Introduction
Cadmium (Cd) is the most widespread environmental
pollutant, which exerts diverse toxic effects in various tissues
and organs of humans and animals (1). The human population is exposed to Cd
toxicity through food, water and the air (2). The main sources of Cd include
agricultural and industrial pollution (3). Cd toxicity is dependent on its
biological characteristics, and it is defined as non-biodegradable
with an extensive biological half-life, particularly below the
earth (4).
It has previously been reported that exposure to low
doses of Cd [1–2 mg/kg body weight (BW)] predominantly affects the
testes, and no other organs (5).
The mechanism underlying Cd toxicity to the testes remains unclear.
One theory hypothesized that Cd toxicity induced failure in the
circulatory system due to vascular damage, particularly in the
testes, which may result in the inability to utilize zinc (6). Cd may therefore replace zinc in the
bloodstream of the testes. Zinc, manganese and selenium are
important for testicular function and fertility, whereas Cd, cobalt
and mercury are very toxic, and impair testicular function and
reduce fertility (7).
It is well-known that Cd toxicity induces oxidative
stress via the production of free radicals, which are harmful to
cells. Free radicals may damage protein, lipid, enzymes and DNA,
and therefore must be neutralized by antioxidants before entering
cells (8). Antioxidants serve as
potent scavengers for free radicals and prevent the occurrence of
disease (9). Recently, the use of
natural products and flavonoids, including curcumin and cinnamon,
for the treatment of diabetes and toxicity has been widely reported
due to their protective effects (10,11).
Such findings suggest that identifying herbal medicines for the
protection of antioxidants, and the treatment of toxicity and human
disease is required. Among such natural products recently
identified is the grape seed extract (GSE).
GSE is one of the most powerful antioxidants, which
contains high levels of bioflavonoids, vitamin C and vitamin E
(12). GSE protects cells from
damage by regulating cell oxidative damage (13), reducing organ injury, improving the
balance between oxidants and antioxidants, and reducing the release
of inflammatory mediators (14,15).
In addition, GSE has been reported to exert anticarcinogenic
effects (16). It is well-known
that the organs most affected by Cd are the testes, and the present
study aimed to determine the beneficial effects of GSE on human
health. Therefore, the present study was designed to examine the
possible ameliorative and protective effects of GSE against chronic
Cd-induced testicular dysfunction.
Materials and methods
Materials
CdCl2, diethyl ether, ethidium bromide
and agarose were purchased from Sigma-Aldrich (St. Louis, MO, USA).
GSE was purchased from GNC (Jeddah, Saudi Arabia). The adult male
Wistar rats (age, 3 months; weight, 250–280 g) were obtained from
King Fahd Center for Scientific Research, King Abdulaziz University
(Jeddah, Saudi Arabia). Kits for the detection of catalase,
glutathione reductase (GSH-R), glutathione peroxidase (GSH-Px) and
malondialdehyde (MDA) were purchased from Bio-Diagnostic (Giza,
Egypt). The DNA ladder (100 bp) was purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). QIAzol reagent and oligo dT
primers were obtained from Qiagen, Inc. (Valencia, CA, USA).
Animal treatments and experimental
design
All procedures for animal handling and treatments
were approved by the Ethical Committee Office of the Scientific
Deanship of Taif University (Taif, Saudi Arabia). A total of 40
male adult Wistar rats (n=10/group; age, 3 months; weight, 250–280
g) were used in the present study. For acclimation, the rats were
handled manually for 2 weeks prior to the experiment. The rats were
maintained under a light/dark cycle (12–12 h) and received ad
libitum access to food and water. The healthy male Wistar rats
were randomly divided into four groups (n=10/group) as follows:
Control group, which received a balanced diet; Cd chloride
(CdCl2) group, which orally received 5 mg
CdCl2/kg BW/day dissolved in water, based on a previous
study (17); GSE group, which
orally received GSE (400 mg/kg BW/day) dissolved in water (18); and the protective group, which
orally received a mixture of GSE and CdCl2 dissolved in
water as described for each individual group. All treatments were
administered for 3 consecutive months. At the end of the
experiment, the rats were sacrificed by inhalation of diethyl
ether. Blood samples were collected for the extraction of serum by
centrifugation for 10 min at 4,000 × g, and testicular tissues were
collected for RNA extraction and immunohistochemistry.
Serum testosterone assays
Alterations in the serum levels of testosterone were
measured using a commercial kit (Testosterone ELISA kit; cat. no.
ab108666; Abcam, Tokyo, Japan) that was purchased from CliniLab
(Cairo, Egypt). All kit procedures were followed as stated in the
manufacturer's protocol.
Serum antioxidant levels
Serum levels of catalase, GSH-R, GSH-Px and MDA were
measured spectrophotometrically using specific commercial kits. The
assays were conducted according to the manufacturer's
protocols.
Testicular histopathology and
immunohistochemistry
Sections (4 µM) were cut using a microtome
from the left and right testes of the rats, and were processed for
general histological staining using hematoxylin and eosin stain
(Sigma-Aldrich), based on previously stated protocols (19). The sections were deparaffinized in
xylene, and were dehydrated in various ascending concentrations of
ethanol. Endogenous peroxidase activity was blocked with 3%
hydrogen peroxide for 15 min. After heating for 20 min in 0.01
mol/l citrate buffer using a microwave pressure cooker, the
sections were allowed to cool to room temperature. Blocking of
non-specific binding was conducted using normal horse serum
(Sigma-Aldrich) for 20 min at room temperature. Subsequently, the
testicular sections were incubated with the following primary
antibodies for 30 min: Anti-B-cell lymphoma 2-associated X protein
(Bax; sc-493; mouse monoclonal; clone no. 2D2; Thermo Fisher
Scientific, Inc.) and anti-Ki-67 (sc-7846; Dako, Glostrup, Denmark)
at dilutions of 1:1,000 for each antibody. Subsequently, the
immunoperoxidase technique [avidin-biotin complex (ABC) kit; Lab
Vision Corporation, Fremont, CA, USA) was used to stain the
testicular sections, and the binding sites were detected with ABC
chromogen. For rinsing between each step, phosphate-buffered saline
was used. Finally, all sections were counterstained with
hematoxylin and eosin stain and images were viewed microscopically
(Wolfe-S90982; Carolina Biological Supply Co., Burlington, NC, USA)
and images captures using a Canon Power-Shot SX500 IS digital
camera (Canon, Inc., Tokyo, Japan) (20).
Analysis of gene expression
Total RNA was extracted from testicular samples
using QIAzol reagent as previously described (10,11).
Integrity of the RNA was visualized using 1.5% denatured agarose
gel electrophoresis, followed by ethidium bromide staining. Total
RNA (2 µg) in a total volume of 11 µl, together with
0.5 ng oligo dT primers and sterilized diethylpyrocarbonate (DEPC)
water, was used to synthesize cDNA. Briefly, the mixture was
incubated in a T100™ Thermal Cycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 70°C for 10 min for denaturation.
Subsequently, 2 µl 10X reverse transcription (RT)-buffer,
100 U Moloney Murine Leukemia Virus Reverse Transcriptase (Thermo
Fisher Scientific, Inc.), 1 µl 10 mM dNTPs and 5 µl
DEPC water was added (total volume, 20 µl). This mixture was
then incubated in the thermal cycler at 37°C for 1 h, and at 70°C
for 10 min in order to induce inactivation of the enzyme. For
RT-polymerase chain reaction (PCR) analysis, specific primers were
used (Table I), which were
designed using Oligo-4 software and were synthesized by Macrogen,
Inc. (Seoul, South Korea). In a total volume of 25 µl [1
µl synthesized cDNA, 1 µl each primer (10 pM), 12.5
µl PCR master mix (Promega Corporation, Madison, WI, USA)
and 9.5 µl sterilized deionized water], PCR was conducted.
The PCR cycling conditions were set as follows: Initial
denaturation for 1 cycle at 95°C for 4 min, followed by 27 cycles
(each consisting of denaturation at 94°C for 1 min, annealing as
stated in Table I for each gene,
and extension at 72°C for 1 min) with a final extension step at
72°C for 7 min. G3PDH was used as an internal control. PCR products
were separated by 1.5% agarose gel electrophoresis for 30 min (Bio
Basic Inc., Markham, ON, Canada) and were stained with ethidium
bromide in Tris-borate-EDTA buffer. The gels were visualized under
ultraviolet light and subsequently photographed using an InGenius
version 3.0 gel documentation system (Syngene, Cambridge, UK). The
band intensities were densitometrically quantified and calculated
using ImageJ software version 1.47 (http://imagej.en.softonic.com/).
 | Table IPCR conditions and primer sequence of
the indicated genes. |
Table I
PCR conditions and primer sequence of
the indicated genes.
| Gene | Product size
(bp) | Annealing temp.
(°C) | Direction | Sequence
(5′-3′) |
|---|
| GST | 575 | 55 | Sense |
GCTGGAGTGGAGTTTGAAGAA |
| | | Antisense |
GTCCTGACCACGTCAACATAG |
| SOD | 410 | 55 | Sense |
AGGATTAACTGAAGGCGAGCAT |
| | | Antisense |
TCTACAGTTAGCAGGCCAGCAG |
| P450scc | 688 | 55 | Sense |
CGCTCAGTGCTGGTCAAAA |
| | | Antisense |
TCTGGTAGACGGCGTCGAT |
| P450c17 | 302 | 55 | Sense |
GACCAAGGGAAAGGCGT |
| | | Antisense |
GCATCCACGATACCCTC |
| 3β-HSD | 547 | 55 | Sense |
CCGCAAGTATCATGACAGA |
| | | Antisense |
CCGCAAGTATCATGACAGA |
| 17β-HSD | 653 | 55 | Sense |
TTCTGCAAGGCTTTACCAGG |
| | | Antisense |
ACAAACTCATCGGCGGTCTT |
| AR | 570 | 55 | Sense |
TTACGAAGTGGGCATGATGA |
| | | Antisense |
ATCTTGTCCAGGACTCGGTG |
| FSHR | 490 | 55 | Sense |
GAGTCATCCCGAAAGGATCA |
| | | Antisense |
TAAAATGACTGGCCCAGAGG |
| StAR | 389 | 58 | Sense |
TTGGGCATACTCAACAACCA |
| | | Antisense |
ATGACACCGCTTTGCTCAG |
| G3PDH | 309 | 52 | Sense |
AGATCCACAACGGATACATT |
| | | Antisense |
TCCCTCAAGATTGTCAGCAA |
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Experiments were repeated 3 times. One-way analysis of
variance and Fisher post-hoc descriptive test were used to analyze
the data using SPSS software version 11.5 for Windows (SPSS, Inc.,
Chicago, IL, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
Protective effects of GSE on
CdCl2-induced alterations in the serum levels of
testosterone
Serum testosterone levels were 0.52±0.2 ng/ml in the
control rats. Treatment with CdCl2 significantly
decreased the testosterone levels (0.07±0.02 ng/ml). Notably, GSE
increased testosterone levels (3.4±0.39 ng/ml). Co-treatment with
GSE and CdCl2 exhibited a protective effect, and the
testosterone levels were significantly increased in this group,
compared with the CdCl2-treated rats (P<0.05;
3.6±0.42 ng/ml).
Protective effects of GSE on
CdCl2-induced alterations in the serum and mRNA
expression levels of antioxidants and MDA
Treatment with CdCl2 for 3 months
resulted in a significant decrease in the levels of catalase, GSH-R
and GSH-Px (P<0.05; Table II).
In addition, CDCl2 significantly increased the levels of
MDA. Co-treatment with GSE and CdCl2 inhibited and
normalized these alterations. Treatment with GSE alone exhibited
good antioxidant activity (Table
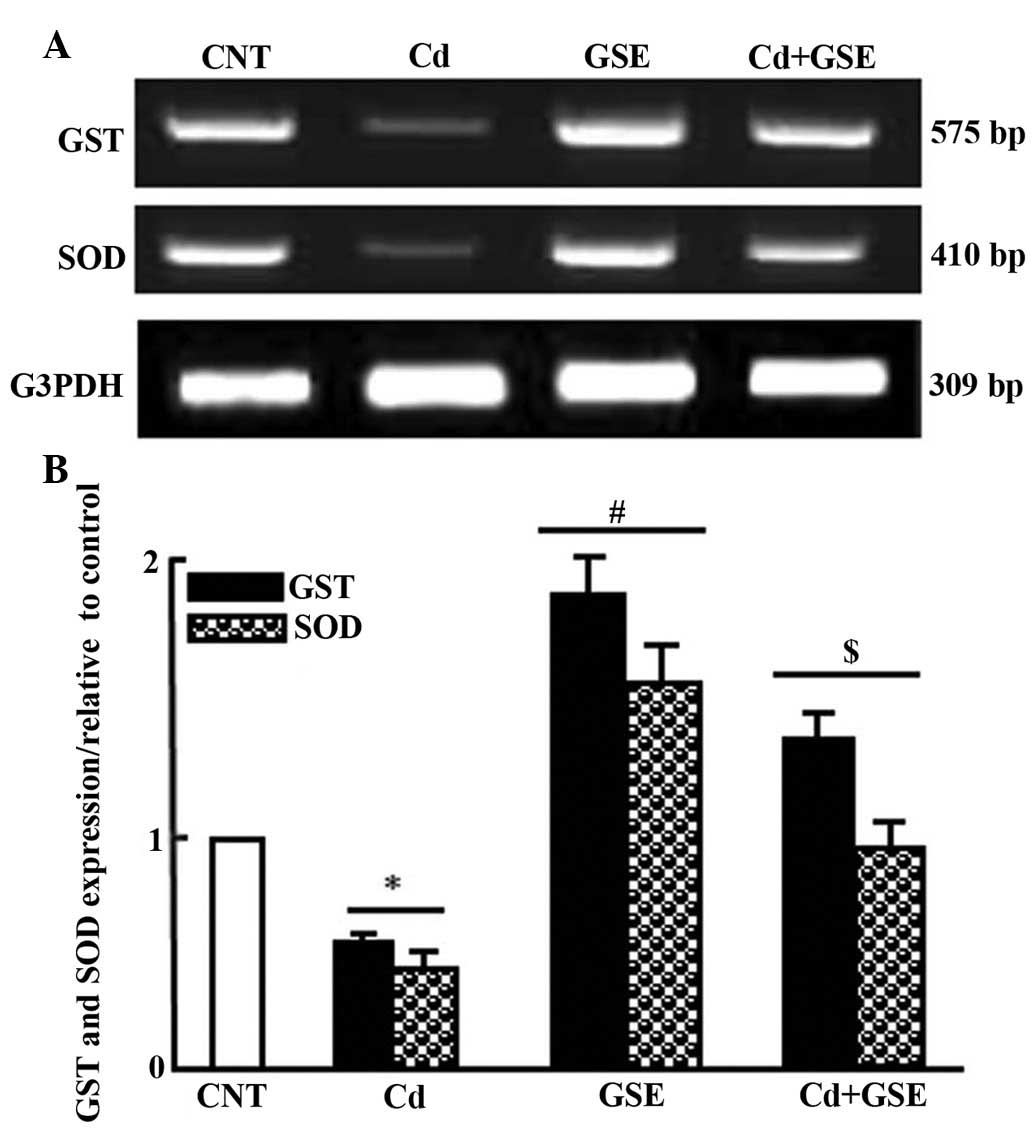
II). Consistent with the alterations to serum levels, treatment
with CdCl2 significantly downregulated the mRNA
expression levels of superoxide dismutase (SOD) and glutathione
S-transferase (GST), the expression of which was normalized
following co-treatment with GSE and CdCl2 (P<0.05;
Fig. 1A and B).
 | Table IISerum alterations in antioxidant and
MDA levels following CdCl2 administration for 3 months
in Wistar rats. |
Table II
Serum alterations in antioxidant and
MDA levels following CdCl2 administration for 3 months
in Wistar rats.
| Factor | Control |
CdCl2 | GSE |
GSE+CdCl2 |
|---|
| Catalase (U/g
protein) | 31.2±2.7 | 16.8±2.6a | 35.6±2.1a | 27.1±1.9b |
| GSH-R (U/g
protein) | 75.1±6.4 | 26.2±4.4a | 78.8±10.1a | 42.9±4.5b |
| GSH-Px (U/g
protein) | 77.7±7.7 | 31.2±12.2a | 58±4.7a | 41±4.9b |
| MDA (nmol/g
protein) | 27.2±3.3 | 85.6±7.8a | 35.5±5.2a | 55.1±8.3b |
Protective effects of GSE on
CdCl2-induced alterations in the testicular
histopathology of Wistar rats
As shown in Fig. 2,
the testes consist of numerous seminiferous tubules (STs), which
are lined with spermatogenic cells. Interstitial connective tissue
and Leydig cells are located between the STs (Fig. 2A). The STs of the GSE group were
characterized by highly active spermatocytes and spermatozoa
(Fig. 2B). However, the testes of
the CdCl2 group exhibited congestion and edema in the
interstitial blood vessels (Fig.
2C). The STs of the CdCl2 group were characterized
by irregular arrangement of the epithelial lining, degeneration and
sloughing of spermatogenic cells, and central accumulation in the
STs (Fig. 2D). The testes of the
protective group (GSE+CdCl2) exhibited improved
histological ST structure; however, low levels of edema and
congestion persisted in the interstitial tissue (Fig. 2E).
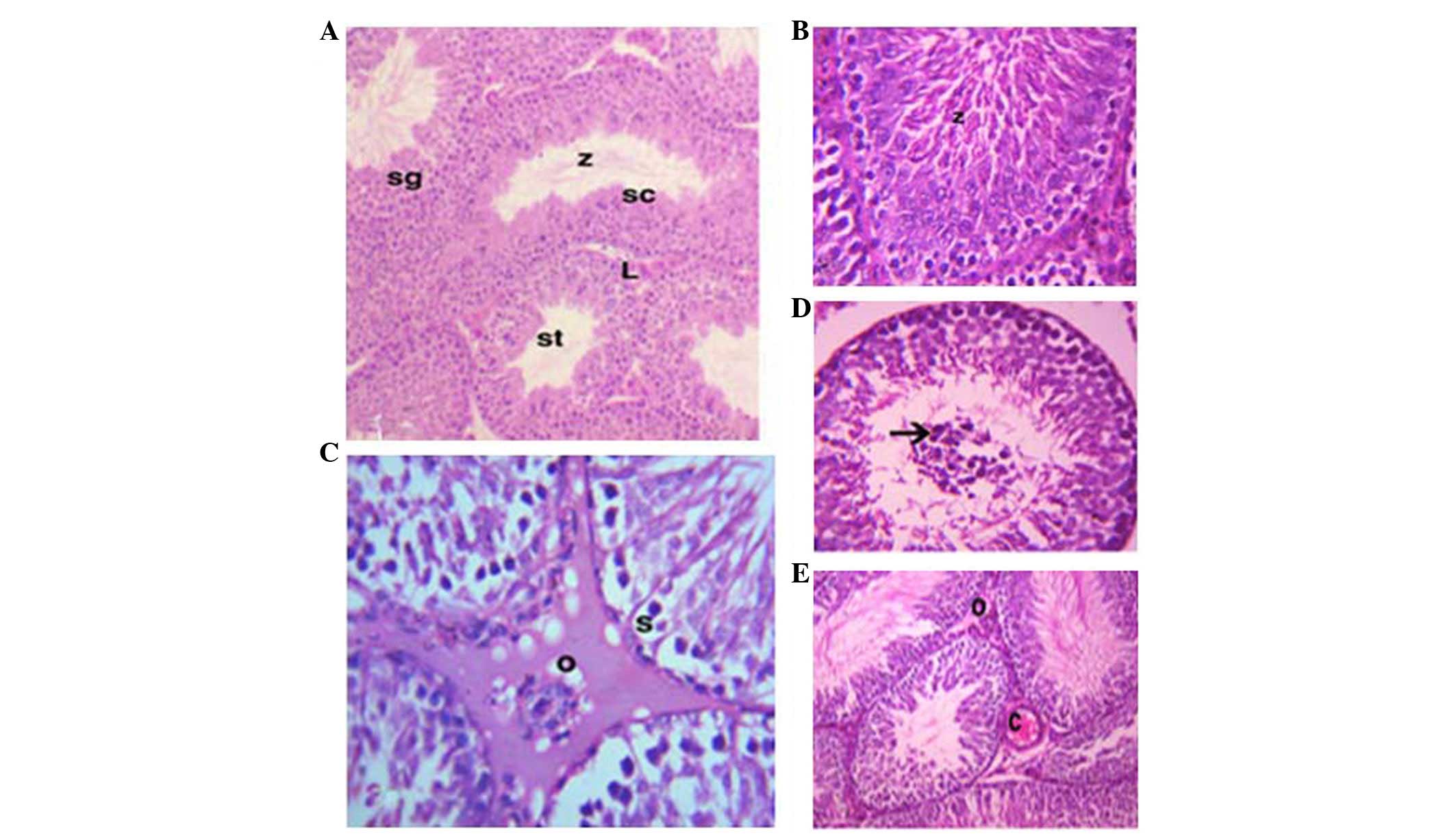 | Figure 2Photomicrographs of the testes. (A)
In the control group, numerous seminiferous tubules (st),
spermatogonia (sg), spermatocytes (sc) and spermatozoa (z) were
detected. Interstitial connective tissue and Leydig cells (L) were
observed [hematoxylin & eosin (H&E); magnification, ×10).
(B) In the grape seed extract (GSE) group activated spermatocytes
and spermatozoa (z) were detected (H&E; magnification, ×40).
(C) In the cadmium chloride (CdCl2) group, edema (o) and
sloughing of spermatogonia (s) were detected (H&E;
magnification, ×40). (D) Accumulation of the sloughed cells was
detected in the center of the seminiferous tubules (arrow)
(H&E; magnification, ×40). (E) In the co-treated group (GSE and
CdCl2), the seminiferous tubules were characterized by
congestion (C) and slight edema (o) (H&E; magnification,
×10). |
Protective effects of GSE on
CdCl2-induced alterations in the expression of Bax and
Ki-67 in the testes of Wistar rats
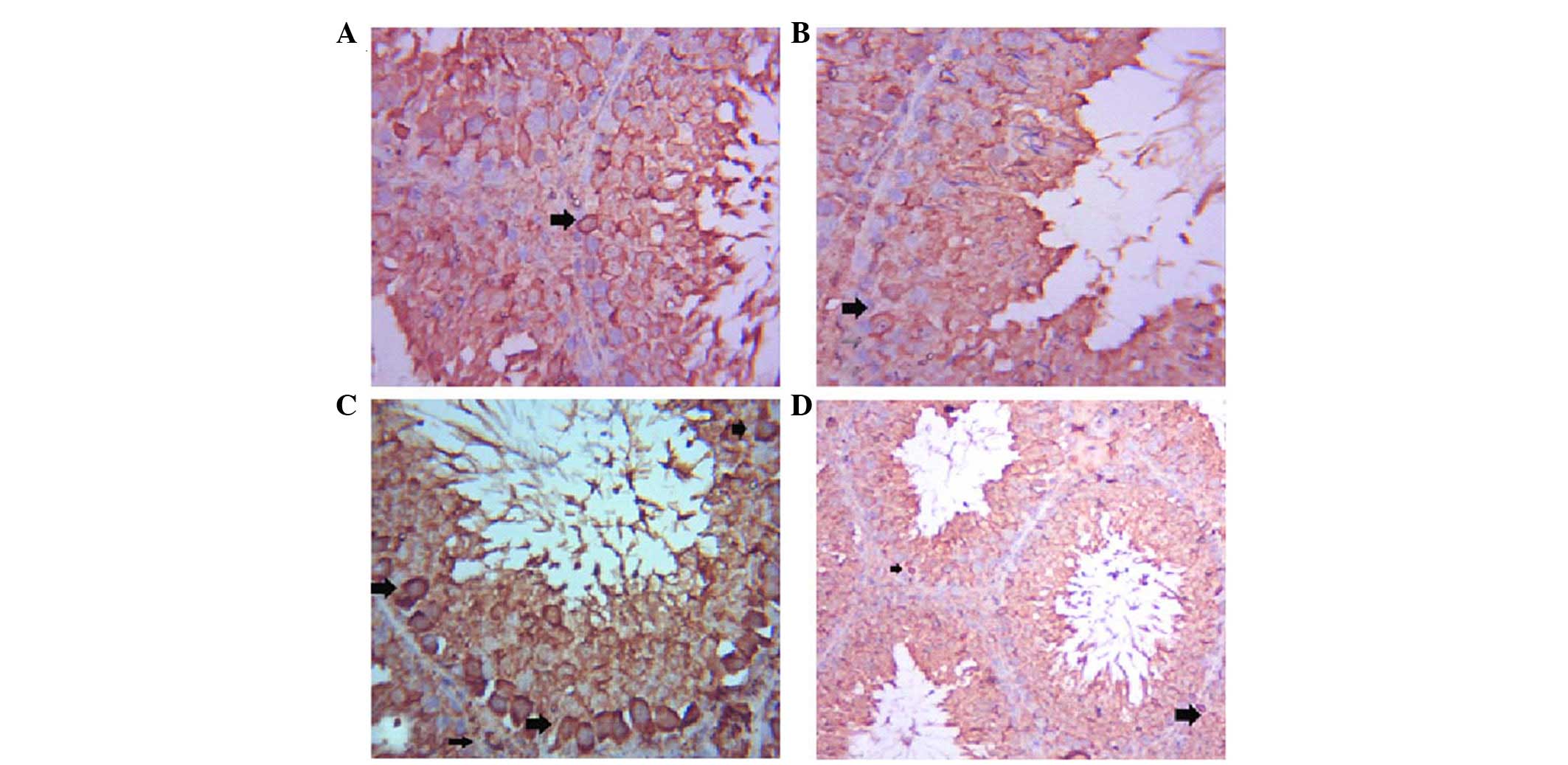
The testes of the control group exhibited positive
Bax immunostaining of a few spermatogenic cells (Fig. 3A), whereas Bax expression was
decreased in the spermatogenic cells of the GSE group (Fig. 3B). Immunostaining of the ST cells
of the CdCl2 group was strongly positive (Fig. 3C), whereas co-treatment with GSE
induced a marked decrease in Bax immunoreactivity in ST cells
(Fig. 3D).
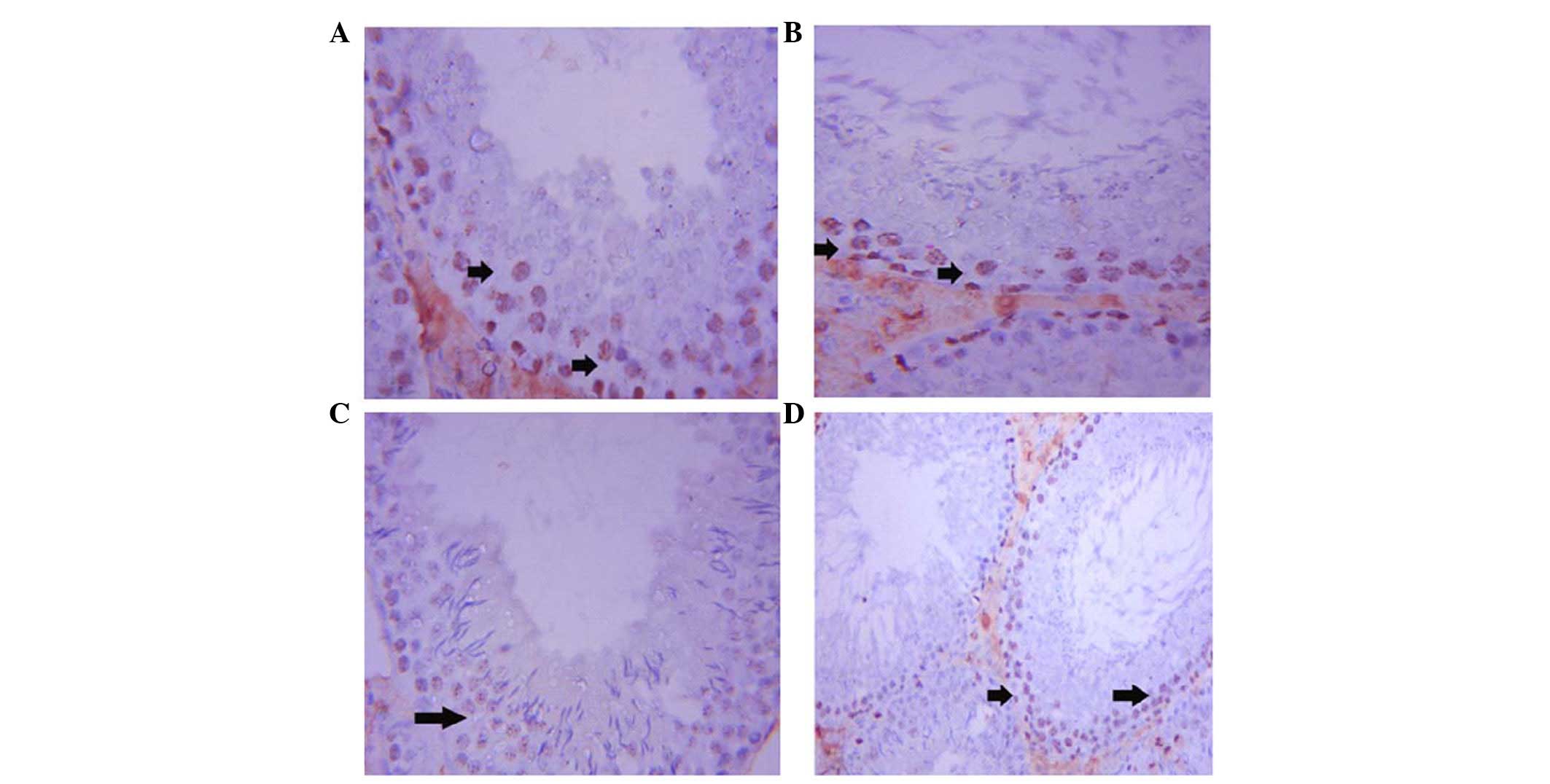
Ki-67 expression was upregulated in the ST cells of
the control rats. Immunostaining was localized to the spermatogonia
and spermatocytes (Fig. 4A). In
addition, Ki-67 expression was activated in the ST cells of the GSE
group (Fig. 4B). Conversely,
immunostaining of Ki-67 was weak in the ST cells of the
CdCl2 group (Fig. 4C).
Co-treatment with GSE and CdCl2 led to an improvement in
Ki-67 expression in ST cells; however, the staining was not as
strong as reported in the control rats (Fig. 4D).
Protective effects of GSE on
CdCl2-induced alterations in the expression levels of
steroidogenesis-associated genes in the testes of Wistar rats
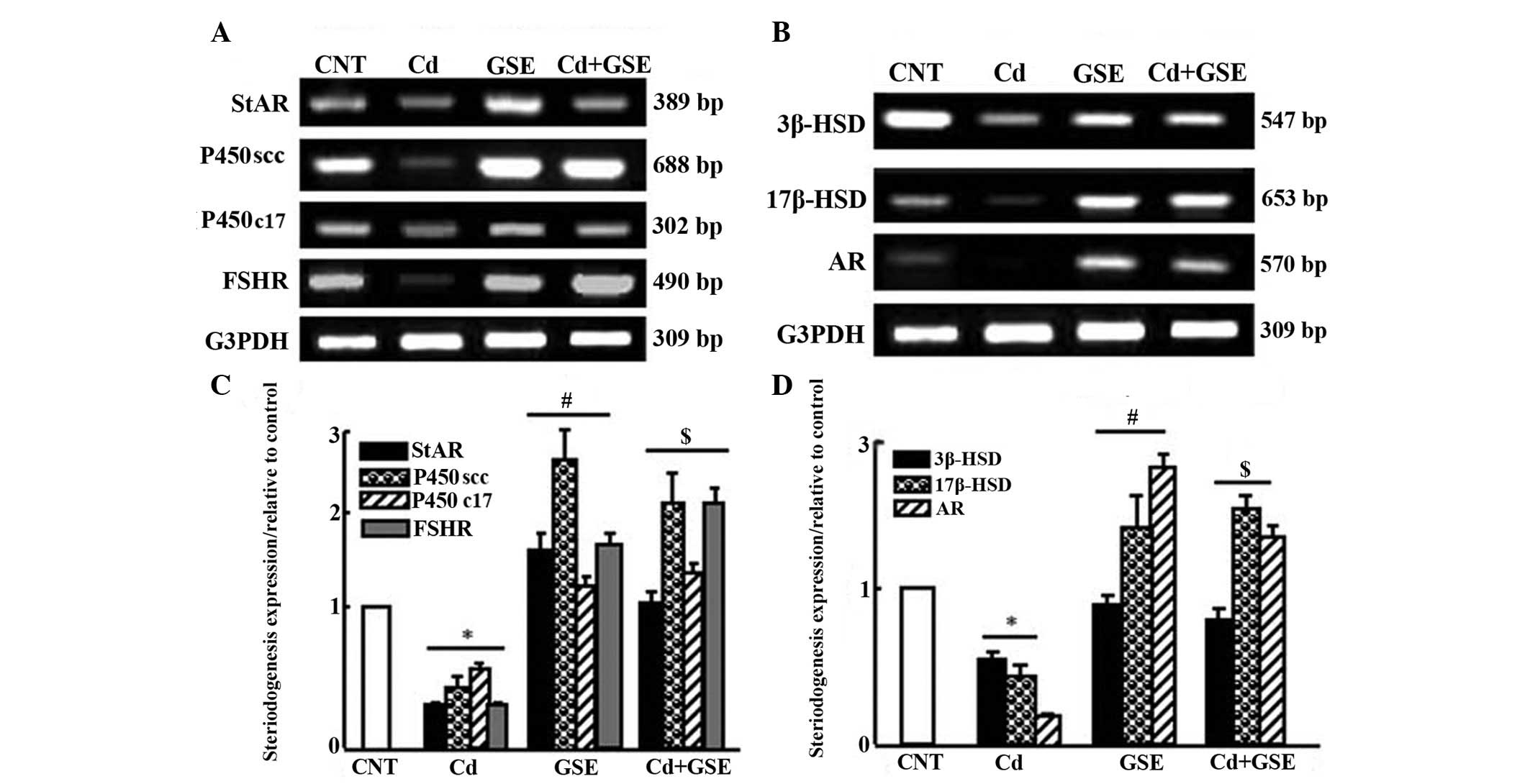
The expression levels of steroidogenesis-associated
genes were downregulated following treatment with CdCl2
for 3 months (Fig. 5). There was a
significant downregulation in the mRNA expression levels of
cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc),
cytochrome P450 17A1 (P450c17), 3β-hydroxysteroid dehydrogenase
(3β-HSD), 17β-HSD, androgen receptor (AR), steroidogenic acute
regulatory protein (StAR), and follicle-stimulating hormone
receptor (FSHR) in the CdCl2 group (P<0.05; Fig. 5A and B). Notably, GSE exhibited a
stimulatory effect on the expression of steroidogenesis-associated
enzymes. Co-treatment with CdCl2 and GSE normalized and
significantly upregulated the mRNA expression levels of
steroidogenesis-associated genes, which were decreased in the
CdCl2 group, as determined by densitometric analysis
(P<0.05; Fig. 5C and D).
Discussion
The present study demonstrated that CdCl2
toxicity induced serious alterations in the testes, which were
protected against by co-administration of GSE. CdCl2
administration induced significant decreases in the serum levels of
testosterone and antioxidants, increased the expression of an
apoptosis-associated biomarker in the testes, and downregulated the
mRNA expression levels of steroidogenesis-associated enzymes in the
rat testes. Furthermore, GSE ameliorated all alterations induced by
CdCl2 toxicity. It is well-known that Cd is considered
one of the most toxic elements, and has a toxicity grade of seven
(21). Humans are exposed to Cd
toxicity by either inhalation or ingestion; skin absorption of Cd
is relatively insignificant (22,23).
Cd is reported to be contained in industrial emissions, cigarette
smoke and agricultural fertilizers (23). One method of Cd toxicity occurs
through Cd-containing pigments, which are included in utensils and
electrode materials contained within nickel-Cd batteries. Another
method occurs through handling of contaminated food and water, and
by smoking (24). During exposure
to Cd, it may accumulate in the liver, kidneys, reproductive
organs, and other tissues (25,26).
Cd accumulation may occur in the testes, where it
induces testicular oxidative stress by two mechanisms: By reacting
with the sulfhydryl groups of various proteins or by glutathione
depletion (27), as confirmed in
the findings of the present study (Table II and Fig. 1). Oxidative stress may induce cell
proliferation via alterations in the DNA repair mechanism (28). The results of the present study
confirmed that GSE exerts potential effects against oxidative
stress, and may inhibit the production of free radicals, as
described in a previous study (29). Treatment with Cd decreased
antioxidant levels and increased MDA levels. It has previously been
reported that Cd acts through overproduction of ROS and enhanced
lipid peroxidation (30).
Cd toxicity alters testicular function and reduces
fertility by lowering sperm count and motility (31). In addition, Cd toxicity may cause
the formation of multinucleated giant cells. Giant cell formation
is caused by additional DNA replication of the primary
spermatocytes that fail to undergo meiosis (32). In addition, chronic Cd toxicity
leads to impairment of the H2O2 removal
system, which leads to inhibition of steroidogenesis in the Leydig
cells due to an accumulation of H2O2
(33).
It has been demonstrated that low doses of
CdCl2 induces alterations in testicular tissues,
represented by mild interstitial fibrosis, while high doses of
CdCl2 result in edema, degeneration, calcification of
the tunica albuginea and hypertrophy of interstitial cells
(34). The present study used the
optimal dose of CdCl2 to induce toxicity without lethal
effects. The results demonstrated that congestion and edema were
present in the interstitial blood vessels, and sloughing of the
spermatogenic cells resulted in central accumulation in the STs.
These findings are consistent with those reported in other studies
(31,35,36).
The reported histopathological changes are mainly caused by
disturbances in vascular permeability as a result of Cd toxicity
(37).
Bax is a proapoptotic marker, which is increased in
the early stages of intoxication and disease. Conversely, Ki-67
expression is a marker for cell proliferation. High expression of
Ki-67 has been reported in cells during G2 and early M
stages of cell growth (38,39).
The results of the present study confirmed that Bax markers were
increased in the spermatogenic cells of the
CdCl2-treated group, while Ki67 was decreased. The
expression of Bax and Ki67 is a mechanism of the body that acts
against the degenerative effects caused by CdCl2 in the
testes; the administration of GSE counteracted these effects. These
findings were supported by the results of previous studies
(38,40). Cd has been shown to exert
biological effects on the Sertoli cells of piglets, resulting in
DNA damage and the appearance of apoptotic cells (41). Apoptosis of the germ cells of the
testes can be induced by intrinsic and extrinsic pathways (42). Bax expression has been detected in
Leydig cells, indicating that Bax, a promotor of cell death, is
often positively regulated by the tumour-suppressor gene p53
(43).
Androgenesis in the testes involves multi-critical
steps that start with the synthesis of cholesterol, followed by its
transport within the steroidogenic testicular tissues and its
metabolism to form steroid biosynthesis. Steroidogenic cells (SCs)
acquire cholesterol either by de novo synthesis or from the
high density lipoproteins (HDL) or low density lipoproteins (LDL)
that circulate in the blood (44).
Scavenger receptor class B member 1 (SRB-1) is a specific receptor
for lipoproteins and is located on the surface of SCs. SRB-1 aids
LDL and HDL uptake by SCs (44).
The increased production of luteinizing hormone (LH) and
follicle-stimulating hormone (FSH) allows the synthesis of
cholesterol by activating the enzymes of cholesterol biosynthesis,
including cholesterol ester hydrolase. Furthermore, LH and FSH
production regulates the uptake of cholesterol esters by SCs
through the enhancement of the expression of HDL and LDL receptors,
which recognize SRB-1 (45). The
present study detected a marked decrease in the serum levels of
testosterone, possibly due to Cd-induced decreased synthesis and
availability of cholesterol for steroidogenesis. Consequently,
decreases in cholesterol biosynthesis result in downregulation of
steroid biosynthesis (46).
Following cholesterol biosynthesis, StAR transports
it to the inner mitochondrial membrane (IMM) (47). The results of the present study
detected a decrease in the mRNA expression levels of StAR in the
testicular tissues of Cd-treated rats. This decrease may be due to
a decrease in serum levels of LH, since LH is responsible for
steroidogenesis through StAR activation in SCs (45,48).
In agreement with these findings, previous studies have confirmed
the role of StAR expression and activity in steroidogenesis
(45,48). In the IMM, pregnenolone is produced
by the action of cholesterol on P450scc, which is a major enzyme
that regulates steroidogenesis (49).
The present study detected a significant reduction
in the levels of the P450scc enzyme in Cd-treated rats, which may
result from a decrease in availability of cholesterol in the IMM
due to a reduction in FSH receptor, which regulates P450scc levels
(46). Therefore, a significant
decrease in the expression of P450scc is another factor that may
contribute to an attenuation in steroidogenesis. In addition, it
has been reported that a decrease in StAR activity is associated
with a decrease in steroidogenesis (49). Notably, GSE counteracted the
Cd-induced inhibitory effects.
CdCl2 administration downregulates the
expression of 3β-HSD and 17β-HSD enzymes, and serves a critical
role in steroidogenesis. A direct effect of endocrine disruptors on
enzyme levels has also been reported in previous studies (50,51).
Steroidogenic enzymes and steroidogenesis can be disrupted at
various levels; however, StAR is the enzyme most affected by up- or
downregulation of steroidogenesis and its related enzymes (52). Furthermore, the expression levels
of AR were downregulated in the rats treated with Cd. The decreased
serum levels of testosterone may be explained by this
downregulation in AR expression. In conclusion, GSE ameliorated
Cd-induced alterations in antioxidants, histopathology,
immunoreactivity of an apoptotic marker, and mRNA expression of
steroidogenesis-associated genes. Future in vitro studies
are required in order to outline the direct beneficial effect of
GSE on lyedig cell function, and its availability for treatment of
testicular dysfunction during CdCl2 toxicity.
References
|
1
|
No authors listed. Meeting of the IARC
working group on beryllium, cadmium, mercury and exposures in the
glass manufacturing industry. Scand J Work Environ Health.
19:360–363. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodruff TJ, Carlson A, Scwartz JM and
Giudice LC: Proceedings of the Summit on Environmental Challenges
to Reproductive Health and Fertility: Executive summary. Fertil
Steril. 89:281–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Järup L and Akesson A: Current status of
cadmium as an environmental health problem. Toxicol Appl Pharmacol.
238:201–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goyer R: Toxic effect of metal: Casarett
and doult's toxicology. The Basic Science of Poisons. 5th edition.
Klaassen CD: McGraw-Hill; New York: pp. 691–736. 1995
|
|
5
|
Prozialeck WC, Edwards JR and Woods JM:
The vascular endothelium as a target of cadmium toxicity. Life Sci.
79:1493–1506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amara S, Abdelmelek H, Garrel C, Guiraud
P, Douki T, Ravanat J, Favier A, Sakly M and Ben Rhounia K:
Preventive effect of zinc against cadmium-induced oxidative stress
in the rat testis. J Reprod Dev. 54:129–134. 2008. View Article : Google Scholar
|
|
7
|
Anderson MB, Pedigo NG, Katz RP and George
WJ: Histopathalogy of testes from mice chronically treated with
cobalt. Reprod Toxicol. 6:41–50. 1992. View Article : Google Scholar
|
|
8
|
Naskar S, Islam A, Mazumder UK, Saha P,
Haldar PK and Gupta M: In vitro and in vivo antioxidant potential
of hydromethanolic extract of Phoenix dactylifera fruits. J Sci
Res. 2:144–157. 2010.
|
|
9
|
Islam MR, Shahnaj MP, Raihan MO, Hasan SMR
and Islam ME: In vitro and in vivo antioxidant potential of
ethanolic extract of Syzigium jambos (L.) bark. IJRAP. 2:810–815.
2011.
|
|
10
|
Soliman MM, Attia HF, Hussein MM, Hassan
ME and Ismail TA: Protective effect of N-acetylcystiene against
titanium dioxide nanoparticles modulated immune responses in male
albino rats. Am J Immunol. 9:148–158. 2013. View Article : Google Scholar
|
|
11
|
Soliman MM, Baiomy AA and Yassin MH:
Molecular and histopathological study on the ameliorative effects
of curcumin against lead acetate-induced hepatotoxicity and
nephrototoxicity in Wistar rats. Biol Trace Elem Res. 167:91–102.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sachs A: A natural alternative for
treating colds, infections, herpes, candida and many other
ailments. The Authoritative Guide to Grapefruit Extract. Stay
Healthy Naturally. Life rhythm; Mendocino, California: pp. 775–795.
1997
|
|
13
|
El-Ashmawy IM, Saleh A and Salama OM:
Effects of marjoram volatile oil and grape seed extract on ethanol
toxicity in male rats. Basic Clin Pharmacol Toxicol. 101:320–327.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehirli O, Ozel Y, Dulundu E, Topaloglu U,
Ercan F and Sener G: Grape seed extract treatment reduces hepatic
ischemia-reperfusion injury in rats. Phytother Res. 22:43–48. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alkhedaide AQ: The anti-inflammatory
effect of grape seed extract in rats exposed to cadmium chloride
toxicity. Int J Adv Res. 3:298–305. 2015.
|
|
16
|
Cetin A, Kaynar L, Koçyiğit I, Hacioğlu
SK, Saraymen R, Oztürk A, Orhan O and Sağdiç O: The effect of grape
seed extract on radiation-induced oxidative stress in the rat
liver. Turk J Gatroenterol. 19:92–98. 2008.
|
|
17
|
El-Demerdash FM, Yousef MI, Kedwany FS and
Baghdadi HH: Cadmium-induced changes in lipid peroxidation, blood
hematology, biochemical parameters and semen quality of male rats:
Protective role of vitamin E and beta-carotene. Food Chem Toxicol.
42:1563–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li SG, Ding YS, Niu Q, Xu SZ, Pang LJ, Ma
RL, Jing MX, Feng GL, Liu JM and Guo SX: Grape seed
proanthocyanidin extract alleviates arsenic-induced oxidative
reproductive toxicity in male mice. Biomed Environ Sci. 28:272–280.
2015.PubMed/NCBI
|
|
19
|
Bancroft JD and Gamble A: General stains.
Theory and Practice of Histological Techniques. 5th edition.
Churchill Livingstone; Edinburgh: pp. 116–117. 2002
|
|
20
|
Kiernan JA: Histological and Histochemical
Methods: Theory and Practice. 4th edition. Scion Publishing Ltd;
Banbury: pp. 211–235. 2008
|
|
21
|
Fay RM and Mumtaz MM: Development of a
priority list of chemical mixtures occurring at 1188 hazardous
waste sites, using the HazDat database. Food Chemical Toxicol.
34:1163–1165. 1996. View Article : Google Scholar
|
|
22
|
Mead MN: Cadmium confusion: Do consumers
need protection? Environ Health Perspect. 118:a528–a534. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zalups RK and Ahmad S: Molecular handling
of cadmium in transporting epithelia. Toxicol Appl Pharmacol.
186:163–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waisberg M, Joseph P, Hale B and
Beyersmann D: Molecular and cellular mechanisms of cadmium
carcinogenesis. Toxicology. 192:195–117. 2003. View Article : Google Scholar
|
|
25
|
Godt JF, Scheidig C, Grosse-Siestrup V,
Esche P, Brandenburg P, Reich A and Groneberg DA: The toxicity of
cadmium and resulting hazards for human health. J Occup Med
Toxicol. 1:222006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takamure Y, Shimada H, Kiyozumi M,
Yasutake A and Imamura Y: A possible mechanism of resistance to
cadmium toxicity in male Long-Evans rats. Environ Toxicol
Pharmacol. 21:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valko M, Morris H and Cronin MT: Metals,
toxicity and oxidative stress. Curr Med Chem. 12:1161–1208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beyersmann D and Hartwig A: Carcinogenic
metal compounds: Recent insight into molecular and cellular
mechanisms. Arch Toxicol. 82:493–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dulundu E, Ozel Y, Topaloglu U, Toklu H,
Ercan F, Gedik N and Sener G: Grape seed extract reduces oxidative
stress and fibrosis in experimental biliary obstruction. J
Gastroenterol Hepatol. 22:885–892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sen Gupta R, Sen Gupta E, Dhakal BK,
Thakur AR and Ahnn J: Vitamin C and vitamin E protect the rat
testes from cadmium-induced reactive oxygen species. Mol Cells.
17:132–139. 2004.PubMed/NCBI
|
|
31
|
Yang HS, Han DK, Kim JR and Sim JC:
Effects of alpha-tocopherol on cadmium-induced toxicity in rat
testis and spermatogenesis. J Korean Med Sci. 21:445–451. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang HJ, Lee SH, Jin Y, Choi JH, Han CH
and Lee MH: Genotoxicity and toxicological effects of acrylamide on
reproductive system in male rats. J Vet Sci. 6:103–109.
2005.PubMed/NCBI
|
|
33
|
Diemer T, Allen JA, Hales KH and Hales DB:
Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells
and inhibits steroidogenic acute regulatory (StAR) protein and
steroidogenesis. Endocrinology. 144:2882–2891. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ekhoye EI, Nwangwa EK and Aloamaka CP:
Changes in some testicular biometric parameters and testicular
function in cadmium chloride administered Wistar rats. Br J Med Med
Res. 3:2031–2041. 2013. View Article : Google Scholar
|
|
35
|
Elgawish RAR and Ghanem ME: Effect of long
term cadmium chloride exposure on testicular functions in male
albino rats. Am J Anim Vet Sci. 9:182–188. 2014. View Article : Google Scholar
|
|
36
|
El-Shahat AE, Gabr A, Meki AR and Mehana
ES: Altered testicular morphology and oxidative stress induced by
cadmium in experimental rats and protective effect of simultaneous
green tea extract. Inter J Morphol. 27(3): 757–764. 2009.
|
|
37
|
de Souza Predes F, Diamante MA and Dolder
H: Testis response to low doses of cadmium in Wistar rats. Int J
Exp Pathol. 91:125–131. 2010. View Article : Google Scholar
|
|
38
|
Sakr SA and Nooh HZ: Effect of Ocimum
basilicum extract on cadmium-induced testicular histomorphometric
and immunohistochemical alterations in albino rats. Anat Cell Biol.
46:122–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sasaki K, Murakami T, Kawasaki M and
Takahashi M: The cell cycle associated change of the Ki-67 reactive
nuclear antigen expression. J Cell Physiol. 133:579–584. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Falana BA, Ogundele OM, Duru FI, Oshinubi
AA and Falode DT: Role of Se+Zn in regeneration (Ki-67) following
Pb toxicity (p53andcad) in the germinal epithelium of adult Wistar
rats. Pak J Biol Sci. 16:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang M, He Z, Wen L, Wu J, Yuan L, Lu Y,
Guo C, Zhu L, Deng S and Yuan H: Cadmium suppresses the
proliferation of piglet Sertoli cells and causes their DNA damage,
cell apoptosis and aberrant ultrastructure. Reprod Biol Endocrinol.
8:972010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Taylor MF, Woolveridge I, Metcalfe AD,
Streuli CH, Hickman JA and Morris ID: Leydige cell apoptosis in the
rat testes after administration of the cytotoxin ethane
dimethanesulphonate: Role of the Bcl-2 family members. J
Endocrinol. 157:317–326. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao G, Zhao L, Stangl H, Hasegawa T,
Richardson JA, Parker KL and Hobbs HH: Developmental and hormonal
regulation of murine scavenger receptor, class B, type 1. Mol
Endocrinol. 13:1460–1473. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fauser BCJM: Molecular biology of
steroidogenesis. Molecular Biology in Reproductive Medicine. Fauser
BCJM, Rutherford AJ, Strauss JF and Van Steirteghem A: 1st edition.
Parthenon Publishing; New York: pp. 234–251. 1999
|
|
46
|
Barlow NJ, Phillips SL, Wallace DG, Gaido
KW and Foster PM: Quantitative changes in gene expression in fetal
rat testes following exposure to di(n-butyl) phthalate. Toxicol
Sci. 73:431–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hasegawa T, Zhao L, Caron KM, Majdic G,
Suzuki T, Shizawa S, Sasano H and Parker KL: Developmental roles of
the steroidogenic acute regulatory protein (StAR) as revealed by
StAR knockout mice. Mol Endocrinol. 14:1462–1471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Murugesan P, Muthusamy T, Balasubramanian
K and Arunakaran J: Effects of vitamins C and E on steroidogenic
enzymes mRNA expression in polychlorinated biphenyl (Aroclor 1254)
exposed adult rat Leydig cells. Toxicology. 232:170–182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Omura T and Morohashi K: Gene regulation
of steroidogenesis. J Steroid Biochem Mol Biol. 53:19–25. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lyssimachou A, Jessen BM and Arukwe A:
Brain cytochrome P450 aromatase gene isoforms and activity levels
in atlantic salmon after waterborne exposure to nominal
environmental concentrations of the pharmaceutical ethynylestradiol
and antifoulant tributyltin. Toxicol Sci. 91:82–92. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin TC, Chien SC, Hsu PC and Li LA:
Mechanistic study of polychlorinated biphenyl 126-induced CYP11B1
and CYP11B2 up-regulation. Endocrinol. 147:1536–1544. 2006.
View Article : Google Scholar
|
|
52
|
Rice S, Mason HD and Whitehead SA:
Phytoestrogens and their low dose combinations inhibit mRNA
expression and activity of aromatase in human granulosa-luteal
cells. J Steroid Biochem Mol Biol. 101:216–225. 2006. View Article : Google Scholar : PubMed/NCBI
|