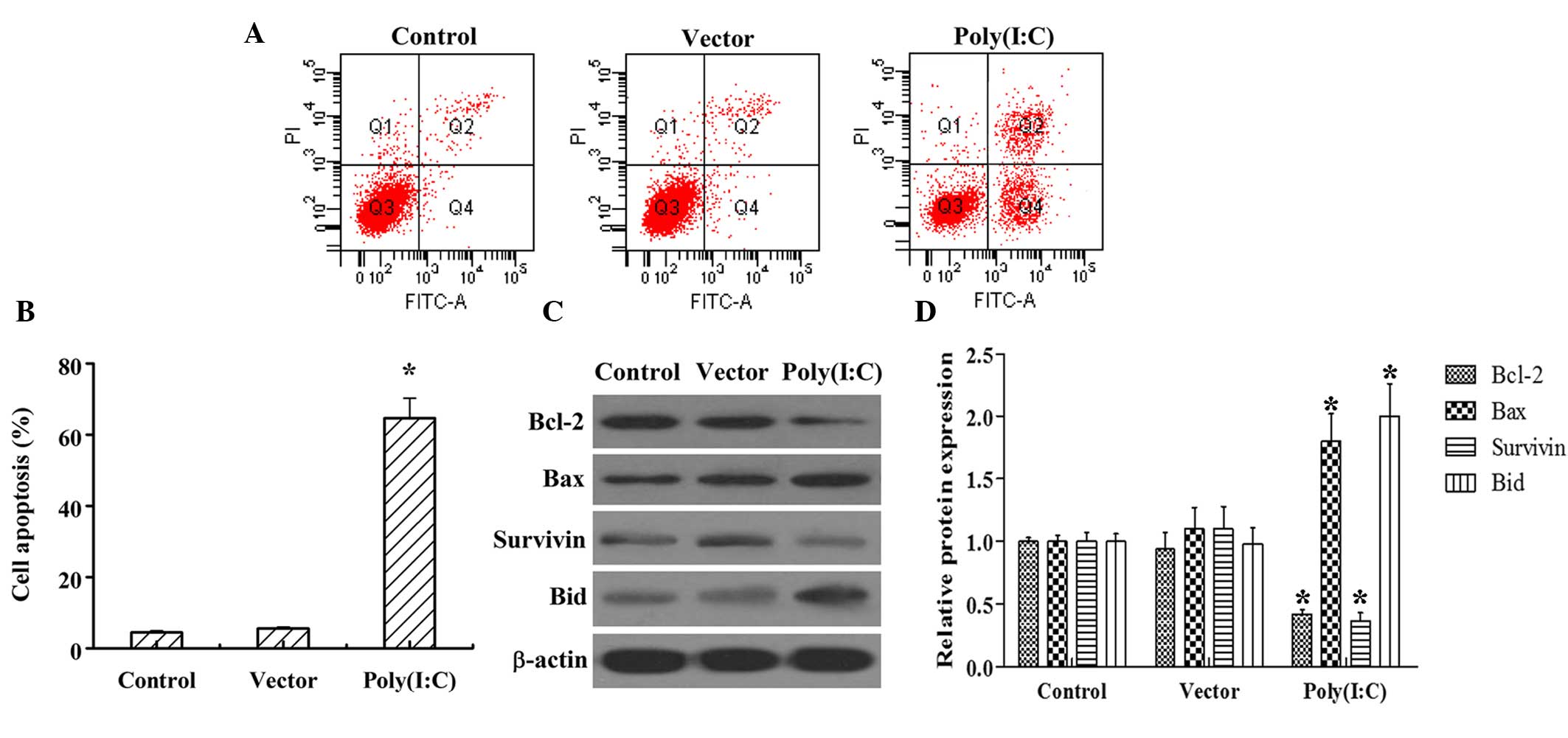
The effects of poly (I:C) transfected into the HeLa
cervical cancer cell line was first examined. Flow cytometry
following Annexin V/PI staining revealed that poly (I:C)
transfection increased the percentage of apoptosis cells between
4.5 and 65%, compared with those in the control group (Fig. 1A and B). In addition, poly (I:C)
transfection markedly increased the protein levels of the
pro-apoptotic Bax and Bid, whereas it decreased the protein levels
of anti-apoptotic Bcl-2 and Survivin, compared with the control
group. However, vector transfection had no marked effect on either
cervical cancer cell apoptosis or the protein levels of the
apoptosis-associated markers, Bacl-2, Bax, Survivin or Bid
(Fig. 1C and D).
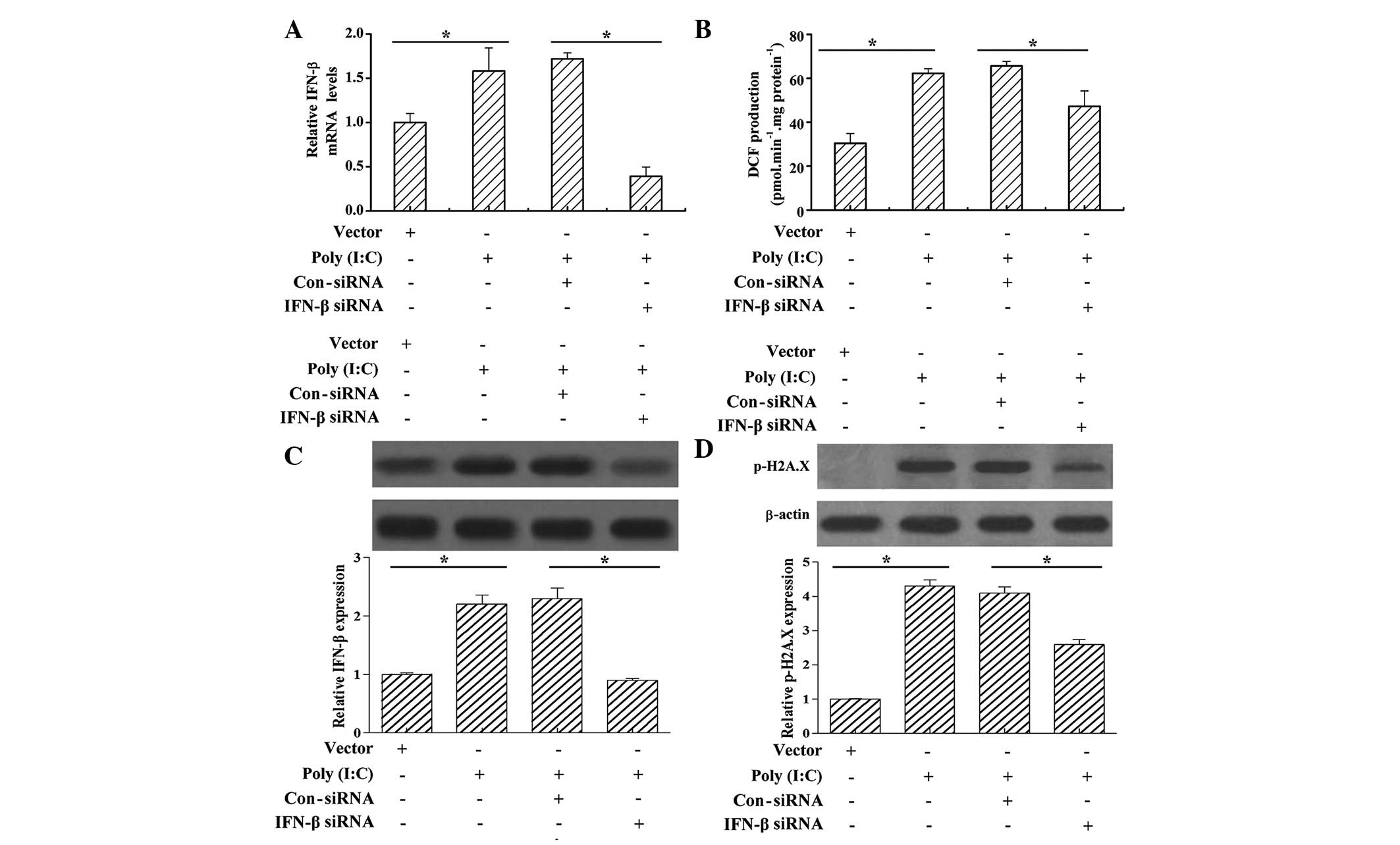
Poly (I:C) transfection increases the
mRNA and protein levels of IFN-β, the production of ROS and DNA
damage in cervical cancer cells
IFN-β has been reported to be involved in apoptosis
in cancer (26). In order to
elucidate whether IFN-β is involved in poly (I:C)
transfection-induced cervical cancer cell apoptosis, the present
study determined the mRNA and protein levels of IFN-β following
poly (I:C) transfection. It was found that vector transfection had
no significant effect on the mRNA and protein levels of IFN-β;
however, poly (I:C) transfection significantly promoted the mRNA
and protein levels of IFN-β, compared with the vector control
(Fig. 2A and C). An increase in
ROS stress can induce apoptosis in cancer cells (27); in order to investigate whether poly
(I:C) induced cancer cell apoptosis through the induction of
oxidative stress, the present study determined whether poly (I:C)
transfection triggered the generation of ROS. The results
demonstrated that the staining intensity of carboxy-H2DCFDA
increased significantly in the HeLa cells following poly (I:C)
transfection, compared with the vector control, and this increase
was inhibited by IFN-β siRNA treatment (Fig. 2B). Excessive ROS production has the
potential to damage cellular macromolecules, including DNA,
eventually leading to cell death (28). In order to investigate whether poly
(I:C) transfection also induced DNA damage, the present study
examined DNA damage by analyzing the phosphorylation levels of
γH2A.X at Ser 139. The western blot analyses showed that the levels
of p-γH2A.X increased in the HeLa cells following poly (I:C)
transfection; however, treatment with IFN-β siRNA subsequent to
poly (I:C) transfection decreased the levels of p-γH2A.X (Fig. 2D).
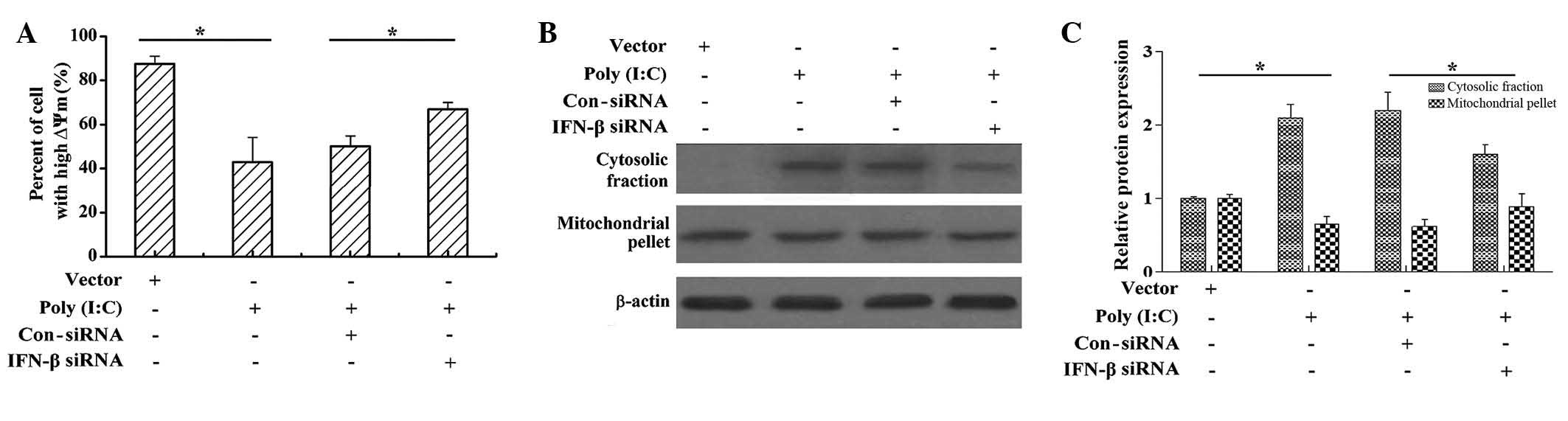
Poly (I:C) transfection increases
mitochondrial outer membrane permeabilization (MOMP) and cytochrome
c release in cervical cancer cells
MOMP is often required for activation of the caspase
proteases, which cause apoptotic cell death (29). To understand the underlying
mechanism by which poly (I:C) transfection induces apoptosis, as
well as the role of IFN-β in this process, the present study
investigated the change in ∆Ψm, which is the cause of MOMP.
Following poly (I:C) transfection, the number of cells exhibiting a
high ΔΨm decreased, compared with those transfected with the vector
(Fig. 3A); and IFN-β siRNA
treatment following poly (I:C) transfection markedly increased the
number of cells with a high ΔΨm. This data suggested that poly
(I:C) transfection may disrupt the ΔΨm, and that IFN-β is involved
in this process (Fig. 3A). To
further confirm the involvement of the mitochondrial signaling
pathway during poly (I:C) transfection-induced apoptosis, the
present study measured the release of cytochrome c from the
mitochondria into cytosol, a hallmark of mitochondria-mediated
apoptosis. As shown in Figs. 3B and
C, poly (I:C) transfection increased the content of cytosolic
cytochrome c and decreased the content of mitochondrial
cytochrome c; however, IFN-β siRNA treatment following poly
(I:C) transfection decreased the content of cytosolic cytochrome
c and increased the content of mitochondrial cytochrome
c. These results suggested that IFN-β attenuated the poly
(I:C)-induced release of cytochrome c from mitochondria into
cytosol.
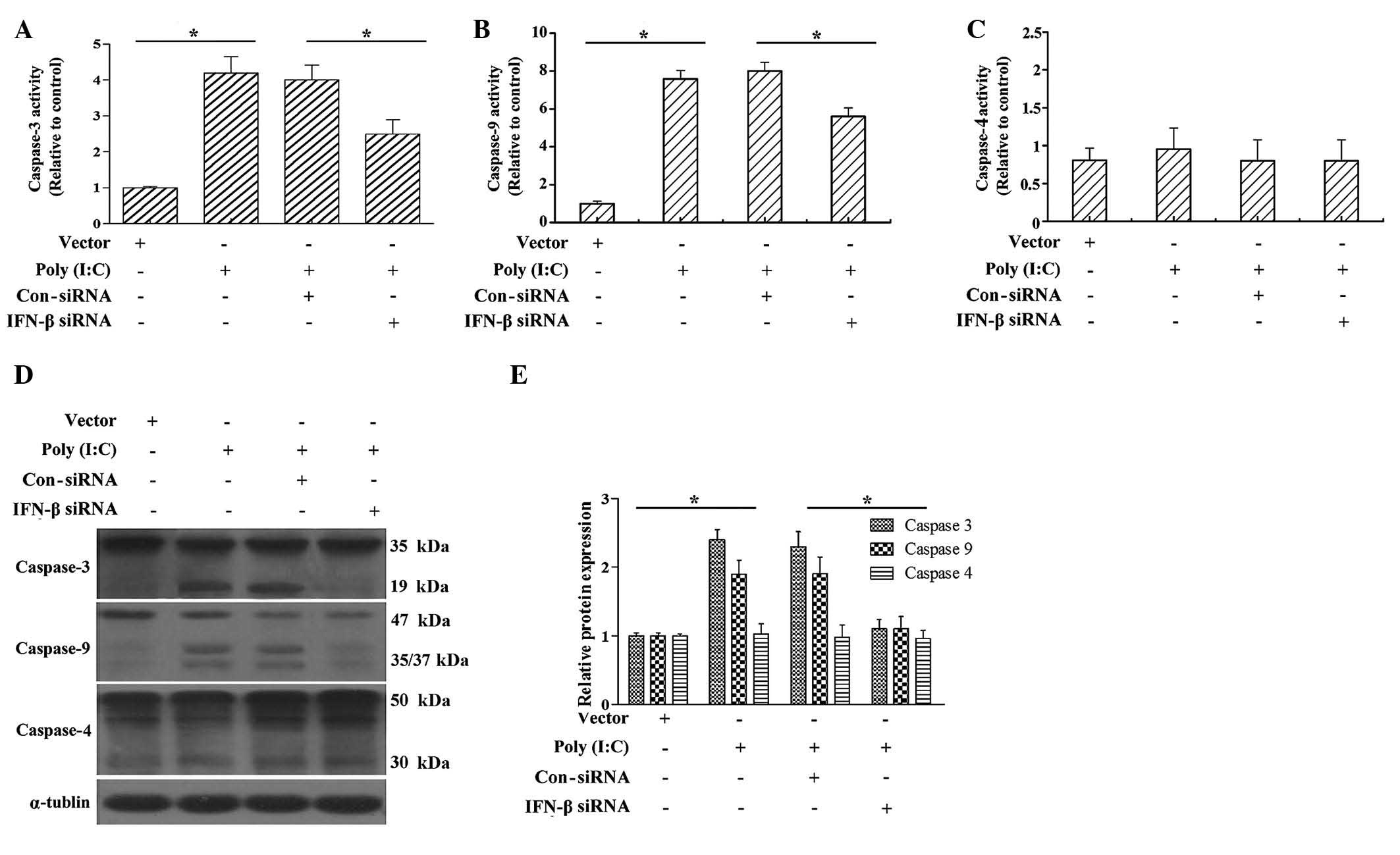
Poly (I:C) transfection induces caspase-9
and caspase-3 activation
The release of cytochrome c from the
mitochondria into the cytosol is a key event for caspase activation
(6). In order to further confirm
whether poly (I:C) induced caspase activation, and whether IFN-β
was involved in the process of caspase activation, the present
study subsequently examined caspase-9 and caspase-3 activity and
processing. As shown in Fig. 4,
poly (I:C) transfection significantly increased the activities of
caspase-3 and caspase-9, and IFN-β siRNA markedly decreased the
poly (I:C)-induced increases in caspase-3 and caspase-9 activity
(Fig. 4A and B). The results of
the western blot analysis showed that poly (I:C) transfection also
promoted the cleavage of caspase-3 and caspase-9.
Caspase-4 activation is required for endoplasmic
reticulum (ER) stress-induced apoptosis in human cells (30). In order to investigate whether poly
(I:C) transfection also induces cervical cancer cell apoptosis
through the ER-mediated pathway, caspase-4 activity and processing
were examined following poly (I:C) transfection. The results showed
that poly (I:C) transfection marginally increased caspase-4
activity, however, the difference was not significant when compared
with the vector-transfected group (Fig. 4C). In addition, IFN-β siRNA had no
significant effect on caspase-4 activity (Fig. 4C). These results suggested that
poly (I:C) induced the cervical cancer cell apoptosis predominantly
through the mitochondrial-mediated pathway.
IFN-β siRNA inhibits poly (I:C)
transfection-induced cervical cancer cell apoptosis
The results described above suggested that poly
(I:C) induced cervical cancer cell apoptosis, and that IFN-β was
involved in this progress. In order to confirm the involvement of
IFN-β in poly (I:C)-induced cervical cancer cell apoptosis, the
present study determined the levels of cervical cancer cell
apoptosis following poly (I:C) transfection and IFN-β siRNA
treatment. The results showed that poly (I:C) transfection
significantly induced cervical cancer cell apoptosis, compared with
vector transfection (Fig. 5).
However, IFN-β siRNA sharply attenuated the cervical cancer cell
apoptosis induced by poly (I:C) transfection (Fig. 5). These results demonstrated that
poly (I:C) induced cervical cancer cell apoptosis partly by
promoting the expression of IFN-β.
Discussion
Poly (I:C) is an analogue of dsRNA, which has been
demonstrated to be effective in antitumor immunotherapy (31,32).
Poly (I:C) had been reported to suppress murine B16F10 melanoma
growth (9) and induce apoptosis in
human hepatocellular carcinoma (10). The results of the present
demonstrated that poly (I:C) transfection induced apoptosis in the
HeLa cervical cancer cell line. The Bcl-2 family of proteins
consists of anti-apoptotic proteins, including Bcl-2 and Survivin,
and pro-apoptotic molecules, including Bax and Bid (33–35).
In the present study, it was also demonstrated that poly (I:C)
trans-fection was associated with the upregulation of Bax and Bid,
and downregulation of Bcl-2 and Survivin in the HeLa cells. These
results indicated that poly (I:C) transfection induced cervical
cancer cell apoptosis.
ROS accumulation has been shown to be important in
mediating apoptosis, and DNA damage is considered to be the most
common type of ROS-mediated damage (27). It has been reported that poly (I:C)
transfection induces ROS-triggered apoptosis in human renal cell
carcinoma (19). A similar
observation was made in the present study, in which poly (I:C)
transfection resulted in ROS production and DNA fragmentation,
which may be contributing factors in apoptosis of the HeLa cells.
Mitochondria are the major organelles for ROS production, and
excessive ROS accumulation contributes to cell/tissue injury or
death (36–38). In the intrinsic pathway, MOMP,
which leads to the release of proapoptotic proteins from the
mitochondrial intermembrane space, including cytochrome c,
promote caspase activation following their release from the
mitochondria into the cytosol (6).
The present study confirmed that poly (I:C) transfection decreased
∆Ψm in the HeLa cells, and induced the release of cytochrome
c from the mitochondria into the cytosol. The release of
cytochrome c induces an initiator caspase, for example
caspase-9 activation, which subsequently triggers the cleavage and
activation of caspase-3 and caspase-7 (6). The present study also demonstrated
that poly (I:C) transfection induced caspase-9 and caspase-3
activation in the HeLa cells. To elucidate whether poly (I:C)
transfection induced apoptosis via the ER stress-mediate apoptosis
pathway, caspase-4 activity and processing were examined, with the
results demonstrating that poly (I:C) transfection induced a
marginal effect on caspase-4 activation. Together, these results
suggested that the apoptosis of cervical cancer cells induced by
poly (I:C) was predominantly triggered via the mitochondrial
apoptotic pathway.
IFNs are a family of natural glycoproteins, which
consist of IFN-α, IFN-β and IFN-γ (17), and IFN-β has been reported to be
induced apoptosis in melanoma cell lines (26). In the present study, it was
demonstrated that poly (I:C) transfection induced the mRNA
expression of IFN-β in the HeLa cells. In addition, IFN-β knockdown
significantly attenuated poly (I:C) transfection-induced ROS
production, DNA damage, MOMP and cytochrome c release, as
well as caspase-9 and caspase-3 activation, in the HeLa cells.
These results suggested that IFN-β is likely to be involved in poly
(I:C)-induced aoptosis in HeLa cells. The results of the present
study also demonstrated that IFN-β knockdown significantly restored
poly (I:C) transfection-induced apoptosis in the HeLa cells. This
result confirmed that poly (I:C) transfection induced HeLa
apoptosis through the IFN-β signaling pathway.
In conclusion, the results of the present study
indicated that poly (I:C) contributed to the apoptosis of cervical
cancer cells via the mitochondrial apoptotic pathway. In addition,
poly (I:C) transfection regulated cervical cancer cell apoptosis
through the IFN-β signaling and the intrinsic mitochondrial
apoptotic pathway. However, the exact regulatory mechanism of poly
(I:C) and IFN-β in cervical cancer cells requires further
clarification. These findings indicate that poly (I:C) may be
considered a competitive candidate for the treatment of cervical
cancer.
References
|
1
|
Einstein MH, Schiller JT, Viscidi RP,
Strickler HD, Coursaget P, Tan T, Halsey N and Jenkins D:
Clinician's guide to human papillomavirus immunology: Knowns and
unknowns. Lancet Infect Dis. 9:347–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chauhan SC, Jaggi M, Bell MC, Verma M and
Kumar D: Epidemiology of human papilloma virus (hpv) in cervical
mucosa. Methods Mol Biol. 471:439–456. 2009. View Article : Google Scholar
|
|
3
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Y, Zhang J, Zhang Q, Chen P, Song J, Yu
S, Liu H, Liu F, Song C, Yang D and Liu J: Adenosine induces
apoptosis in human liver cancer cells through ROS production and
mitochondrial dysfunction. Biochem Biophys Res Commun. 448:8–14.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Bio. 11:621–632. 2010. View
Article : Google Scholar
|
|
7
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh KK: Mitochondria damage checkpoint,
aging and cancer. Ann NY Acad Sci. 1067:182–190. 2006. View Article : Google Scholar
|
|
9
|
Fujimura T, Nakagawa S, Ohtani T, Ito Y
and Aiba S: Inhibitory effect of the polyinosinic-polycytidylic
acid/cationic liposome on the progression of murine B16F10
melanoma. Eur J Immunol. 36:3371–3380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Lin A, Sui Q, Zhang C, Tian Z and
Zhang J: Phosphorothioate modification of the TLR9 ligand CpG ODN
inhibits poly (I:C)-induced apoptosis of hepatocellular carcinoma
by entry blockade. Cancer Lett. 355:76–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng S, Geng J, Sun R, Tian Z and Wei H:
Polyinosinic-pol ycytidylic acid liposome induces human hepatoma
cells apoptosis which correlates to the up-regulation of RIG-I like
receptors. Cancer Sci. 100:529–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sottini A, Capra R, Serana F, Chiarini M,
Caimi L and Imberti L: Interferon-beta therapy monitoring in
multiple sclerosis patients. Endocr Metab Immune Disord Drug
Targets. 9:14–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cha L, de Jong E, French MA and Fernandez
S: IFN-α exerts opposing effects on activation-induced and
IL-17-induced proliferation of T cells that may impair homeostatic
maintenance of CD4+ T cell numbers in treated HIV
infection. J Immunol. 193:2178–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong B, Li H, Lu Y, Zhang M, Zheng Y, Qian
J and Yi Q: USP18 is crucial for IFN-γ-mediated inhibition of B16
melanoma tumorigenesis and antitumor immunity. Mol Cancer.
13:1322014. View Article : Google Scholar
|
|
15
|
Ryu H, Oh JE, Rhee KJ, Baik SK, Kim J,
Kang SJ, Sohn JH, Choi E, Shin HC and Kim YM: Adipose
tissue-derived mesenchymal stem cells cultured at high density
express IFN-β and suppress the growth of MCF-7 human breast cancer
cells. Cancer Lett. 352:220–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuzuka T, Miller K, Pickel L, Doi C,
Ayuzawa R and Tamura M: The synergistic induction of
cyclooxygenase-2 in lung fibroblasts by angiotensin II and
pro-inflammatory cytokines. Mol Cell Biochem. 320:163–171. 2009.
View Article : Google Scholar
|
|
17
|
Takano S, Ishikawa E, Matsuda M, Yamamoto
T and Matsumura A: Interferon-β inhibits glioma angiogenesis
through downregulation of vascular endothelial growth factor and
upregulation of interferon inducible protein 10. Int J Oncol.
45:1837–1846. 2014.PubMed/NCBI
|
|
18
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harashima N, Minami T, Uemura H and Harada
M: Transfection of poly (I:C) can induce reactive oxygen
species-triggered apoptosis and interferon-β-mediated growth arrest
in human renal cell carcinoma cells via innate adjuvant receptors
and the 2–5A system. Mol Cancer. 13:2172014. View Article : Google Scholar
|
|
20
|
Siegal FP, Kadowaki N, Shodell M,
Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S and Liu YJ: The
nature of the principal type 1 interferon-producing cells in human
blood. Science. 284:1835–1837. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Laggner H, Hermann M, Gmeiner BM and
Kapiotis S: Cu2+ and Cu+ bathocuproine
disulfonate complexes promote the oxidation of the ROS-detecting
compound dichlorofluorescin (DCFH). Anal Biochem Chem. 385:959–961.
2006. View Article : Google Scholar
|
|
23
|
Yim HY, Yang Y, Lim JS, Lee MS, Zhang DE
and Kim KI: The mitochondrial pathway and reactive oxygen species
are critical contributors to interferon-α/β-mediated apoptosis in
Ubp43-deficient hematopoietic cells. Biochem Biophys Res Commun.
423:436–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rottenberg H and Wu S: Quantitative assay
by flow cytometry of the mitochondrial membrane potential in intact
cells. Biochim Biophys Acta. 1404:393–404. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Zhang SP and Cai YQ: Cytoprotective
effects of selenium on cadmium-induced LLC-PK1 cells apoptosis by
activating JNK pathway. Toxicol In Vitro. 21:677–684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chawla-Sarkar M, Leaman DW and Borden EC:
Preferential induction of apoptosis by interferon (IFN)-beta
compared with IFN-alpha2: Correlation with TRAIL/Apo2 L induction
in melanoma cell lines. Clin Cancer Res. 7:1821–1831.
2001.PubMed/NCBI
|
|
27
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Update. 7:97–110. 2004. View Article : Google Scholar
|
|
28
|
Hensley K, Robinson KA, Gabbita SP,
Salsman S and Floyd RA: Reactive oxygen species, cell signaling and
cell injury. Free Radical Bio Med. 28:1456–1462. 2000. View Article : Google Scholar
|
|
29
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C, Wei J, Li Y, He X, Zhou Q, Yan J,
Zhang J, Liu Y, Liu Y and Shu HB: Transmembrane Protein 214
(TMEM214) mediates endoplasmic reticulum stress-induced caspase 4
enzyme activation and apoptosis. J Biol Chem. 288:17908–17917.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pimm MV and Baldwin RW: Treatment of
transplanted rat tumours with double-stranded RNA (BRL 5907). II
Treatment of pleural and peritoneal growths. Br J Cancer.
33:166–171. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pimm MV, Embleton MJ and Baldwin RW:
Treatment of transplanted rat tumours with double-stranded RNA (BRL
5907). I Influenced of systemic and local administration. Br J
Cancer. 33:154–165. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang BH, Xia F, Pop R, Dohi T, Socolovsky
M and Altieri DC: Developmental control of apoptosis by the
immunophilin aryl hydrocarbon receptor-interacting protein (aip)
involves mitochondrial import of the survivin protein. J Biol Chem.
286:16758–16767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Croker BA, O'Donnell JA, Nowell CJ,
Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang
JG, et al: Fas-mediated neutrophil apoptosis is accelerated by Bid,
Bak and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci
USA. 108:13135–13140. 2011. View Article : Google Scholar
|
|
36
|
Kang J and Pervaiz S: Mitochondria: Redox
metabolism and dysfunction. Biochem Res Int. 2012:8967512012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoboue ED and Devin A: Reactive oxygen
species-mediated control of mitochondrial biogenesis. Int J Cell
Biol. 2012:4038702012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yadav N and Chandra D: Mitochondrial and
postmitochondrial survival signaling in cancer. Mitochondrion.
16:18–25. 2014. View Article : Google Scholar
|