Introduction
Ovarian cancer has the lowest survival rate of all
gynecological malignancies (1).
Ovarian cancers predominantly include three types of tumor,
epithelial tumors, germ cell tumors and stromal tumors (2). Of all ovarian cancers, 85–90% are
epithelial ovarian carcinomas (3,4) and
~70% of patients are diagnosed at an advanced stage with pelvic or
lymph node metastasis. Only 1/4 patients are diagnosed at an early
stage and the worldwide five-year survival rate of patients with
advanced ovarian cancer is 20–25% (5,6). It
is difficult to detect ovarian cancer at an early stage
predominantly due to its inherent metastatic nature resulting in a
poor prognosis (7).
Surgery combined with chemotherapy is an important
therapeutic strategy for ovarian cancer. Cisplatin is a first line
platinum-based chemotherapeutic agent, which exerts marked
antitumor activity in a number of solid tumors (8–12).
It is also one of the most commonly used agents for the treatment
of ovarian cancer. However, the extensive application of cisplatin
may result in adverse gastrointestinal toxicity, including severe
nausea and vomiting, renal toxicity and neurotoxicity (13). Notably, long-term use of cisplatin
results in drug resistance, which is a major obstacle in cancer
chemotherapy (14–16). Investigation into therapeutic
strategies with increased efficacy in order to decrease the side
effects of treatment or reduce drug resistance in ovarian cancer is
required.
Recently, natural products extracted from medicinal
plants have drawn more attention in cancer therapy. Oridonin is
extracted from the Chinese herb Rabdosia rubescens, and is a
natural compound with the structure of a tetracycline diter-penoid
(17,18). It has been reported to exert
antitumor effects and is widely used, in China, in the clinical
treatment of a number of tumor types. Qi et al (19) reported that oridonin effectively
induced cell apoptosis of pancreatic cancer cells, and a
nanosuspension was more effective than free oridonin on
G2/M-phase cell cycle arrest and apoptosis in the PANC-1
human pancreatic cancer cell line. Gao et al (20) demonstrated that oridonin induces
apoptosis and senescence by increasing hydrogen peroxide and
glutathione depletion in colorectal cancer cells. Furthermore, a
study demonstrated that autophagy preceded apoptosis in
oridonin-treated MCF-7 human breast cancer cells (21). In lung cancer patients, oridonin
also suppressed mammalian target of rapamycin (mTOR) signaling and
the growth of lung cancer tumors, suggesting inhibition of mTORC1
may be an effective target for improving the therapeutic outcome of
treatment with oridonin (22).
In the present study, two cisplatin-resistant
ovarian cancer cell lines, A2780/DDP and SKOV3/DDP, were used to
investigate the underlying mechanism of combined therapy with
oridonin and cisplatin. It is beneficial to elucidate the molecular
mechanism of disease progression to aid in the development of
therapeutic strategies that reverse drug resistance.
Materials and methods
Therapeutic agents and cell lines
Oridonin and cisplatin were obtained from the
Shifeng Biocorporation (Shanghai, China).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT)
was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
A2780/DDP cisplatin-resistant human ovarian cancer subline (Huiying
Corporation, Shanghai, China) and SKOV3/DDP (Yunnan Tumor Hospital,
Kunming, China) were used. The non-resistant cells lines, A2780 and
SKOV3 were obtained from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China). The cells were cultured in Dulbecco's modified
Eagle's medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) enriched with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) at 37°C and 5% CO2. Cisplatin was
obtained from Qilu Pharmaceutical Co., Ltd. (Jinan, China) and 0.5
µg/ml cisplatin was added into the medium to maintain
chemoresistance in the resistant cells.
MTT assay
The inhibitory effect of oridonin alone, cisplatin
alone and oridonin + cisplatin on A2780/DDP and SKOV3/DDP human
ovarian cancer cells was measured using the MTT assay. Cells were
transferred to cispl-atin-free medium 3 days prior to the
experiments. The cells (1.0×104 cells/well) were plated
into 96-well plates and allowed to attach overnight. The cells were
treated with different concentrations of oridonin (10, 40, 80 and
160 µmol/l) or cisplatin (1, 2, 5, 10, 20, 40, 80, 200, 500
or 1,000 µM) for 24, 48 and 72 h, respectively. Control
cells were administered an equal quantity of dimethyl sulfoxide
(DMSO). MTT (20 µl; 5 mg/ml) was added to each well and
incubated for 4 h at 37°C in the dark. The supernatant was removed
and the formazan crystals were dissolved in 100 µl DMSO and
mixed thoroughly prior to determining absorbance at a wavelength of
490 nm using an AquaMate-Plus ultraviolet spectrophotometer (Thermo
Fisher Scientific, Inc.). All in vitro experiments were
conducted in triplicate.
Apoptosis rate analysis
Flow cytometry was used to detect the apoptosis
rate. A2780/DDP cells (2×104 cells/well) were plated
into 6-well plates. They were cultured for 6 to 8 h and treated
with cisplatin alone, oridonin alone, or cisplatin in combination
of oridonin for 48 h. The oridonin concentrations used were: 0, 10,
20, 40, 80 and 160 µmol/l and 50 µM cisplatin. The
cells were analyzed following treatment with RNase (Sigma-Aldrich)
and stained with Annexin V and propidium iodide (PI; Sigma-Aldrich)
for flow cytometry (BD FACSCalibur™; BD Biosciences, Franklin
Lakes, NJ, USA).
Western blotting
Western blotting was performed as described
previously (23–25). Briefly, a total protein extract for
each tissue sample or cell line was dissolved in lysis buffer and
equal quantities of protein (60 µg) were analyzed by
immu-noblotting. Rabbit anti-human polyclonal antibodies against
Bcl-2 (cat. no. sc-492), rabbit anti-human polyclonal antibodies
against Bax (cat. no. 526), rabbit anti-human polyclonal antibodies
against MMP-2 (cat. no. sc-10736) and goat anti-human polyclonal
IgG antibodies against MMP-9 (cat. no. sc-6840) were purchased from
Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and used at a
dilution of 1:1,000. The horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody was obtained from Abgent, Inc. (San
Diego, CA, USA; cat. no. ASS1006).
Statistical analysis
All data were processed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis was performed using
analysis of variance and Student's t-test for continuous data. The
data are presented as the mean ± standard error of the mean and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Half maximal inhibitory concentration
(IC50) value of cisplatin in ovarian cancer cells
In order to detect the anti-tumor effects of
cisplatin on ovarian cancer cells, the A2780 and SKOV3 human
ovarian cancer cell lines and the cisplatin-resistant sublines,
A2780/DDP and SKVO3/DDP, were used as cell models. The ovarian
cancer cells were treated with increasing concentrations of
cisplatin and the inhibitory rate was determined by an MTT assay.
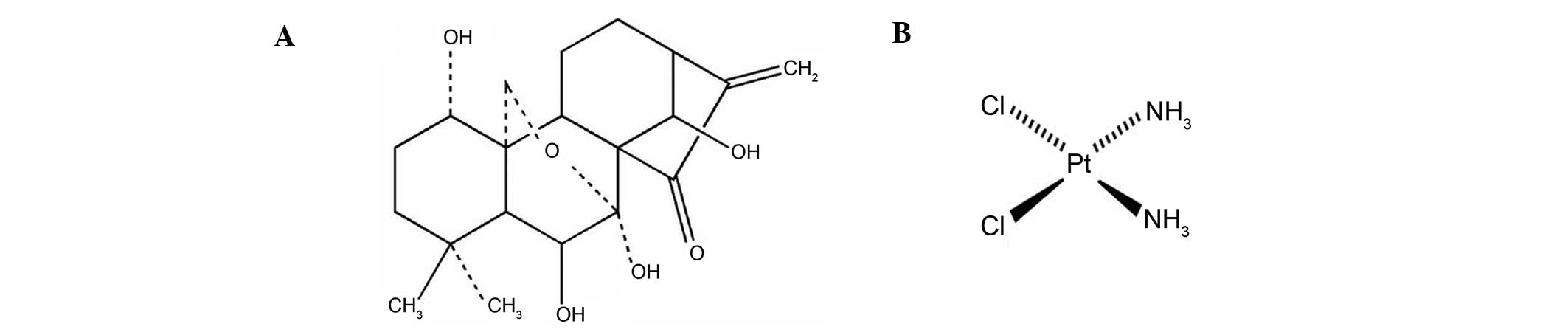
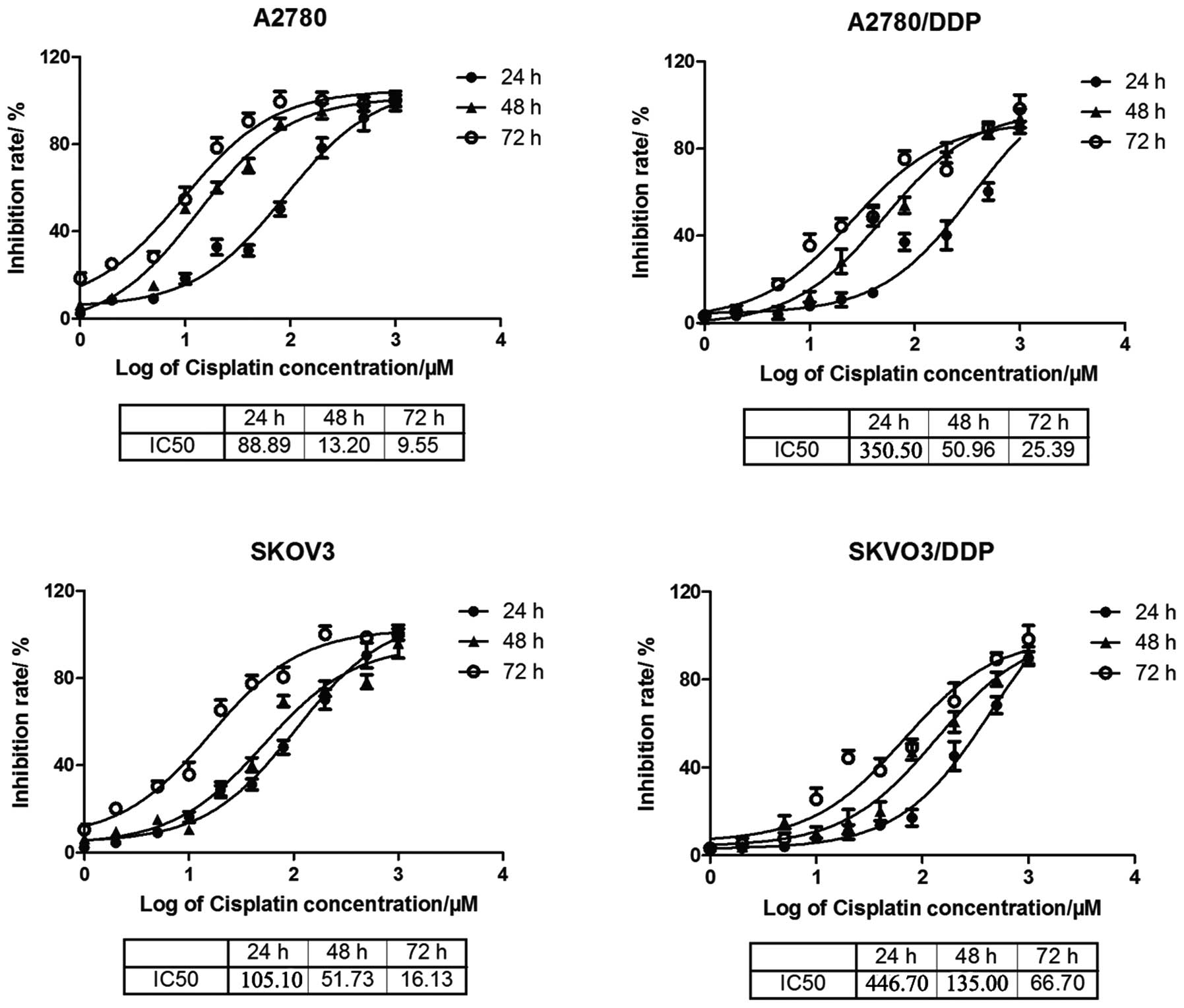
The structure of oridonin and cisplatin is presented in Fig. 1. Cisplatin had an increasing
antitumor effect in A2780 and A2780/DDP human ovarian cancer cell
lines in a dose- and time-dependent manner. As presented in
Fig. 2, the IC50 values
of cisplatin were 88.89, 13.20 and 9.55 µM for 24, 48 and 72
h in the A2780 sensitive cell line, respectively. However, the
IC50 values were 350.50, 50.96 and 25.39 µM in
the A2780/DDP cisplatin-resistant cell line following treatment for
24, 48 and 72 h, respectively. IC50 values in SKOV3 and
SKOV3/DDP were 105.10, 51.73, 16.13 and 446.70, 135.00, 66.70
µM, respectively, for 24, 48 and 72 h.
Oridonin synergistically increases the
antitumor effects of cisplatin in A2780/DDP cells
The inhibitory effects of oridonin in ovarian cancer
cells were also detected by an MTT assay. The A2780/DDP cisplatin
resistant ovarian cancer cells were treated with increasing
concentrations (10, 20, 40, 80 and 160 µM) of oridonin for
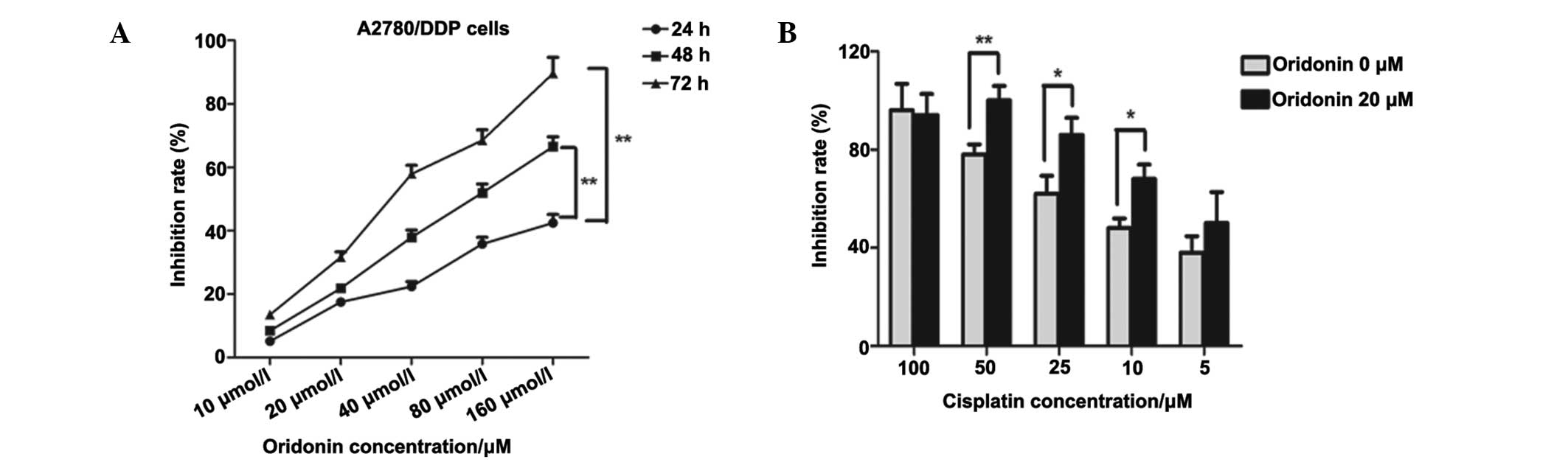
24, 48 and 72 h, respectively. As presented in Fig. 3A, the inhibitory effects increased
in a dose-dependent manner.
In order to determine whether oridonin exerts
synergistic antitumor effects with cisplatin in ovarian cancer
cells, 20 µM was selected as the appropriate concentration
for oridonin as the inhibition rate was effective at ~30% but
relatively low. The concentrations of cisplatin used were 5, 10,
25, 50 and 100 µM. Compared with the group treated with
oridonin alone, the inhibitory effects were significantly increased
at 10, 25 (P<0.05) and 50 µM (P<0.01), which
demonstrated that oridonin and cisplatin demonstrate synergistic
antitumor effects in ovarian cancer cells.
IC50 values of cisplatin were
decreased when administered in combination with 20 µM oridonin in
A2780/DDP and SKOV3/DDP cells
Results from the present study demonstrated that
oridonin synergistically increased the antitumor effects of
cisplatin in A2780/DDP and SKOV3/DDP cells. The cells were treated
with increasing concentrations of cisplatin in combination with 20
µM oridonin for 48 h. The samples had two replicates and the
experiments were conducted twice. The untreated cells were used as
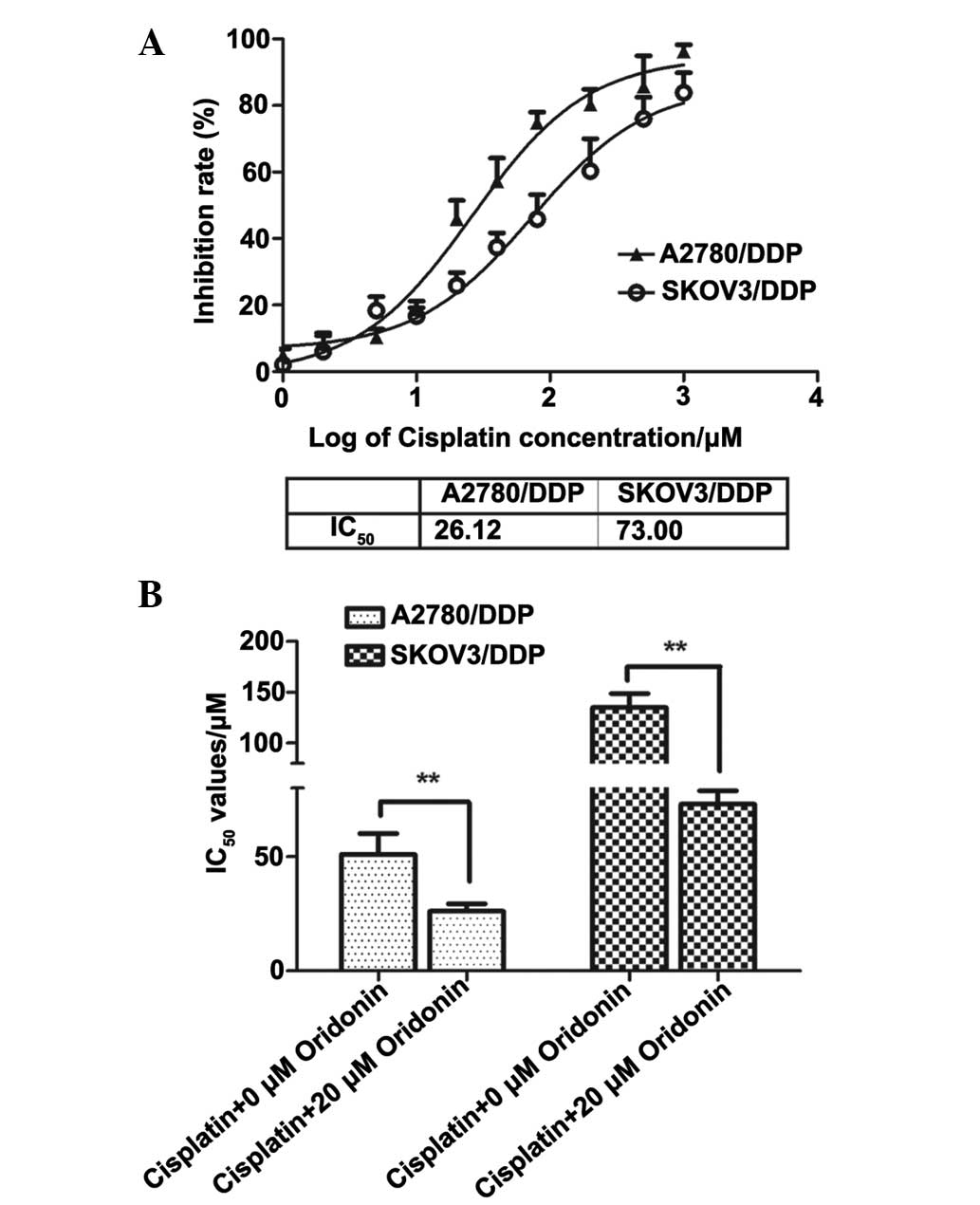
negative controls. As presented in Fig. 4, the IC50 values were
calculated as 26.12 and 73.00 µM for 48 h. The ratio of
IC50 values was downregulated by ~1.95 and 1.84-fold in
A2780/DDP and SKOV3/DDP cells treated with cisplatin + 20 µM
of oridonin, respectively, which demonstrated that oridonin
significantly (P<0.01) decreased the resistance to cisplatin in
A2780/DDP and SKOV3/DDP cells.
Oridonin and cisplatin synergistically
induces cell apoptosis in A2780/DDP cells
The present study demonstrated that the cell death
rates were significantly increased in the cisplatin and oridonin
group. In order to identify whether cell apoptosis in A2780/DDP
cells was induced by treatment with oridonin alone, cisplatin alone
and oridonin in combination with cisplatin for 48 h,
fluorescence-activated cell sorting (FACS) analysis was performed
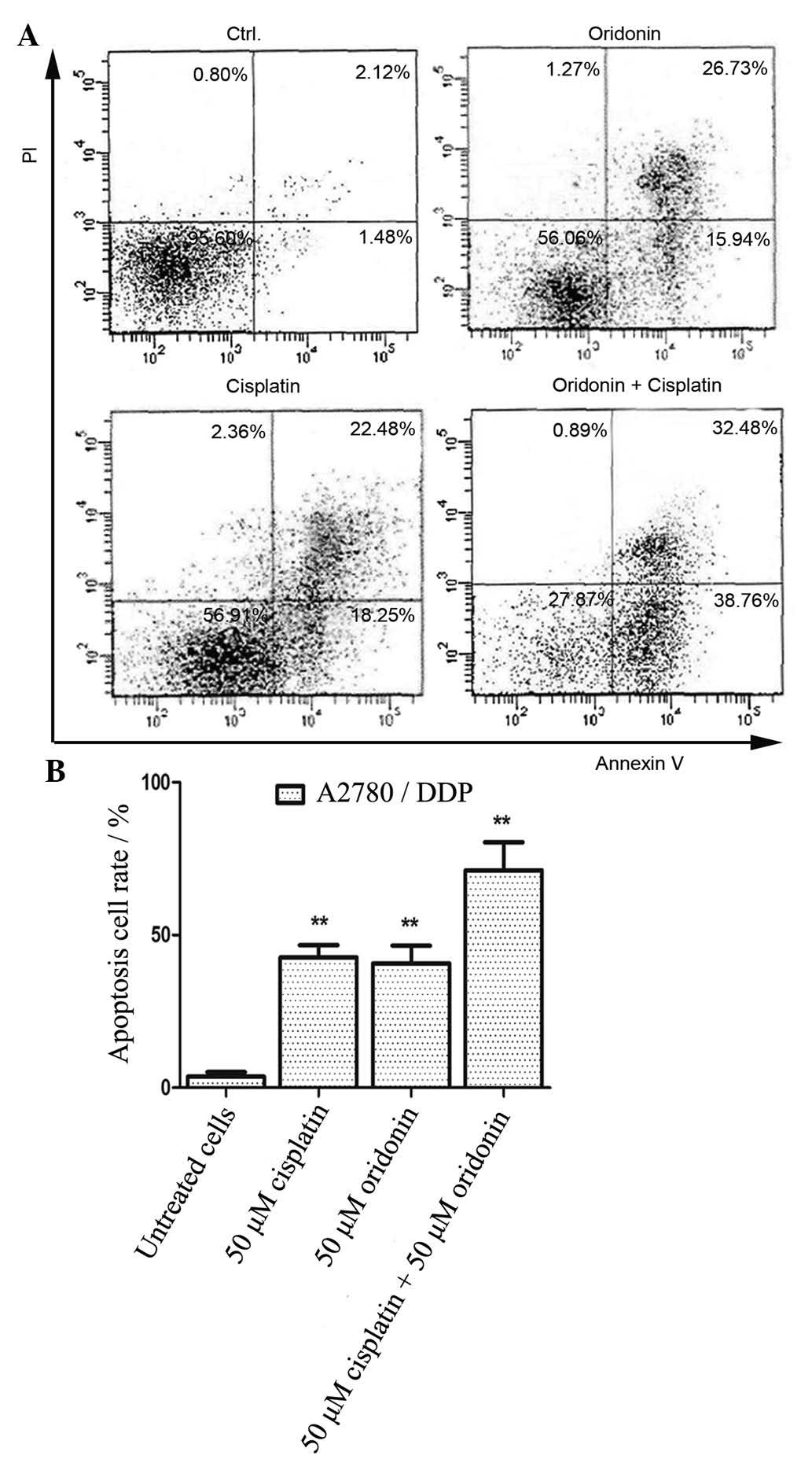
to detect the cell apoptosis rate in A2780/DDP cells. As presented
in Fig. 5A, the cells were treated
with 50 µM cisplatin alone, 50 µM oridonin alone, or
50 µM cisplatin + 50 µM oridonin for 48 h, and the
late apop-tosis rate was 32.48% in the oridonin + cisplatin group,
which was markedly higher than that of the oridonin or cisplatin
alone groups. As presented in Fig.
5B, the early and late cell apoptosis rates were 42.67, 40.73
and 71.24% in the oridonin, cisplatin and oridonin + cisplatin
groups, respectively. All the results demonstrated that oridonin
and cisplatin act synergistically with cisplatin in inducing cell
apoptosis in A2780/DDP cells.
Oridonin induces cell apoptosis of
ovarian cancer cells and induces cell-cycle arrest at the
G0/G1 phase
In order to further elucidate the underlying
mechanism of cell apoptosis in cisplatin-resistant ovarian cancer
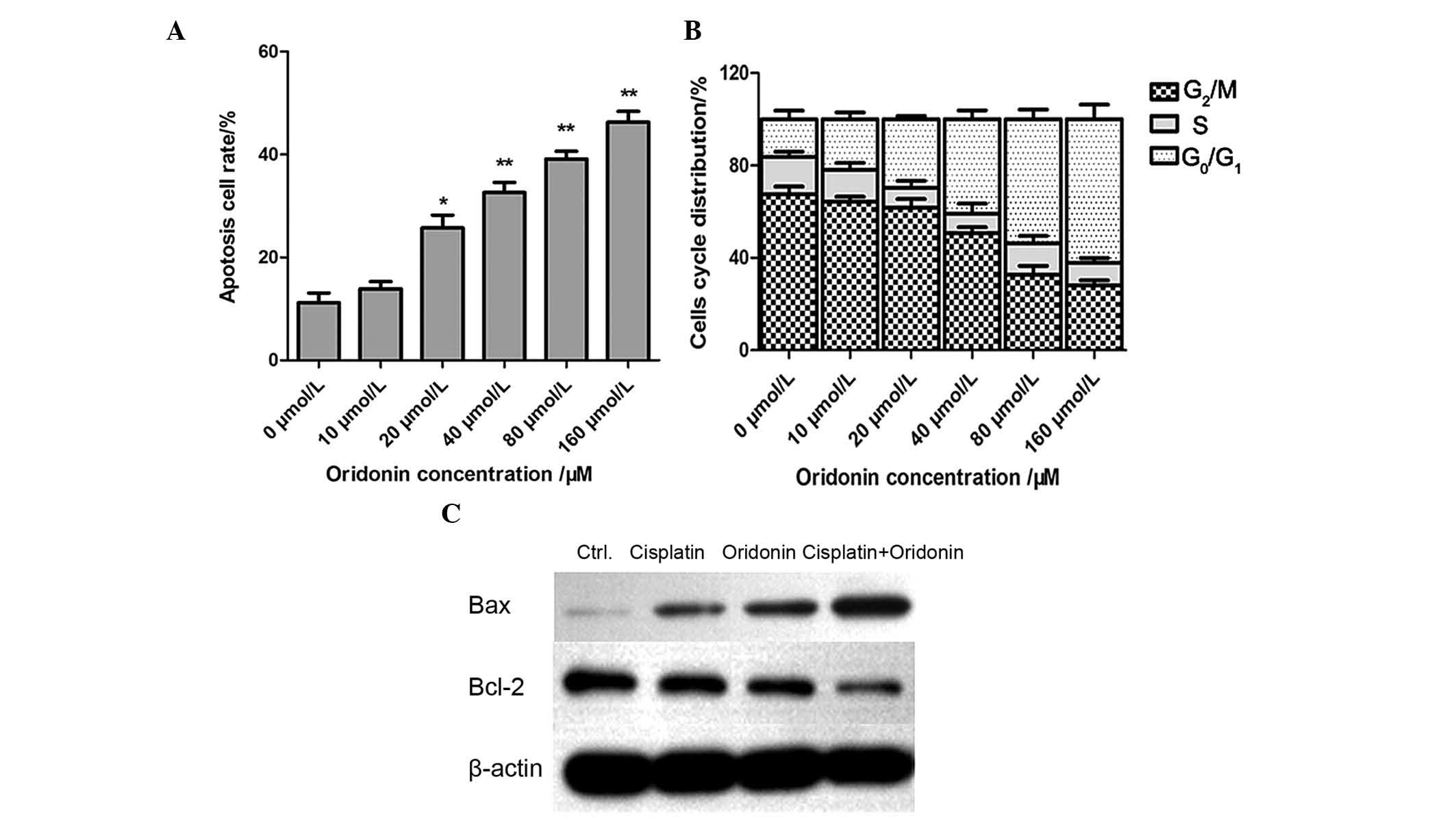
cells, the A2780/DDP cells were treated with oridonin at 10, 20,
40, 80 and 160 µM for 48 h. As presented in Fig. 6A, the result demonstrated that
oridonin induced cell apoptosis of A2780/DDP cells, which increased
in a dose-dependent manner.
The cell cycle distribution of A2780/DDP cells was
also analyzed by the PI-staining method. As presented in Fig. 6B, G0/G1 phase
arrest of the cells was induced and the number of cells in
G0/G1 phase increased in a dose-dependent
manner, while the number of cells in G2/M phase
decreased and the number in S phase was not markedly changed.
Oridonin and cisplatin synergistically
downregulated the expression levels of Bcl-2 and upregulated the
expression levels of Bax
The expression levels of Bcl-2 family proteins were
detected by western blot analysis. As presented in Fig. 6C, treatment with oridonin resulted
in downregulation of Bcl-2 protein expression levels and the
upregulation of Bax protein expression levels. This was consistent
with cells that were treated with cisplatin alone. Notably, in
A2780/DDP cells treated with 20 µM oridonin and 10 µM
cisplatin for 48 h, the ratio of Bax/Bcl-2 was markedly higher than
that of cells treated with cisplatin or oridonin alone. All data
indicated that oridonin and cisplatin synergistically downregulated
the protein expression levels of Bcl-2 and upregulated the protein
expression levels of Bax.
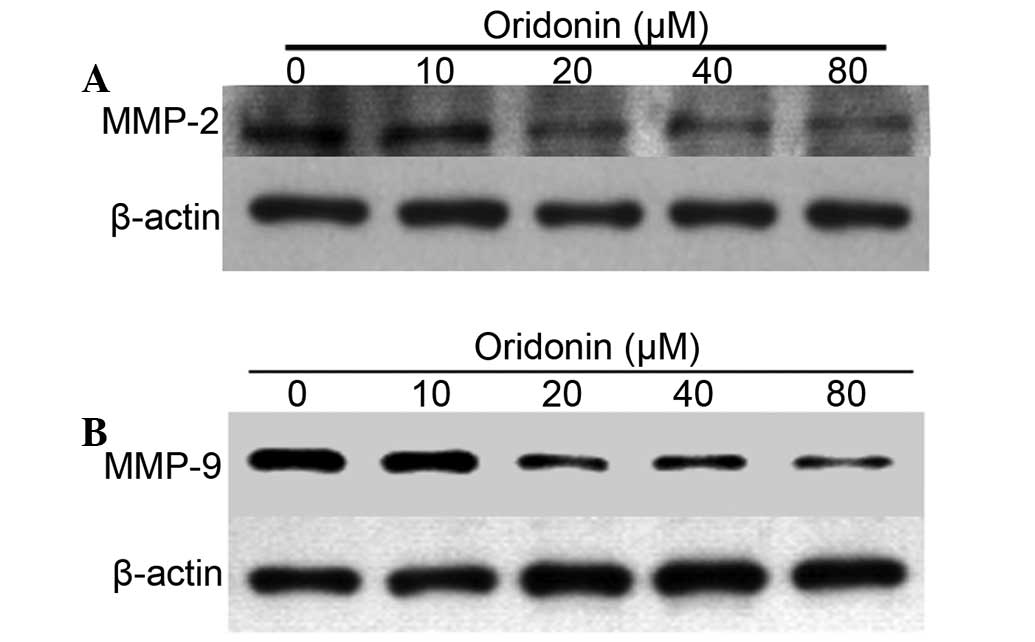
Expression levels of MMP-2 and MMP-9
decreased in a dose-dependent manner with oridonin treatment
MMPs, including MMP-2 and MMP-9, are involved in the
invasion and metastasis in a number of types of human malignancy,
as degradation of collagen IV in the basement membrane and
extracellular matrix facilitates tumor progression. The expression
levels of MMP-2 and MMP-9 in A2780/DDP cells treated with an
increasing concentration of oridonin were detected. A2780/DDP cells
were treated with increasing concentrations (10, 20, 40 and 80
µM) of oridonin for 48 h. As presented in Fig. 7, the protein expression levels of
MMP-2 and MMP-9 decreased with increasing concentration of oridonin
suggesting that oridonin may suppress the invasion and metastasis
of human ovarian cancer cells.
Discussion
Adverse side-effects of chemotherapy and resistance
to chemotherapeutic agents are a key problem in ovarian cancer
therapy (26). Cisplatin
resistance is a major obstacle in the treatment of ovarian cancer
and novel chemotherapeutic strategies are urgently required
(4,27). The present study aimed to
investigate a novel method to reverse cisplatin-resistance using
combination therapy with oridonin and cisplatin in human ovarian
cancer cells. The cisplatin-resistant A2780/DDP and SKOV3/DDP
ovarian cancer cell lines were used as cell models. Results from
the present study demonstrated that oridonin had a synergistic role
with cisplatin to inhibit proliferation and induce cell apoptosis
of cisplatin-resistant ovarian cancer cells.
The combined therapy of oridonin and cisplatin has a
synergistic antitumor effect, which may decrease the dose required
of a single therapeutic agent used. It is effective to kill tumor
cells at a relatively lower dose in order to decrease the side
effects of chemotherapeutic agents. The results demonstrated that
cell death significantly increased in the cisplatin + oridonin
group partly as a result of increased cell apoptosis. The apoptosis
rate was 71.24% in the oridonin + cisplatin group, markedly higher
than the rates of 42.67 and 40.73% in the oridonin and cisplatin
groups, respectively. This was consistent with the detection of
protein expression levels by western blot analysis. The
downregulated protein expression level of Bcl-2 and upregulated
protein expression level of Bax demonstrated that combination
therapy with oridonin and cisplatin promoted cell apoptosis in
drug-resistant A2780/DDP cells. Cell phase was detected by FACS,
and the results demonstrated oridonin induces cell-cycle arrest in
G0/G1 phase and the apoptosis rate increased
in a dose-dependent manner with oridonin. All the results
demonstrated that combined therapy is an effective method to
inhibit the proliferation of human ovarian cancer cells.
Notably, the combined treatment of oridonin and
cisplatin effectively reversed the cisplatin resistance. The
IC50 values were significantly decreased from 50.97 to
26.12 µM in the A2780/DDP cells and, consistently, in the
SKOV3/DDP cells, the IC50 values were decreased from
135.20 to 73.00 µM at 48 h.
MMPs are involved in the invasion and metastasis of
human malignancies, and the present study demonstrated the
expression levels of MMP-2 and MMP-9 were decreased in A2780/DDP
cells treated with increasing concentration of oridonin. The data
indicated that oridonin may suppress the invasion and metastasis of
human ovarian cancer cells, which may be an effective therapeutic
strategy to inhibit the spread of drug-resistant ovarian cancer
cells.
In conclusion, combination therapy with oridonin and
cisplatin was an useful method to treat cisplatin-resistant ovarian
cancer cells. The two compounds exerted synergistic antitumor
effects and effectively reversed the cisplatin resistance in human
ovarian cancer cells.
References
|
1
|
Ozga M, Aghajanian C, Myers-Virtue S,
McDonnell G, Jhanwar S, Hichenberg S and Sulimanoff I: A systematic
review of ovarian cancer and fear of recurrence. Palliat Support
Care. 13:1–10. 2015. View Article : Google Scholar
|
|
2
|
Ye H, Karim AA and Loh XJ: Current
treatment options and drug delivery systems as potential
therapeutic agents for ovarian cancer: A review. Mater Sci Eng C
Mater Biol Appl. 45:609–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zafrakas M, Grimbizis G, Timologou A and
Tarlatzis BC: Endometriosis and ovarian cancer risk: A systematic
review of epidemiological studies. Front Surg. 1:142014. View Article : Google Scholar
|
|
4
|
Yoshida H, Teramae M, Yamauchi M, Fukuda
T, Yasui T, Sumi T, Honda K and Ishiko O: Association of copper
transporter expression with platinum resistance in epithelial
ovarian cancer. Anticancer Res. 33:1409–1414. 2013.PubMed/NCBI
|
|
5
|
Redman C, Duffy S and Dobson C: Improving
early detection of ovarian cancer. Practitioner. 255:27–30.
2011.PubMed/NCBI
|
|
6
|
Walters Haygood CL, Arend RC, Straughn JM
and Buchsbaum DJ: Ovarian cancer stem cells: Can targeted therapy
lead to improved progression-free survival? World J Stem Cells.
6:441–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gallo D, Fruscella E, Ferlini C, Apollonio
P, Mancuso S and Scambia G: Preclinical in vivo activity of a
combination gemcitabine/liposomal doxorubicin against
cisplatin-resistant human ovarian cancer (A2780/CDDP). Int J
Gynecol Cancer. 16:222–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song T, Kim MK, Lee YY, Choi CH, Kim TJ,
Lee JW, Kim BG and Bae DS: Phase II study of ifosfamide and
cisplatin for the treatment of recurrent ovarian cancer. Cancer
Chemother Pharmacol. 72:653–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Musiani D, Hammond DE, Cirillo L, Erriquez
J, Olivero M, Clague MJ and Di Renzo MF: PIM2 kinase is induced by
cisplatin in ovarian cancer cells and limits drug efficacy. J
Proteome Res. 13:4970–4982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziyan W and Yang L: MicroRNA-21 regulates
the sensitivity to cisplatin in a human osteosarcoma cell line. Ir
J Med Sci. 185:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lockhart AC, Sundaram S, Sarantopoulos J,
Mita MM, Wang-Gillam A, Moseley JL, Barber SL, Lane AR, Wack C,
Kassalow L, et al: Phase I dose-escalation study of cabazitaxel
administered in combination with cisplatin in patients with
advanced solid tumors. Invest New Drugs. 32:1236–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costello BA, Borad MJ, Qi Y, Kim JP,
Northfelt DW, Erlichman C and Alberts SR: Phase I trial of
everolimus, gemcitabine and cisplatin in patients with solid
tumors. Inves New Drugs. 32:710–716. 2014. View Article : Google Scholar
|
|
13
|
Joo WD, Lee JY, Kim JH, Yoo HJ, Roh HJ,
Park JY, Kim DY, Kim YM, Kim YT and Nam JH: Efficacy of taxane and
platinum-based chemotherapy guided by extreme drug resistance assay
in patients with epithelial ovarian cancer. J Gynecol Oncol.
20:96–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HS, Kim TJ, Chung HH, Kim JW, Kim BG,
Park NH, Song YS, Bae DS and Kang SB: In vitro extreme drug
resistance assay to taxanes or platinum compounds for the
prediction of clinical outcomes in epithelial ovarian cancer: A
prospective cohort study. J Cancer Res Clin Oncol. 135:1513–1520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karam AK, Chiang JW, Fung E, Nossov V and
Karlan BY: Extreme drug resistance assay results do not influence
survival in women with epithelial ovarian cancer. Gynecol Oncol.
114:246–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Wang J, Cai K, Jiang L, Zhou D,
Yang C, Chen J, Chen D and Dou J: Downregulation of gene MDR1 by
shRNA to reverse multidrug-resistance of ovarian cancer A2780
cells. J Cancer Res Ther. 8:226–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis, inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao FH, Hu XH, Li W, Liu H, Zhang YJ, Guo
ZY, Xu MH, Wang ST, Jiang B, Liu F, et al: Oridonin induces
apoptosis and senescence in colorectal cancer cells by increasing
histone hyperacetylation and regulation of p16, p21, p27 and c-myc.
BMC Cancer. 10:6102010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi X, Zhang D, Xu X, Feng F, Ren G, Chu Q,
Zhang Q and Tian K: Oridonin nanosuspension was more effective than
free oridonin on G2/M cell cycle arrest and apoptosis in the human
pancreatic cancer PANC-1 cell line. Int J Nanomedicine.
7:1793–1804. 2012.PubMed/NCBI
|
|
20
|
Gao FH, Liu F, Wei W, Liu LB, Xu MH, Guo
ZY, Li W, Jiang B and Wu YL: Oridonin induces apoptosis and
senescence by increasing hydrogen peroxide and glutathione
depletion in colorectal cancer cells. Int J Mol Med. 29:649–655.
2012.PubMed/NCBI
|
|
21
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Autophagy preceded apoptosis in oridonin-treated human
breast cancer MCF-7 cells. Biol Pharm Bull. 30:859–864. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YY, Lv YF, Lu L and Cai L: Oridonin
inhibits mTOR signaling and the growth of lung cancer tumors.
Anticancer Drugs. 25:1192–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding Y, Wang Y, Chen J, Hu Y, Cao Z, Ren P
and Zhang Y: P21 overexpression sensitizes osteosarcoma U2OS cells
to cisplatin via evoking caspase-3 and Bax/Bcl-2 cascade. Tumour
Biol. 35:3119–3123. 2014. View Article : Google Scholar
|
|
24
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu HF, Chie YJ, Yang MS, Lee CS, Fu JJ,
Yang JS, Tan TW, Wu SH, Ma YS, Ip SW and Chung JG: Apigenin induces
caspase-dependent apoptosis in human lung cancer A549 cells through
Bax- and Bcl-2-triggered mitochondrial pathway. Int J Oncol.
36:1477–1484. 2010.PubMed/NCBI
|
|
26
|
Huq F, Yu JQ, Beale P, Chan C, Arzuman L,
Nessa MU and Mazumder ME: Combinations of platinums and selected
phyto-chemicals as a means of overcoming resistance in ovarian
cancer. Anticancer Res. 34:541–545. 2014.PubMed/NCBI
|
|
27
|
Gamarra-Luques CD, Hapon MB, Goyeneche AA
and Telleria CM: Resistance to cisplatin and paclitaxel does not
affect the sensitivity of human ovarian cancer cells to
antipro-gestin-induced cytotoxicity. J Ovarian Res. 7:452014.
View Article : Google Scholar
|