Introduction
Inflammation, which is a reactive response of the
body to exogenous microbes or harmful damage, is predominantly
achieved by increased recruitment of immune cells to injured or
infected tissues (1). Inflammation
is regulated by a cascade of numerous molecular interactions and
biochemical reactions, which are responsible for propagation and
maturation of the inflammatory response. Various cytokines and
proinflammatory mediators, including interleukin (IL)-1β, tumor
necrosis factor-α (TNF-α), IL-6, nitric oxide (NO) and
prostaglandins (PGs), are involved in the inflammatory response.
IL-1β, investigated for its fever-inducing and inflammatory
properties, is considered a typical proinflammatory cytokine
(2). Furthermore, TNF-α is
regarded as a central cytokine in the development of several
autoimmune diseases (3). The
ability to suppress IL-1β and TNF-α production in vitro and
in vivo has been widely applied to screen anti-inflammatory
agents (4). IL-6 is a pleiotropic
cytokine that is involved in the regulation of immune responses,
the acute-phase reaction of inflammation, and hematopoiesis
(5). Inhibitors of these cytokines
are widely used in the treatment of numerous autoimmune diseases.
Cyclooxygenases (COX) are key enzymes responsible for inflammation,
and the conversion of arachidonic acid to PGs and thromboxane. PGs
are derived from arachidonic acid, the conversion of which is
catalyzed by COX-2 (6), and are
essential for generation of the inflammatory response (7). COX-2 is induced by several
inflammatory stimuli, including cytokines and growth factors,
whereas COX-1 is constitutively expressed in various tissues
(8). In addition, inducible nitric
oxide synthase (iNOS) sustainably produces a high output of NO,
which is an important inflammatory reaction in activated
macrophages (9).
Macrophages are critical for the initiation,
maintenance and resolution of the inflammatory response, including
the overproduction of proinflammatory mediators, such as TNF-α,
IL-1β, IL-6, NO and PGE2 (10). Chamaecyparis obtusa is a
type of tropical tree species found in Japan and the southern
regions of South Korea. Essential oil extracted from the pruned
leaves and twigs of C. obtusa (EOCO) contains various types
of monoterpenes, including α-terpinyl acetate, β-phellandrene,
β-myrcene, limonene, bornyl acetate, γ-terpinene, β-thujaplicin and
α-terpineol (11,12). Although the biological activities
of EOCO remain to be elucidated, antimicrobial, antifungal and
anti-inflammatory effects have previously been reported (13,14).
EOCO has been demonstrated to reduce the production of
PGE2, and the mRNA expression levels of TNF-α and COX-2
in LPS-stimulated peripheral blood mononuclear cells from rats
(15). In addition, β-thujaplicin,
an active constituent from C. obtusa, has been reported to
decrease NO, PGE2, IL-6 and TNF-α levels, and suppress
iNOS, COX2 and nuclear factor-κB expression in LPS-stimulated RAW
264.7 cells (16). However, the
anti-inflammatory effects of C. obtusa have yet to be
confirmed in animal models of inflammation.
In the present study, the anti-inflammatory effects
of EOCO were investigated on carrageenan-induced paw edema and
thioglycollate-induced peritonitis mouse models. The levels of
IL-1β, IL-6 and TNF-α were then detected in paw homogenates and
peritoneal fluid. In addition, the inhibitory effects of EOCO were
determined on the secretion of proinflammatory mediators, and the
expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells.
Materials and methods
Animals
Male C57BL/6 (B6) and ICR mice (weight, 20–30 g)
were purchased from Orient Bio, Inc. (Seongnam, Korea). The mice
were maintained under specific pathogen-free conditions at the
Catholic Research Institute of Medical Science of the Catholic
University of Korea (Seoul, Korea), and were fed standard mouse
chow and water. The mice were maintained in Plexiglass cages at a
constant temperature of 22±2°C and a relative humidity of 55±5%,
with a 12 h dark-light cycle for at least 1 week prior to the
experimental session. All experimental procedures were approved by
the Animal Research Ethics Committee of the Catholic University of
Korea.
EOCO treatment
EOCO used in the present study was obtained from
Fosto Company (Seoul, Korea). EOCO was emulsified in dimethyl
sulfoxide (DMSO; 1:1, v/v; Sigma-Aldrich, St. Louis, MO, USA) and
was administered intraperitoneally (i.p.) at two different
concentrations (5 or 10 mg/kg) in distilled water. EOCO was
administered 1 h prior to injection with one of the
inflammation-inducing chemical agents: Carrageenan (n=7 per group)
or thioglycollate (n=6 per group). The control mice were treated
with the same volume of distilled water containing DMSO.
Carrageenan-induced paw edema model
The initial hind paw thickness of the ICR mice was
determined using a pocket thickness gauge (0–5 mm) (17). Each group of mice received
subplantar administration of 50 µl carrageenan (2% w/v;
Sigma-Aldrich) in saline into the right hind paw 1 h following
injection with EOCO or vehicle. Mice in the control group
(carrageenan-negative group) were injected with an equivalent
volume of saline solution at the same time-point as carrageenan was
administered to the other groups. Paw thickness was measured prior
to the carrageenan injection, and at 1, 2, 3, 4 and 5 h following
carrageenan injection. The degree of swelling induced at each
time-point was calculated as the difference between the initial
hind paw thickness and the thickness at the respective hour
following carrageenan injection.
At 5 h, the mice were sacrificed by cervical
dislocation and the skin tissues were removed from the
carrageenan-injected right hind paws of each experimental group.
Subsequent to rinsing in ice-cold phosphate-buffered saline (PBS),
the tissue was homogenized, and the proteins were extracted from
150 mg tissue/ml PBS containing 0.4 M NaCl, 0.05% Tween 20, 0.1 mM
phenylmethylsulfonyl fluoride (PMSF), 10 mM EDTA and complete
protease-inhibitor cocktail (one tablet/50 ml; Roche Diagnostics,
Indianapolis, IN, USA) at 4°C (18). Following centrifugation twice at
12,000 × g for 15 min at 4°C, the supernatant was stored at −70°C
for the determination of cytokine levels.
Thioglycollate-induced peritonitis
model
Mice were injected intraperitoneally with 5 or 10
mg/kg EOCO, 1 h prior to thioglycollate injection. C57BL/6 mice
were administered an i.p. injection of 2 ml sterile 3%
thioglycollate (BD Biosciences, Franklin Lakes, NJ, USA), or PBS as
a control (19). After 4 h, the
mice were sacrificed by cervical dislocation, and the peritoneal
cavity was flushed with 1.0 ml Hanks' buffered salt solution
without Mg2+ and Ca2+ (Welgene, Seoul, South
Korea). Following centrifugation at 1,000 × g for 5 min at room
temperature, the supernatant was stored at −20°C for cytokine
measurement by enzyme-linked immunosorbent assay (ELISA), and the
pelleted peritoneal cells were collected in order to count the cell
numbers. To collect more peritoneal cells, the peritoneal cavity
was washed again with 10 ml Hanks' buffered salt solution. The
collected peritoneal cells from each sample were resuspended in 5
ml Hanks' buffered salt solution, and the number of cells was
determined manually with a hemocytometer using trypan blue (Gibco;
Thermo Fisher Scientific, Inc.) (20).
Cell culture
The RAW 264.7 murine macrophage cell line was
obtained from American Type Culture Collection (Manassas, VA, USA).
The cells were cultured in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% heat inactivated fetal
bovine serum (Wisent, Inc., St. Bruno, QC, Canada) and were
maintained in a humidified 37°C incubator containing 5%
CO2.
Measurement of NO production
The quantity of nitrite in the culture medium was
measured as an indicator of NO production. For NO quantification,
RAW 264.7 cells were seeded at a density of 5×105
cells/well in 6-well plates and were pretreated with EOCO (1, 10,
50 or 100 µg/ml) for 1 h, followed by a 24 h incubation with
LPS (1 µg/ml; Sigma-Aldrich), as described previously
(21). Following 24 h of
stimulation, 100 µl of each supernatant was collected, mixed
with the same volume of Griess reagent (1% sulfanilamide and 0.1%
naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid),
and incubated for 5 min at room temperature. The quantity of
nitrite was determined by measuring the absorbance at a wavelength
of 540 nm using an ELISA plate reader (Synergy™ MX, BioTek
Instruments, Inc., Winooski, VT, USA). Nitrite concentration was
calculated using standard solutions of sodium nitrite
(NaNO2).
Cell viability assay
RAW 264.7 cells (2×104 cells/well) were
seeded onto 96-well flat-bottom plates and were pre-incubated for
15–18 h. The cells were left untreated or were treated with various
concentrations of EOCO (1, 10, 50 or 100 µg/ml), and were
incubated at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h. A total of 10 µl MTT (2.5 mg/ml;
Sigma-Aldrich) was added to each well and the cells were incubated
for a further 4 h. The MTT was then removed and the cells were
lysed by the addition of 100 µl DMSO to each well. The
optical density was measured at a wavelength of 540 nm using a
Synergy™ MX microplate reader.
ELISA
The expression levels of IL-1β, TNF-α and IL-6 were
determined in paw homogenates, peritoneal fluid and the supernatant
of lysed LPS-stimulated RAW 264.7 cells using ELISA kits (R&D
Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer's protocols.
Western blot analysis
The stimulated RAW 264.7 cells were washed twice
with PBS and lysed in lysis buffer [(0.5 M NaCl, 20 mM Tris-HCl,
0.25% Triton X-100, 1 mM ethylene glycol tetraacetic acid, 1 mM
EDTA, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM benzamidine, 300
µM Na3VO4, 2 µM PMSF, 1 mM
dithiothreitol and one protease inhibitor cocktail tablet] on ice
for 1 h, followed by centrifugation at 12,000 × g for 20 min at
4°C. The protein concentration of the lysates was determined using
the Bradford dye-binding assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A total of 50 µg protein from the
supernatants was separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and was transferred to
nitrocellulose membranes (GE Healthcare Life Sciences, Chalfont,
UK) for 130 min at 100 V. Subsequently, the membranes were blocked
with 5% skim milk in Tris-buffered saline with Tween 20 (TBS-T; 20
mM Tris, 500 mM NaCl, KCl, pH 7.5, and 0.05% Tween 20) for 2 h at
room temperature. The membranes were then agitated in blocking
buffer containing rabbit anti-iNOS immunoglobulin (Ig)G (1:1,000
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no.
sc-8310), rabbit polyclonal anti-COX-2 (1:1,000; Cayman Chemical
Company, Ann Arbor, MI, USA; cat. no. 160106) or mouse monoclonal
anti-β-actin (1:10,000; ABM, Inc., Richmond, BC, Canada) antibodies
overnight in a cold room. Membranes were washed with TBS-T at 10
min intervals for 30 min and were then incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:1,000; Santa
Cruz Biotechnology, Inc.; cat. no. sc-2004) or HRP-conjugated goat
anti-mouse IgG (1:10,000; Santa Cruz Biotechnology, Inc., cat. no.
sc-2005) for 2 h at room temperature. Immunoreactive proteins were
visualized using the enhanced chemiluminescence detection system
(GE Healthcare Life Sciences) on a LAS-4000 (Fujifilm, Tokyo,
Japan). Densitometric values for each band were determined using
ImageJ software, version 1.48 (National Institutes of Health,
Bethesda, MD, USA), and were statistically analyzed.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Comparisons between the groups were performed with
Mann-Whitney U-tests. All data were analyzed using SAS 9.1 software
(SAS Institute, Cary, NC, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of EOCO on carrageenan-induced
paw edema
To determine the anti-inflammatory effects of EOCO,
a carrageenan-induced paw edema mouse model was used. Mice were
treated with EOCO (i.p., 5 or 10 mg/kg) or vehicle (control) 1 h
prior to carrageenan injection, and carrageenan-induced paw
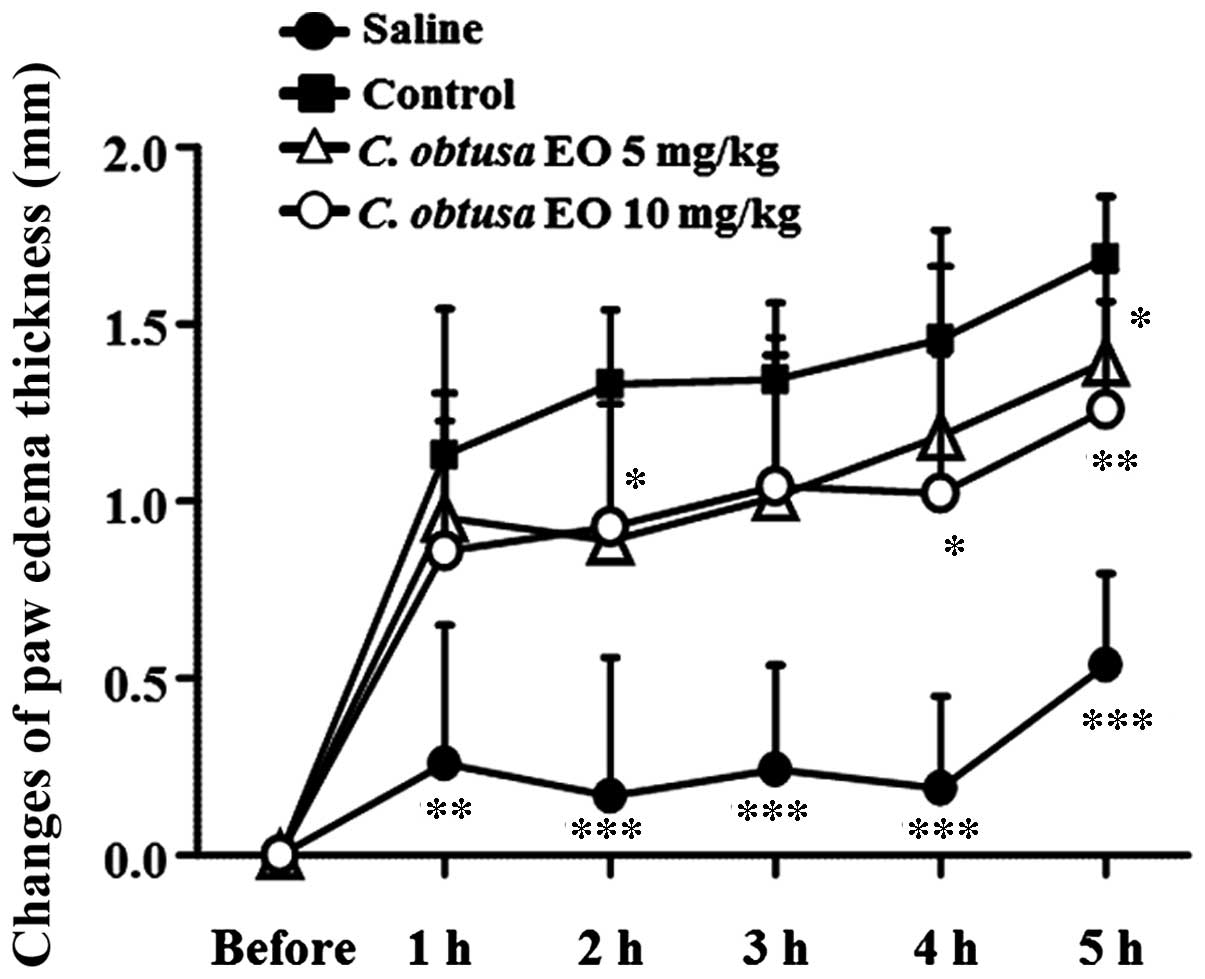
thickness was measured. As presented in Fig. 1, paw thickness increased sharply 1
h after the subplantar injection of carrageenan, and consecutively
increased until 5 h after the injection. Administration of 5 mg/kg
EOCO (i.p.) significantly reduced paw edema 2 and 5 h following
carrageenan injection, as compared with the control group
(P<0.05). In addition, treatment with 10 mg/kg EOCO
significantly inhibited carrageenan-induced paw edema 4 and 5 h
following injection (P<0.05 and P<0.01, respectively).
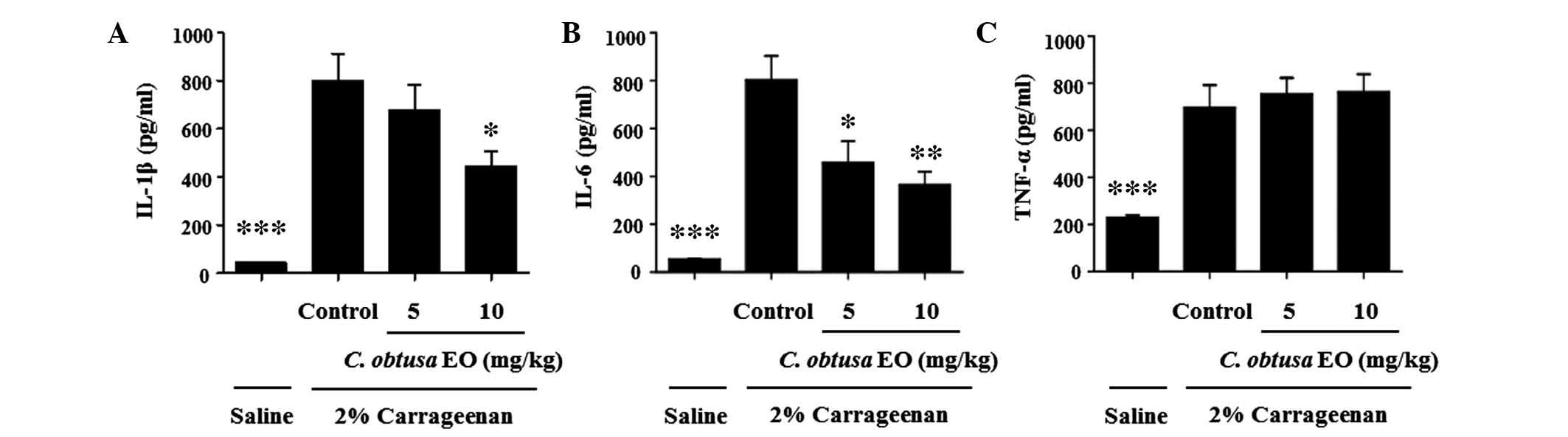
To investigate the mechanism underlying the
inhibitory effects of EOCO on the development of
carrageenan-induced paw edema, the levels of proinflammatory
cytokines in the paw tissue were determined. In the paw
homogenates, the levels of IL-1β, IL-6 and TNF-α were significantly
increased by carrageenan injection, as compared with saline
(P<0.01; Fig. 2). The
administration of 10 mg/kg EOCO markedly inhibited the
carrageenan-induced increased levels of IL-1β and IL-6, as compared
with the control group (P<0.05 and P<0.01, respectively).
Conversely, 5 mg/kg EOCO significantly reduced IL-6 levels only.
However, EOCO treatment did not affect the levels of TNF-α
(Fig. 2C).
Effects of EOCO on thioglycollate-induced
peritonitis
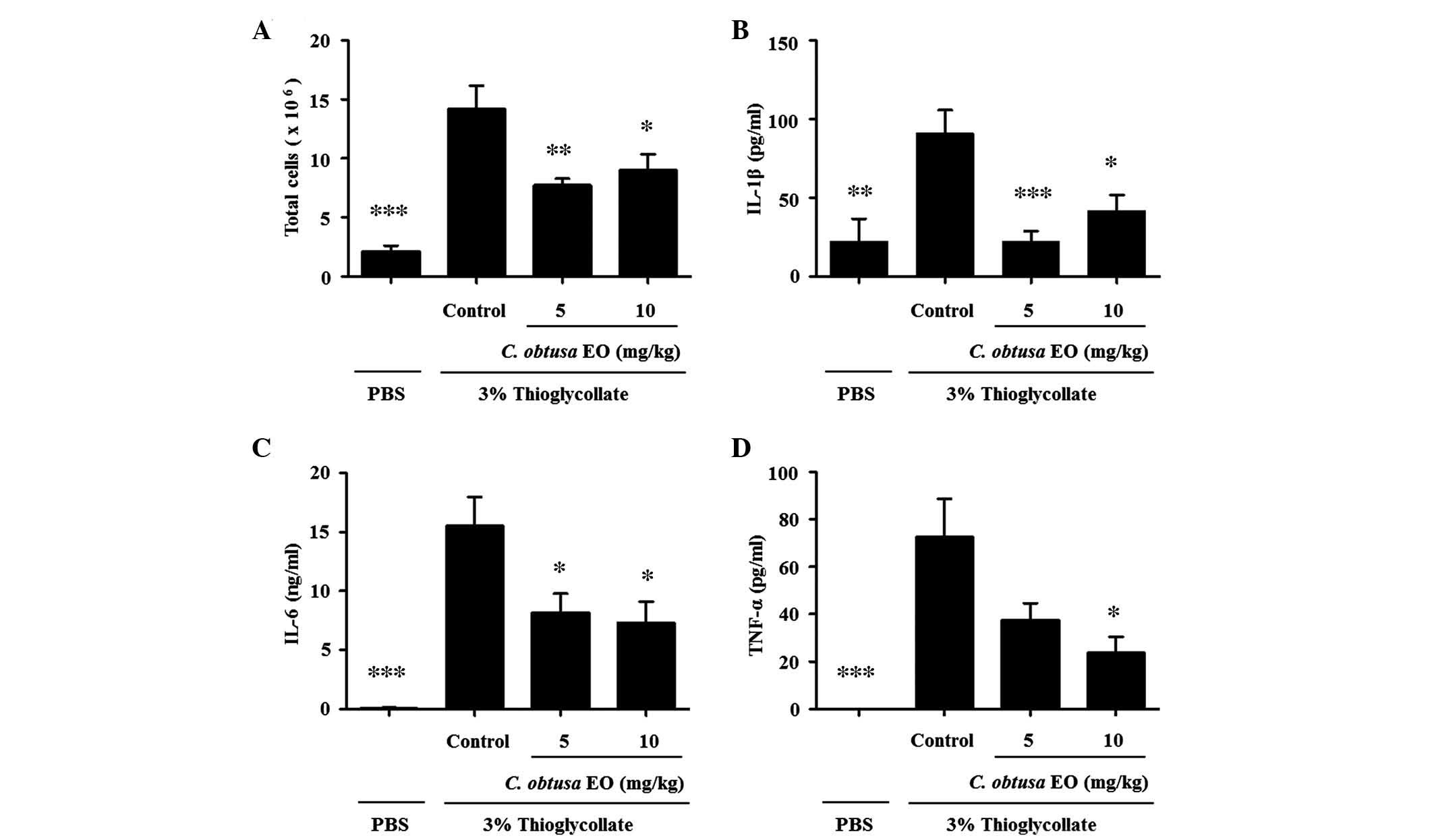
To investigate the anti-inflammatory effects of EOCO
on a mouse model of peritonitis, mice were pretreated with EOCO
(i.p.) 1 h prior to injection with 2 ml 3% thioglycollate (i.p.) to
induce peritonitis. After 4 h, mice were sacrificed and the
peritoneal fluid was collected. Total cells recruited into the
peritoneal cavity following thioglycollate injection were markedly
increased, as compared with in PBS-injected mice (Fig. 3A, P<0.01). Treatment with 5 or
10 mg/kg EOCO significantly reduced the number of total cells
recruited into the peritoneal cavity, as compared with the control
group (P<0.01 and P<0.05, respectively). In addition, the
levels of IL-1β, IL-6 and TNF-α in the peritoneal lavage fluid were
significantly reduced by EOCO treatment, as compared with the
control group (Fig. 3B–D;
P<0.05).
Effects of EOCO on LPS-stimulated RAW
264.7 cells
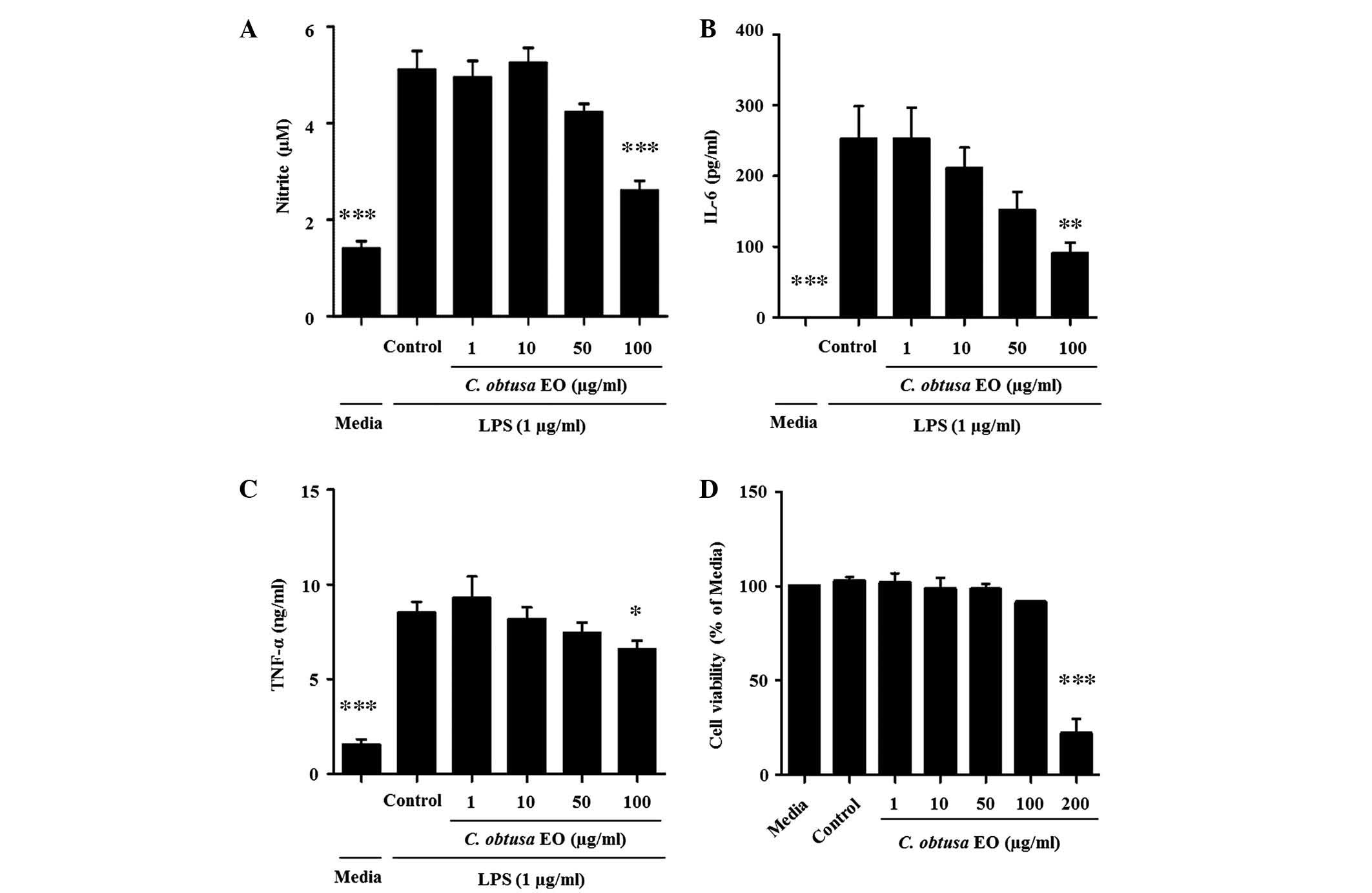
To investigate whether the anti-inflammatory effects
of EOCO in thioglycollate-induced peritonitis were due to the
inhibition of macrophage function, LPS-stimulated RAW 264.7 murine
macrophage cells were treated with EOCO. RAW 264.7 cells were
cultured with LPS (1 µg/ml) in the presence or absence of
EOCO (1, 10, 50 and 100 µg/ml). EOCO decreased the
production of NO, IL-6 and TNF-α by LPS-stimulated RAW 264.7 cells
in a dose-dependent manner, and the inhibition was statistically
significant at a dose of 100 µg/ml (P<0.001, P<0.01
and P<0.05, respectively; Fig.
4A–C). The cytotoxicity of EOCO on RAW 264.7 cells was
determined using the MTT assay. Cells cultured in the presence of
EOCO (1–100 µg/ml) for 24 h demonstrated no change in
viability, as compared with those incubated in culture media only.
However, cell viability was significantly decreased by ~78.8%
following treatment with 200 µg/ml EOCO (Fig. 4D).
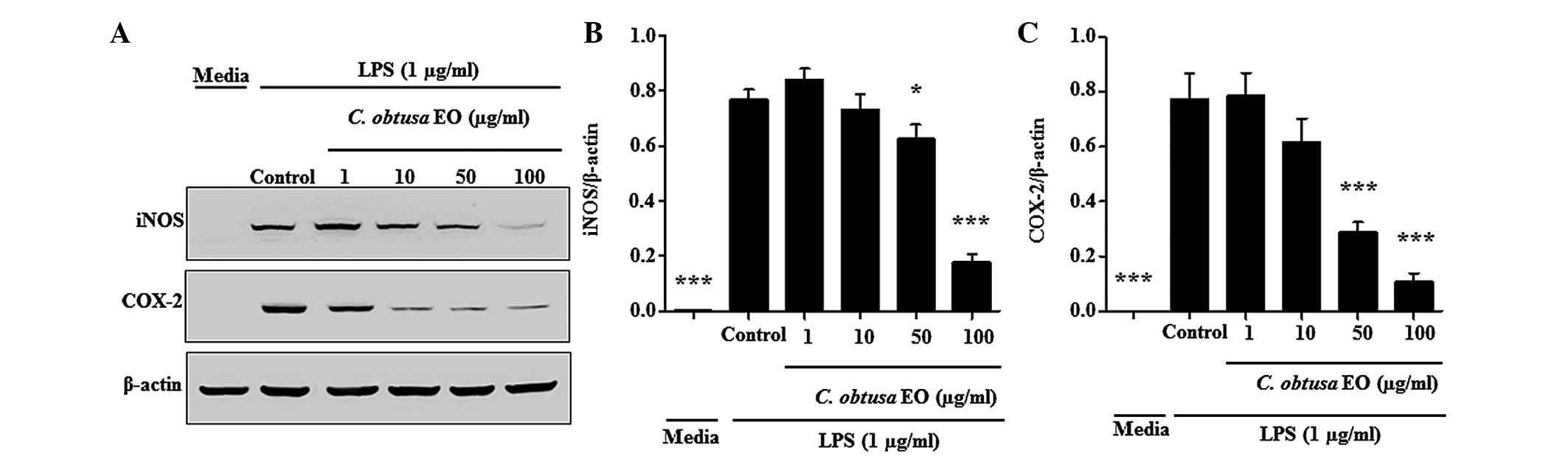
In order to investigate whether the inhibition of NO
production was due to decreased iNOS and COX-2 protein expression,
the effects of EOCO on iNOS and COX-2 protein expression levels
were analyzed by western blotting. As presented in Fig. 5, the protein expression levels of
iNOS and COX-2 in RAW 264.7 cells were significantly increased by
LPS. The LPS-stimulated expression levels of iNOS and COX-2
proteins were significantly inhibited by treatment with 50 and 100
µg/ml of EOCO (Fig. 5).
Discussion
The results of the present study demonstrated the
anti-inflammatory effects of EOCO in vivo and in
vitro. Administration of EOCO significantly suppressed
carrageenan-induced paw edema, and the production of IL-1β and IL-6
in the paws. In the thioglycollate-induced peritonitis model, the
number of total cells, and the levels of IL-1β, IL-6 and TNF-α were
significantly reduced in the peritoneal fluid. Carrageenan-induced
paw inflammation is a commonly used model for the investigation of
novel anti-inflammatory agents (22). The development of paw edema in mice
following injection with the phlogistic agent is hypothesized to be
a biphasic mechanism, of which the first 1–2 h is due to the
release of histamine or serotonin, and the second phase of edema
formation is due to the release of PGs/protease and lysosome, which
peaks at 3 h (22). Local release
of proinflammatory cytokines, including TNF-α, IFN-γ and IL-1β, is
also induced by carrageenan injection (23). In the present study, EOCO exhibited
significant inhibitory effects 2 h after carrageenan treatment,
which continued to 5 h. These results suggested that the underlying
mechanism of action of EOCO may involve blocking PG synthesis
and/or proinflammatory cytokine release.
Murine thioglycollate-induced peritonitis is an
appropriate model with which to study inflammatory events, which
are accompanied by inflammatory mediator production and leukocyte
accumulation. In this model, neutrophil numbers start to increase
and reach peak levels between 4 and 24 h after treatment, whereas
macrophage numbers start to increase at 24 h and reach peak levels
at 3 to 4 days (24). In addition
to leukocyte recruitment, another primary response to inflammation
is the secretion of proinflammatory cytokines, such as IL-1β, IL-6
and TNF-α, by resident or recruited macrophages. The results of the
present study suggested that administration of EOCO may alleviate
thioglycollate-induced peritonitis by inhibiting the function of
intraperitoneal macrophages.
The present study also detected the effects of EOCO
on macrophages. Treatment with EOCO inhibited the production of NO,
IL-6 and TNF-α in LPS-stimulated RAW 264.7 cells. In addition, the
expression levels of iNOS and COX-2 were suppressed by EOCO
treatment.
In conclusion, the results of the present study
demonstrated that EOCO may exert anti-inflammatory effects in
animal models via its ability to decrease the production of
proinflammatory mediators and cytokines. These data suggest that
EOCO may be therapeutically useful for the treatment of
inflammatory diseases.
Acknowledgments
The present study was conducted with the support of
Forest Science & Technology Projects (project no.
S111114L020100) provided by Korea Forest Service.
Abbreviations:
|
C. obtusa
|
Chamaecyparis obtusa
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6
|
|
NO
|
nitric oxide
|
|
COX-2
|
cyclooxygenase-2
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
References
|
1
|
Pomin VH: Sulfated glycans in
inflammation. Eur J Med Chem. 92:353–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luger TA, Stadler BM, Luger BM, Mathieson
BJ, Mage M, Schmidt JA and Oppenheim JJ: Murine epidermal
cell-derived thymocyte-activating factor resembles murine
interleukin 1. J Immunol. 128:2147–2152. 1982.PubMed/NCBI
|
|
3
|
Keffer J, Probert L, Cazlaris H,
Georgopoulos S, Kaslaris E, Kioussis D and Kollias G: Transgenic
mice expressing human tumour necrosis factor: A predictive genetic
model of arthritis. EMBO J. 10:4025–4031. 1991.PubMed/NCBI
|
|
4
|
Dinarello CA: Anti-inflammatory agents:
Present and future. Cell. 140:935–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tilg H, Trehu E, Atkins MB, Dinarello CA
and Mier JW: Interleukin-6 (IL-6) as an anti-inflammatory cytokine:
Induction of circulating IL-1 receptor antagonist and soluble tumor
necrosis factor receptor p55. Blood. 83:113–118. 1994.PubMed/NCBI
|
|
6
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HS, Kim T, Kim MK, Suh DH, Chung HH
and Song YS: Cyclooxygenase-1 and -2: Molecular targets for
cervical neoplasia. J Cancer Prev. 18:123–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pokharel YR, Liu QH, Oh JW, Woo ER and
Kang KW: 4-Hydroxykobusin inhibits the induction of nitric oxide
synthase by inhibiting NF-kappaB and AP-1 activation. Biol Pharm
Bull. 30:1097–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bae D, Seol H, Yoon HG, Na JR, Oh K, Choi
CY, Lee DW, Jun W, Youl Lee K, Lee J, et al: Inhaled essential oil
from Chamaecyparis obtuse ameliorates the impairments of cognitive
function induced by injection of β-amyloid in rats. Pharm Biol.
50:900–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joo SS, Yoo YM, Ko SH, Choi W, Park MJ,
Kang HY, Choi KC, Choi IG and Jeung EB: Effects of essential oil
from Chamaecypris obtusa on the development of atopic
dermatitis-like skin lesions and the suppression of Th cytokines. J
Dermatol Sci. 60:122–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong EJ, Na KJ, Choi IG, Choi KC and Jeung
EB: Antibacterial and antifungal effects of essential oils from
coniferous trees. Biol Pharm Bull. 27:863–866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Q, Kobayashi M, Wakayama Y, Inagaki H,
Katsumata M, Hirata Y, Hirata K, Shimizu T, Kawada T, Park BJ, et
al: Effect of phytoncide from trees on human natural killer cell
function. Int J Immunopathol Pharmacol. 22:951–959. 2009.
|
|
15
|
An BS, Kang JH, Yang H, Jung EM, Kang HS,
Choi IG, Park MJ and Jeung EB: Anti-inflammatory effects of
essential oils from Chamaecyparis obtusa via the cyclooxygenase-2
pathway in rats. Mol Med Rep. 8:255–259. 2013.PubMed/NCBI
|
|
16
|
Shih MF, Chen LY, Tsai PJ and Cherng JY:
In vitro and in vivo therapeutics of β-thujaplicin on LPS-induced
inflammation in macrophages and septic shock in mice. Int J
Immunopathol Pharmacol. 25:39–48. 2012.PubMed/NCBI
|
|
17
|
Paul S, Shin HS and Kang SC: Inhibition of
inflammations and macrophage activation by ginsenoside-Re isolated
from Korean ginseng (Panax ginseng C.A. Meyer). Food Chem Toxicol.
50:1354–1361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Lima FO, Alves V, Barbosa Filho JM,
Almeida JR, Rodrigues LC, Soares MB and Villarreal CF:
Antinociceptive effect of lupeol: Evidence for a role of cytokines
inhibition. Phytother Res. 27:1557–1563. 2013.
|
|
19
|
Call DR, Nemzek JA, Ebong SJ, Bolgos GL,
Newcomb DE and Remick DG: Ratio of local to systemic chemokine
concentrations regulates neutrophil recruitment. Am J Pathol.
158:715–721. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Louis KS and Siegel AC: Cell viability
analysis using trypan blue: Manual and automated methods. Methods
Mol Biol. 740:7–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang GJ, Huang SS and Deng JS:
Anti-inflammatory activities of inotilone from Phellinus linteus
through the inhibition of MMP-9, NF-κB, and MAPK activation in
vitro and in vivo. PLoS One. 7:e359222012. View Article : Google Scholar
|
|
22
|
Posadas I, Bucci M, Roviezzo F, Rossi A,
Parente L, Sautebin L and Cirino G: Carrageenan-induced mouse paw
oedema is biphasic, age-weight dependent and displays differential
nitric oxide cyclooxygenase-2 expression. Br J Pharmacol.
142:331–338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JP, Zhou YM, Ye YJ, Shang XM, Cai YL,
Xiong CM, Wu YX and Xu HX: Topical anti-inflammatory and analgesic
activity of kirenol isolated from Siegesbeckia orientalis. J
Ethnopharmacol. 137:1089–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam D, Harris D and Qin Z: Inflammatory
mediator profiling reveals immune properties of chemotactic
gradients and macrophage mediator production inhibition during
thioglycollate elicited peritoneal inflammation. Mediators Inflamm.
2013:9315622013. View Article : Google Scholar : PubMed/NCBI
|