Introduction
Multiple myeloma (MM) is a malignant tumor type
originating from B cells and is characterized by the increase of
abnormal plasma cells that generate monoclonal immunoglobulin as
well as malignant proliferation in the bone marrow, which cause
fractures and bone marrow failure, resulting in severe clinical
symptoms (1). If left untreated,
the median survival time of advanced MM patients is only six
months, while it is not more than three years in patients receiving
traditional chemotherapy; only 25% of patients survive for more
than five years (2). Therefore, MM
is currently considered to be an incurable disease, which urgently
requires novel approaches to improve the prognosis of patients
(3).
The phosphoinositide-3 kinase (PI3K)/Akt/mammalian
target of rapamycin (mTOR) pathway is an important signaling
pathway that affects cellular energy metabolism, cell size, cell
cycle, cell proliferation as well as cell survival and apoptosis,
and is closely linked to other important signal transduction
pathways (4). Therapies targeting
the PI3K/AKT/mTOR signaling pathway in combination with other drugs
represent promising treatment approaches. The PI3K/AKT/mTOR pathway
has important roles in the survival and growth of MM cells and has
been shown to be the target of natural products with anti-MM
efficacy (5).
Silybin (Fig. 1) is
a biologically active component extracted from the seeds of milk
thistle, Silybum marianum, and its derivatives have been
demonstrated to exhibit marked anti-cancer activity (6). In-vitro studies have shown
that silybin inhibits androgen-dependent and -independent prostate
cancer as well as skin, bladder, lung, colon, breast, ovarian,
renal, liver, cervical and tongue cancer (7–9).
However, the anti-cancer effects of silybin on MM cells and its
underlying mechanisms of action have not yet been fully elucidated.
The present study aimed to assess the ability of silybin to inhibit
the growth and induce apoptosis of human MM cells in vitro.
In addition, the possible involvement of the PI3K/Akt/mTOR
signaling pathway in the anti-MM effects of silybin was
investigated. The present study suggested that silybin is a
promising candidate for the clinical treatment of MM which exerts
its effects via the PI3K/Akt/mTOR pathway.
Materials and methods
Reagents
RPMI-1640 and fetal bovine serum (FBS) were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) and a TRIzol reagent kit were purchased from Invitrogen
(Thermo Fisher Scientific, Waltham, MA, USA). A Cell Apoptosis
Detection kit was obtained from BD Biosciences (Franklin Lakes, NJ,
USA). A bicinchoninic acid (BCA) Protein Assay kit was purchased
from Beyotime Institute of Biotechnology (Haimen, China).
Cell culture and cell viability
assay
The U266 human multiple myeloma cell line was
acquired from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and cultured in (RPMI-1640) with 10%
FBS, 100 U/ml penicillin and 100 mg/ml streptomycin (Amresco, LLC,
Solon, OH, USA) at 37°C in a humidified atmosphere containing 5%
CO2. The cell viability assay was performed according to
the protocol of a previous study (10). Following seeding of cells into
96-well plates at 1×104/well and an overnight incubation
for attachment, cells were incubated with silybin (50, 100 or 200
µM; Sigma-Aldrich; >98% purity) for 12, 24, or 48 h. A
total of 20 µl MTT solution (5 mg/ml) was then added to each
well, followed by culture for another 4 h at 37°C. Subsequently,
the media were removed and 200 µl dimethylsulfoxide
(Amresco, LLC) was added to each well. Following agitation for 20
min, the absorbance of each well was measured using an ELx800
microplate absorbance reader (Bio-Tek Instruments, Winooski, VT,
USA) at λ=570 nm.
Flow cytometry
Following incubation of U266 cells seeded into
six-well plates at 1×106/well with silybin (50, 100 or
200 µM for 24 h), cells were harvested and centrifuged at
1,000 × g for 5 min at 4°C. Subsequent to two washes with ice-cold
phosphate-buffered saline, cell suspensions were incubated with 10
µl fluorescein-conjugated Annexin V (100 mg/ml; BD
Biosciences) for 30 min in the dark. 5 µl propidium iodide
(PI; 100 mg/ml; BD Biosciences) was then added and cells were
incubated for a further 30 min in the dark on ice. Flow cytometry
(FACSCalibur; BD Biosciences) was used to determine the apoptotic
rate.
Western blot analysis
Following treatment of U266 cells with silybin as
described above, cells were lysed and the protein contents were
determined using the BCA Protein Assay kit according to the
manufacturer's instructions. Equal amounts of protein were loaded
into each lane and separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis. Subsequently, proteins were
transferred onto a polyvinylidene difluoride membrane at 4°C for 2
h. The membranes were blocked in 5% non-fat milk for 2 h and then
incubated with anti-PI3K (1:1,000; cat. no. SAB1300969;
Sigma-Aldrich) anti-phosphorylated (p)-Akt (1:1,000; cat. no.
SAB4301414; Sigma-Aldrich), anti-p-mTOR (1:2,000; cat. no.
SAB4301415; Sigma-Aldrich) and β-actin (1:1,000; cat. no. AA128;
Beyotime Institute of Biotechnology) antibodies overnight at 4°C
with agitation. Membranes were then incubated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody
temperature. After washing three times with Tris-buffered saline
with 0.1% Tween 20 for 5 min, membranes were developed using
enhanced chemiluminescence (Tiangen), resolved using a gel analysis
system (Pierce Biotechnology, Inc., Rockford, IL, USA) and exposed
to X-ray film (Kodak, Rochester, NY, USA).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Silybin inhibits the proliferation of
U266 cells
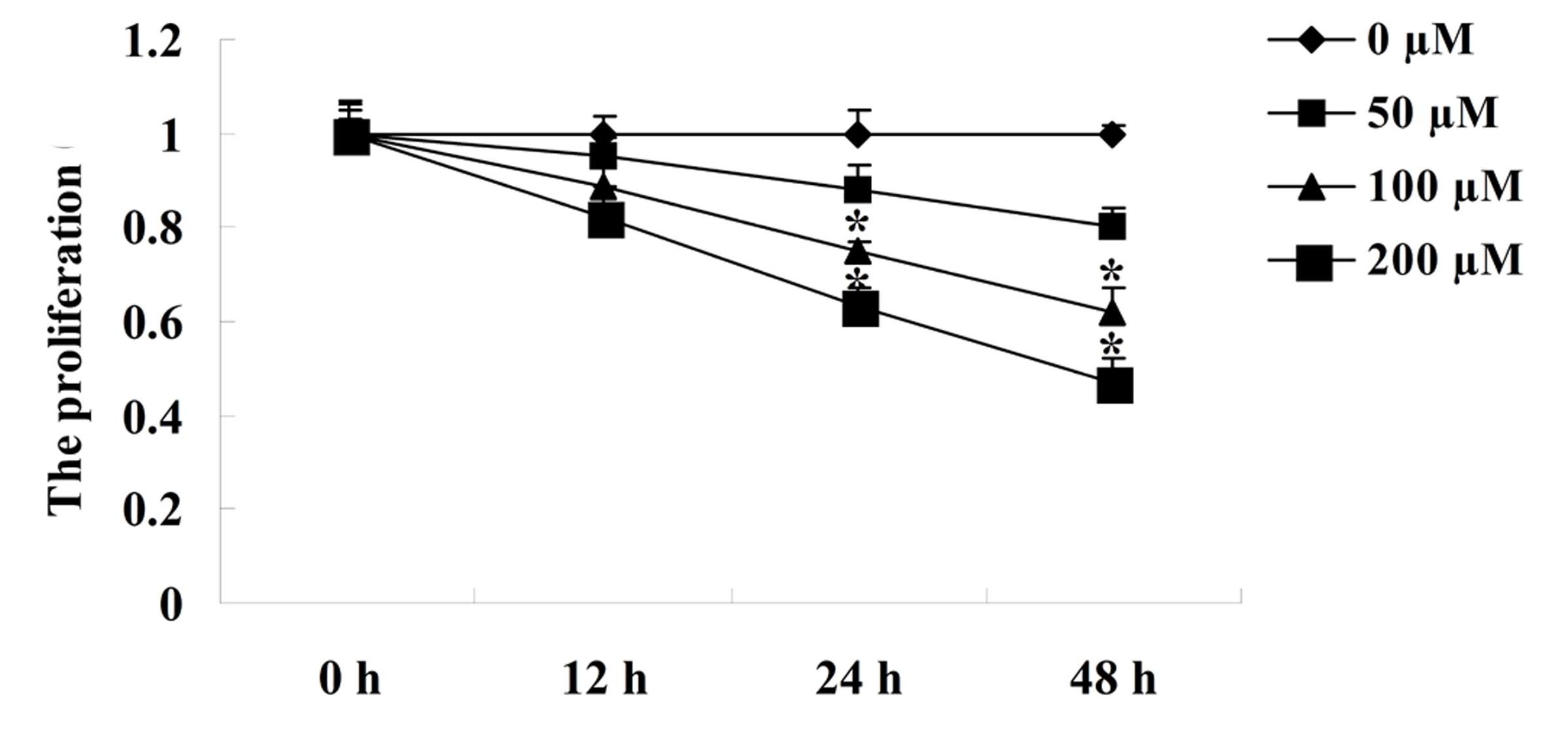
The MTT assay revealed that following incubation
with 100 or 200 µM silybin for 24 or 48 h, the proliferation
of U266 cells was significantly inhibited compared with that of the
untreated cells (P<0.05). Silybin inhibited the proliferation of
U266 cells in a dose- and time-dependent manner (Fig. 2).
Silybin induces apoptosis of U266
cells
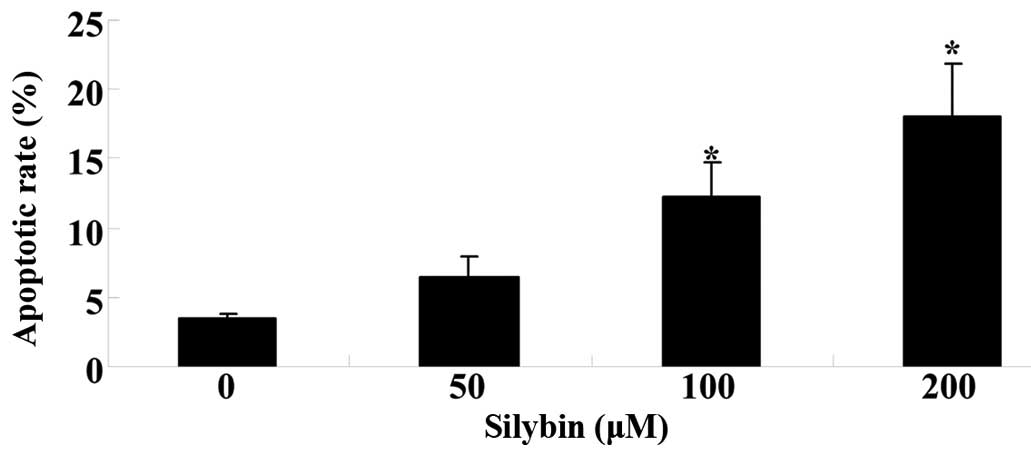
To determine whether silybin induces apoptosis of
U266 cells flow-cytometric analysis following staining with Annexin
V/PI was performed. Silybin (100 or 200 µM) significantly
increased the apoptotic rate of U266 cells following for 24 of
incubation. The induction of U266-cell apoptosis by silybin was
dose-dependent (Fig. 3).
Silybin suppresses PI3K/Akt in U266
cells
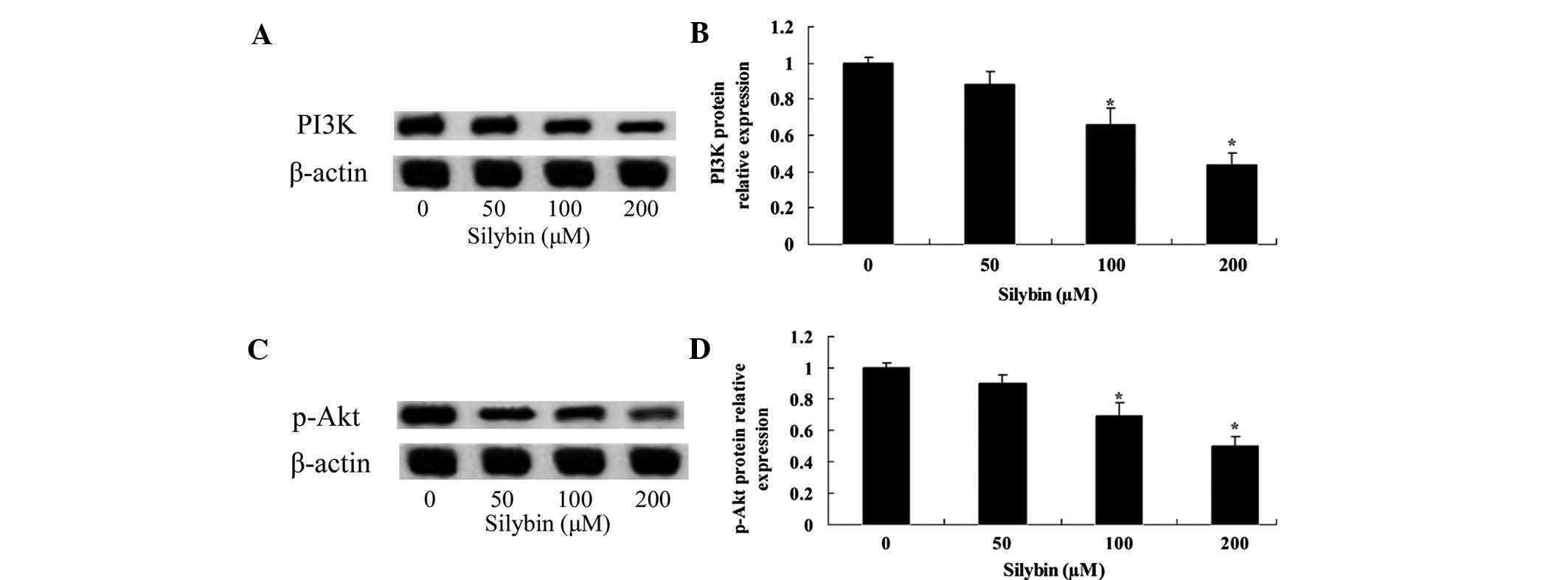
To analyze the underlying molecular mechanisms of
silybin-mediated induction of U266-cell apoptosis, proteins of the
PI3K/Akt signaling pathway were assessed using western blot
analysis. After a 24-h incubation with silybin (100 or 200
µM), protein levels of PI3K and p-Akt in U266 cells were
significantly decreased (Fig.
4A–D). Furthermore, the effects of silybin on PI3K and p-Akt
were in a dose-dependent. These results indicated that silybin may
induce cellular apoptosis of U266 cells via inhibiting the PI3K/Akt
signaling pathway.
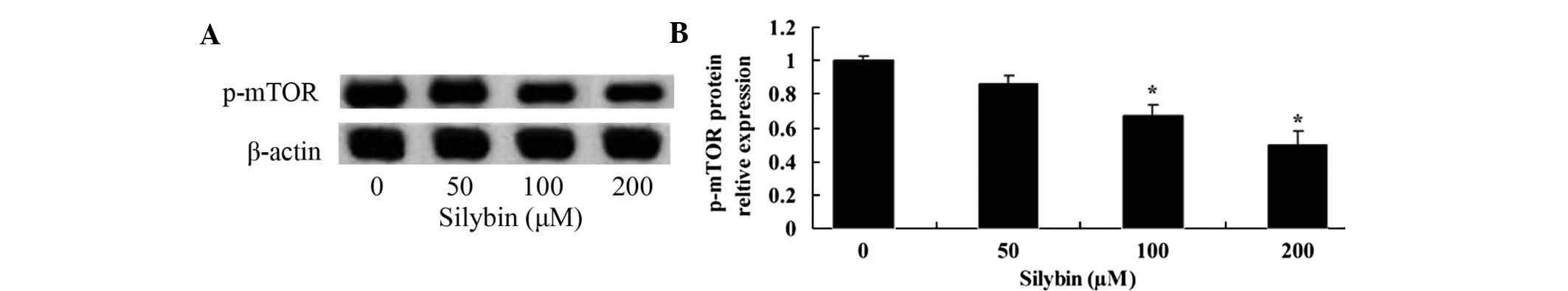
Silybin suppresses mTOR in U266
cells
The present study assessed the involvement of the
mTOR signaling pathway in the mechanism of action of silybin by
using western blot analysis. Following 24 h of incubation, silybin
(100 or 200 µM) significantly reduced the levels of p-mTOR
(Fig. 5A and B). Furthermore, the
effect of silybin on the activation of mTOR were dose-dependent.
These results indicated that the induction of U266-cell apoptosis
by silybin may be mediated via inhibiting PI3K/Akt/mTOR
signaling.
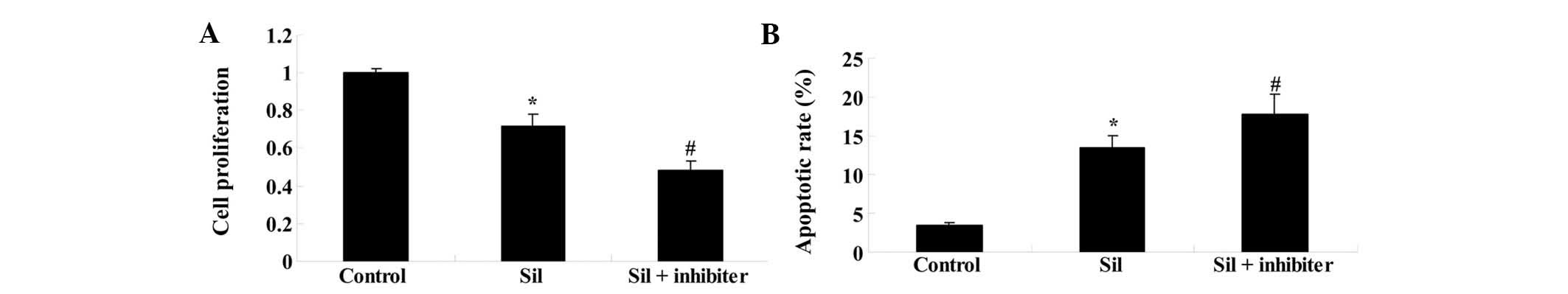
Inhibition of the PI3K/Akt pathway
enhances the potency of silybin in U266 cells
To confirm the involvement of the PI3K/Akt pathway
in the effects of silybin on U266 cells, they were co-treated with
silybin and PI3K inhibitor LY294002 (3 µM) for 24 h. The
reduction in cell proliferation and the induction of apoptosis by
silybin (100 µM) were enhanced by the PI3K inhibitor when
compared to treatment with silybin alone (Fig. 6A and B). These results indicated
that the mechanism of action of silybin in U266 cells involved the
inhibition of the PI3K/Akt pathway.
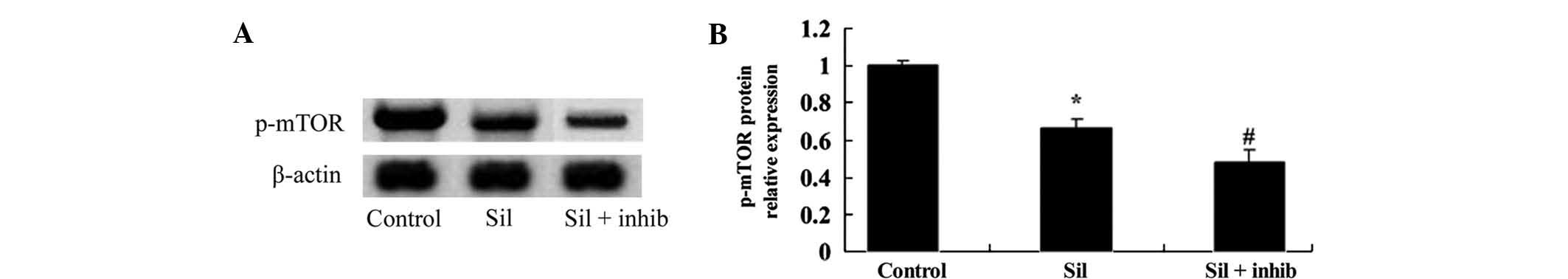
Inhibition of PI3K/Akt enhances
silybin-induced reduction of mTOR activity in U266 cells
To further assess whether inhibition of PI3K/AKT
enhanced silybin-induced apoptosis through modulation of mTOR
activity of U266 cells, they were co-incubated with silybin and
PI3K inhibitor LY294002 for 24 h. The silybin-induced reduction of
p-mTOR levels was enhanced by treatment with PI3K inhibitor
(Fig. 7A and B). These results
indicated that the apoptotic effects of silybin on U266 cells may
be mediated via PI3K/AKT/mTOR signaling.
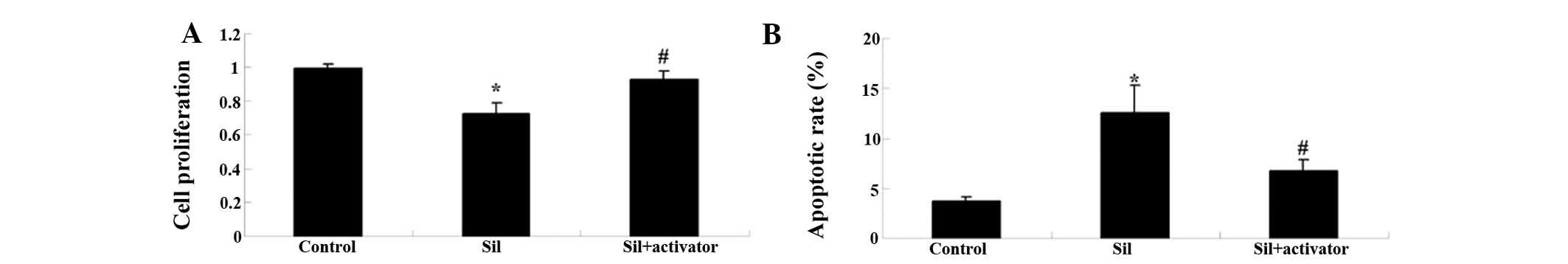
Activation of the PI3K/AKT pathway
reduces the potency of silybin in U266 cells
To further investigate the involvement of the
PI3K/AKT pathway in the anti-MM activity of silybin, U266 cells
were co-treated with silybin and PI3K activator insulin-like growth
factor-1 (IGF-1; 10 µM) for 24 h. The anti-proliferative and
apoptotic effects of silybin on U266 cells were reversed by the
PI3K inhibitor (Fig. 8A and
B).
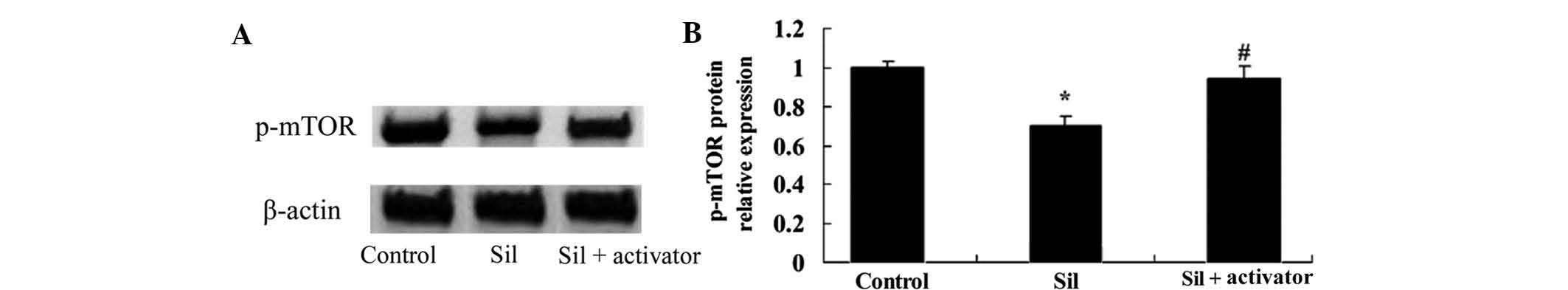
Activation of the PI3K/AKT pathway
attenuates silybin-induced reduction of mTOR activity in U266
cells
To further assess the effects of PI3K/AKT pathway
activation on silybin-induced inhibition of mTOR activity, U266
cells were co-treated with silybin and PI3K activator IGF-1 (10
µM) for 24 h. The silybin-induced reduction of p-mTOR was
attenuated by the PI3K activator (Fig.
9A and B), which further indicated the involvement of the
PI3K/Akt/mTOR pathway in the mechanism of action of silybin.
Discussion
MM is a neoplastic condition featuring malignant
plasma cells, whose main clinical manifestations include
hyper-gammaglobulinemia, renal insufficiency, bone damage and
pancytopenia (11). In spite of MM
currently being considered to be incurable, significant progress
has recently been made in clinical treatments, including the
application of thalidomide, proteasome inhibitors and bone marrow
transplantation, which, however, has not significantly increased
the survival of MM patients (12,13).
Therefore, current research focuses on the discovery of novel
treatments for MM. Recent studies have indicated that silybin
exhibits marked anti-tumor activity and inhibits the growth of
human hepatocellular carcinoma (10,14).
Previous studies by our group reported that silybin inhibited the
growth and induced apoptosis of human MM cells in a dose-dependent
manner. Thus, it was required to explore the mechanisms involved in
the anti-cancer effects of silybin on MM cells and to assess its
potential use in the clinic.
The PI3K/Akt pathway is a vital regulatory pathway
involved in cell growth, proliferation and differentiation, while
the overexpression of PI3K is a crucial step during carcinogenesis.
PI3K is able to activate cyclin-dependent-kinase-4 (CDK4) as well
as CDK2, which mediates the transition of cells into S-phase to
induce DNA synthesis (15).
Furthermore, PI3K controls the expression of p27, which is a
negative regulator of the cell cycle. PI3K/Akt signaling can be
inactivated through phosphorylation of sites Ser280 of checkpoint
kinase 1, and PI3K/Akt can directly activate CDK1, which
facilitates G2/M-phase transition (16). In this context, the findings of the
present study indicated that silybin inhibits cell proliferation
and induces apoptosis through suppression of the PI3K-Akt signaling
pathway. In consistency with this, García-Maceira and Mateo
(17) showed that silybin
inhibited human cervical cancer as well as hepatoma cells through
the PI3K/Akt/mTOR signaling pathway. Zhang et al (18) reported that silybin ameliorated
steatosis and insulin resistance during non-alcoholic fatty liver
disease development via the PI3K/Akt pathway. The results of the
present study indicated that silybin significantly reduced PI3K and
p-Akt protein levels in U266 cells in a dose-dependent manner.
mTOR is a protein kinase associated with the
PI3K/Akt pathway, which can enhance mRNA transcription and
translation through phosphorylative activation of proteins
associated with mRNA translation (19). Akt activates mTOR by
phosphorylating Ser2448 sites of mTOR to enhance the efficiency of
mRNA translation, thereby increasing the expression of proteins
associated with cell growth and differentiation, and accelerating
tumorigenesis (20). The present
study revealed that silybin treatment reduced the protein levels of
p-mTOR in U266 cells in a dose-dependent manner. Raina et al
(21) suggested that silybin
restrained the proliferation of colorectal cancer cells through
inhibiting PIK3CA/Akt-mTOR. Lin et al (22) reported that silybin inhibits
age-associated macular degeneration of the hypoxia-dependent type
via PI3K/Akt/mTOR.
Activated PI3K/Akt may further activate its
downstream molecule mTOR through tuberous sclerosis 1/2 (23). Due to the high homology of the mTOR
carboxy terminus and the PI3K catalytic domain, mTOR is considered
to be a family member of PI3K-associated protein kinases. Upon
activation, mTOR can phosphorylate its two downstream molecules,
namely translation-inhibition molecule EIF-4E binding protein 1
(4E-BP1) and ribosomal protein p70S6K. As 4E-BP1 is inactivated
through phosphorylation, its ability to bind to EIF-4E is lost and
results in the dissociation of the complex and the combination of
EIF-4E with other translation initiation factors to induce protein
translation (24). Following
phosphorylative activation of p70S6K, protein synthesis is
enhanced. Therefore, PI3K/Akt/mTOR is considered to be the main
signal-regulating pathway of protein synthesis and is involved in
the regulation of cell proliferation, differentiation and migration
(11). Of note, in the present
study, inhibition of PI3K enhanced the potency of silybin to reduce
cell proliferation and induce apoptosis, and reduced the protein
levels of p-mTOR in U266 cells. Conversely, activation of PI3K
reduced the anti-proliferative and apoptotic effects of silybin as
well as the reduction of p-mTOR levels in U266 cells. The results
of the present study were consistent with those of previous
studies. For instance, Lin et al (22) reported that silybin inhibited
age-associated macular degeneration of the hypoxia-dependent type
via PI3K/Akt/mTOR. Furthermore, Wang et al (25) showed that silybin exerted effects
against experimental ischemic stroke, which may have been based on
its anti-inflammatory effects mediated through the activation of
Akt/mTOR signaling.
In conclusion, the present study showed that silybin
suppressed cell proliferation and induced apoptosis of human MM
cells. Furthermore, inhibition of PI3K/Akt/mTOR signaling pathways
sensitized human MM cells to silybin treatment. Therefore, silybin
is a promising candidate for the treatment of MM, and inhibition of
the PI3K/Akt/mTOR signaling pathway appears to be an effective
strategy for the enhancement of its efficacy.
References
|
1
|
García-Escobar I, Parrilla L, Ortega LM,
Castellanos D, Pallarés MA and Cortés-Funés H: Clinical experience
with plerixafor as a mobilization regimen for autologous peripheral
blood stem cell transplantation in patients with refractory germ
cell tumors. Mol Clin Oncol. 2:923–926. 2014.PubMed/NCBI
|
|
2
|
Yang G, Geng C, Li Y, Liu A and Chen W:
Multiple myeloma with extramedullary plasmacytoma invading the skin
and eyeballs following autologous stem cell transplantation: A case
report. Exp Ther Med. 6:883–886. 2013.PubMed/NCBI
|
|
3
|
Yang LJ, Chen Y, He J, Yi S, Wen L, Zhao S
and Cui GH: Effects of gambogic acid on the activation of caspase-3
and downregulation of SIRT1 in RPMI-8226 multiple myeloma cells via
the accumulation of ROS. Oncol Lett. 3:1159–1165. 2012.PubMed/NCBI
|
|
4
|
Kwon SJ, Lee JH, Moon KD, Jeong IY, Yee
ST, Lee MK and Seo KI: Isoegomaketone induces apoptosis in SK-MEL-2
human melanoma cells through mitochondrial apoptotic pathway via
activating the PI3K/Akt pathway. Int J Oncol. 45:1969–1976.
2014.PubMed/NCBI
|
|
5
|
Yang Y, Zhou X, Xiao M, Hong Z, Gong Q,
Jiang L and Zhou J: Discovery of chrysoeriol, a PI3K-akt-mTOR
pathway inhibitor with potent antitumor activity against human
multiple myeloma cells in vitro. J Huazhong Univ Sci Technolog Med
Sci. 30:734–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agarwal C, Wadhwa R, Deep G, Biedermann D,
Gažák R, Křen V and Agarwal R: Anti-cancer efficacy of silybin
derivatives-a structure-activity relationship. PLoS One.
8:e600742013. View Article : Google Scholar
|
|
7
|
Tan C, Xu X, Shang Y, Fu X, Xia G and Yang
H: A novel approach for the efficient extraction of silybin from
milk thistle fruits. Pharmacogn Mag. 10:536–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gándara L, Sandes E, Di Venosa G, Prack
McCormick B, Rodriguez L, Mamone L, Batlle A, Eiján AM and Casas A:
The natural flavonoid silybin improves the response to photodynamic
therapy of bladder cancer cells. J Photochem Photobiol B.
133:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahmoodi N, Motamed N and Paylakhi SH: The
comparison of the effects of silybin and
silybin-phosphatidylcholine on viability and ESR expression in
human breast cancer T47D cell line. Cell. J16:299–308. 2014.
|
|
10
|
Zhang S, Yang Y, Liang Z, Duan W, Yang J,
Yan J, Wang N, Feng W, Ding M, Nie Y and Jin Z: Silybin-mediated
inhibition of notch signaling exerts antitumor activity in human
hepatocellular carcinoma cells. PLoS One. 8:e836992013. View Article : Google Scholar
|
|
11
|
Rui M, Huang Z, Liu Y, Wang Z, Liu R, Fu J
and Huang H: Rosiglitazone suppresses angiogenesis in multiple
myeloma via downregulation of hypoxia-inducible factor-1α and
insulin-like growth factor-1 mRNA expression. Mol Med Rep.
10:2137–2143. 2014.PubMed/NCBI
|
|
12
|
Lin M, Zhu J, Shen H and Huang J:
Gastrointestinal bleeding as an initial manifestation in
asymptomatic multiple myeloma: A case report and review of the
literature. Oncol Lett. 5:218–220. 2013.
|
|
13
|
Kabir AL, Rahman MJ, Begum M, Dipta TF,
Baqui MN, Aziz A, Rahman F, Debnath RC and Habib MA: Response of
vincristine, melphalan, cyclophosphamide and prednisolone in
refractory multiple myeloma. Mymensingh Med J. 21:114–119.
2012.PubMed/NCBI
|
|
14
|
Siegel AB, Narayan R, Rodriguez R, Goyal
A, Jacobson JS, Kelly K, Ladas E, Lunghofer PJ, Hansen RJ,
Gustafson DL, et al: A phase I dose-finding study of silybin
phosphatidylcholine (milk thistle) in patients with advanced
hepatocellular carcinoma. Integr Cancer Ther. 13:46–53. 2014.
View Article : Google Scholar
|
|
15
|
Wang L, Wu J, Lu J, Ma R, Sun D and Tang
J: Regulation of the cell cycle and PI3K/akt/mTOR signaling pathway
by tanshinone I in human breast cancer cell lines. Mol Med Rep.
11:931–939. 2015.
|
|
16
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
17
|
García-Maceira P and Mateo J: Silibinin
inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1
signalling pathway in human cervical and hepatoma cancer cells:
Implications for anticancer therapy. Oncogene. 28:313–324. 2009.
View Article : Google Scholar
|
|
18
|
Zhang Y, Hai J, Cao M, Zhang Y, Pei S,
Wang J and Zhang Q: Silibinin ameliorates steatosis and insulin
resistance during non-alcoholic fatty liver disease development
partly through targeting IRS-1/PI3K/akt pathway. Int
Immunopharmacol. 17:714–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong G, Hu L, Liu Y, Bai S, Dai X, Yin L,
Sun Y, Wang X and Hou L: Upregulation of HIF-1α protein induces
mitochondrial autophagy in primary cortical cell cultures through
the inhibition of the mTOR pathway. Int J Mol Med. 34:1133–1140.
2014.PubMed/NCBI
|
|
20
|
Chang Z, Shi G, Jin J, Guo H, Guo X, Luo
F, Song Y and Jia X: Dual PI3K/mTOR inhibitor NVP-BEZ235-induced
apoptosis of hepatocellular carcinoma cell lines is enhanced by
inhibitors of autophagy. Int J Mol Med. 31:1449–1456.
2013.PubMed/NCBI
|
|
21
|
Raina K, Agarwal C, Wadhwa R, Serkova NJ
and Agarwal R: Energy deprivation by silibinin in colorectal cancer
cells: A double-edged sword targeting both apoptotic and autophagic
machineries. Autophagy. 9:697–713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CH, Li CH, Liao PL, Tse LS, Huang WK,
Cheng HW and Cheng YW: Silibinin inhibits VEGF secretion and
age-related macular degeneration in a hypoxia-dependent manner
through the PI-3 kinase/akt/mTOR pathway. Br J Pharmacol.
168:920–931. 2013. View Article : Google Scholar :
|
|
23
|
Han S, Zhang G, Li M, Chen D, Wang Y, Ye W
and Ji Z: L-securinine induces apoptosis in the human promyelocytic
leukemia cell line HL-60 and influences the expression of genes
involved in the PI3K/AKT/mTOR signaling pathway. Oncol Rep.
31:2245–2251. 2014.PubMed/NCBI
|
|
24
|
Liu X, Wang L, Chen J, Ling Q, Wang H, Li
S, Li L, Yang S, Xia M and Jing L: Estrogen receptor β agonist
enhances temozolomide sensitivity of glioma cells by inhibiting
PI3K/AKT/mTOR pathway. Mol Med Rep. 11:1516–1522. 2015.
|
|
25
|
Wang C, Wang Z, Zhang X, Zhang X, Dong L,
Xing Y, Li Y, Liu Z, Chen L, Qiao H, et al: Protection by silibinin
against experimental ischemic stroke: Up-regulated pAkt, pmTOR,
HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci
Lett. 529:45–50. 2012. View Article : Google Scholar : PubMed/NCBI
|