Introduction
The intestinal mucosa is the major location of
nutrient digestion and absorption, as well as the primary gateway
for invasion by pathogenic microorganisms and their toxins
(1). Under physiological
conditions, extra-intestinal tissues and organs are effectively
protected from pathogenic microorganisms and their toxins by the
intestinal mucosa barrier (IMB) (2). The afore-mentioned mechanism involves
the IMB, intestinal mucosa immune barrier, micropopulation barrier,
chemical barrier, slime layer and aqueous layer (3). As a result, when the IMB is damaged,
microorganisms and their toxins can move through the IMB, and enter
the portal vein and lymphatic system, leading to bacterial
translocation, potentially causing systemic inflammatory response
syndrome and multiple organ dysfunction syndrome (4).
Dysfunction of the IMB is closely associated with
diseases of the digestive system. Numerous factors, including
intestinal infection, inflammation and mechanical damage, can
induce abnormal IMB function (5).
IMB dysfunction increases intestinal mucosa permeability, causing
bacteria and antigens to be translocated from the enteric cavity to
the lamina propria mucosae, resulting in the activation of immune
cells and an abnormal mucosal immune response (6). Inflammatory bowel diseases, such as
enteritis, can also damage the IMB by increasing the release of
inflammatory cytokines, further exacerbating the abnormal mucosal
immune response (7). It is widely
understood that IMB dysfunction causes the molecular pathogenesis
of enteritis (7,8).
Glycyrrhiza, a group of perennial leguminous
grasses, have been used in traditional medicine in Asian countries,
including China, India and Japan, for thousands of years, and are
now widely used in Europe and the Middle East (9). Glycyrrhizic acid is water-soluble
compound, consisting of two molecules of glucuronic acid and one
molecule of glycyrrhetinic acid (10). Glycyrrhizic acid, which exhibits a
series of effects, such as liver protection and membrane
stabilization, is one of the major constituents of
Glycyrrhiza root extract. Its biological effects include
anti-inflammatory, anti-ulcer, anti-anaphylaxis, anti-oxidant,
immunoregulatory, membrane stabilization, antiviral and anticancer
activities, as well as liver protective properties (9,10).
The pentacyclic triterpene structure in glycyrrhetinic acid gives
glycyrrhizic acid a similar structure to glucocorticoids and, as
such, similar anti-inflammatory effects (11). The anti-inflammatory effect of
glycyrrhizic acid does not cause severe adverse reactions,
therefore, it is widely used in the treatment of acute and chronic
hepatitis, bronchitis, acquired immune deficiency syndrome and
other diseases (12,13).
Nuclear factor (NF)-κB p65 is a common transcription
factor which is activated by inflammatory cytokines, growth factor
or chemokines in order to regulate transcription (14). Following stimulation by an
inflammatory cytokine or various cytokines, a trimer of NF-κB and
IκBa dissociates in the cytoplasm, and phosphorylation of IκBa
facilitates nuclear localization to induce NF-κB expression, which
is a classical NF-κB pathway (15). Following nuclear entry, NF-κB can
regulate gene expression. Positive and negative feedback loops and
subsequent inflammatory reactions further affect the inflammatory
reaction of the body (16).
Mitogen activated protein kinase (MAPK) signal
transduction pathways are associated with cell proliferation,
differentiation, cell apoptosis and angiogenesis (17). In particular, the p38MAPK signal
transduction pathway regulates stress responses, such as
inflammation and cell apoptosis (18). Following the activation of MAPK
pathways by lipopolysaccharides and other factors, numerous
inflammatory mediators are generated via complex signal conduction
pathways, which promote inflammation. p38MAPK can be activated by
various stimulus along diversified transduction pathways which
activate numerous transcription factors and mediate a range of
biological effects (19).
The present study aimed to investigate the mechanism
by which glycyrrhizic acid prevents enteritis in rats and whether
its effects are mediated via reduction of NF-κB p65 and p38MAPK
expression levels.
Materials and methods
Chemicals
Methotrexate (MTX) was purchased from Shanghai
Zhongxi Sunve Pharmaceutical Co., Ltd. (Shanghai, China). Tumor
necrosis factor-α (TNF-α; MTA00), interleukin-1β (IL-1β; DLB50),
IL-6 (D6050), IL-10 (D1000B), cycloxy-genase-2 (COX-2; KCB4198) and
interferon-γ (IFN-γ; RIF00) enzyme-linked immunosorbent assay
(ELISA) kits were purchased from R&D Systems Europe, Ltd.
(Abingdon, UK). Bicinchoninic acid (BCA) assay kit (P0010S) was
purchased from Beyotime Institute of Biotechnology (Jiangsu,
China).
MTX-induced enteritis rat model and
grouping
A total of 24 adult male Sprague-Dawley rats, aged
6–7 weeks and weighing 220–250 g, were obtained from the Animal
Resource Center of the Linyi People's Hospital (Linyi, China). Rats
were housed at an ambient temperature of 22±1°C, with a 12-h
light-dark cycle, and free access to food and water. The current
study was conducted in accordance with the recommendations of the
Guide for the Care and Use of Laboratory Animals by the National
Institutes of Health (20) and the
protocol was approved by the Ethics Committee of Linyi People's
Hospital. The experimental rats were randomly allocated into three
equal groups: Control, MTX and glycyrrhizic acid groups (n=8 per
group). In the control group, normal rats were peritoneally
injected with 1 ml normal saline. In the MTX and glycyrrhizic acid
groups, enteritis was induced in rats by injection with 20 mg/kg
MTX. In the MTX group, MTX-induced rats were peritoneally injected
with 1 ml normal saline. In the glycyrrhizic acid group,
MTX-induced rats were peritoneally injected with 200 mg
glycyrrhizic acid for 9 weeks (21). After 9 weeks, rats was sacrificed
via decollation and blood samples and intestinal tissue samples
were subsequently harvested. Blood samples were centrifuged at
13,200 × g for 10 min at 4°C to obtain the serum. Serum and
intestinal tissue samples were stored at −80°C prior to
analysis.
Collection of rat blood samples and
measurement of inflammatory mediators by ELISA
Peripheral blood was collected after glycyrrhizic
acid treatment and centrifuged at 13,200 × g for 10 min at 4°C. The
supernatant was collected, and the levels of TNF-α, IL-1β, IL-6,
IL-10, IFN-γ and COX-2 were measured using the described ELISA
kits, according to the manufacturer's protocol.
Measurement of plasma D-lactate
concentration and diamine oxidase (DAO) activity
Peripheral blood supernatant was obtained as
described for the ELISAs. The D-lactate concentration levels and
DAO activity were measured using spectrophotometry (Multiskan MS
plate reader; Thermo Fisher Scientific, Inc., Waltham, MA, USA), as
previously described (22,23).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of intercellular adhesion
molecule-1 (ICAM-1)
Total RNA was extracted from rat intestinal tissue
samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The extracted RNA
(1 µg) was reverse transcribed into cDNA using a SuperScript
III First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.). qPCR was performed on a Gene Amp 2400 PCR system
(PerkinElmer, Inc., Waltham, MA, USA) using a Premix Ex Taq
kit (Takara Bio, Inc., Kyoto, Japan), according to the
manufacturer's protocol as follows: 94°C for 5 min followed by 40
cycles of 95°C for 30 sec, 60°C for 45 sec and 72°C for 30 sec, and
72°C for 10 min. Primer sequences were as follows: ICAM-1 forward
5′-AACGACGCTTCTTTTGCTC-3′ and reverse 5′-CTCTGGCGGTAATAGGTGTAA-3′;
and GAPDH (reference) forward 5′-CGTGTTCCTACCCCCAATGT-3′ and
reverse 5′-TGTCATACTTGGCAGGTTTCT-3′. Relative expression levels
were normalized against GAPDH, using the 2−ΔΔCq method
(24).
Western blotting
Intestinal tissue samples were collected and
incubated with 100 µl tissue lysis buffer (Beyotime
Institute of Biotechnology) for 30 min on ice. Homogenates were
centrifuged at 13,200 × g for 10 min at 4°C, the supernatant was
collected and the protein concentration measured using a the BCA
kit, according to the manufacturer's protocol. Equal concentrations
of protein sample were separated by running on 10% SDS-PAGE gels
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) at 100 V for 75 min and transferred onto polyvinylidene
difluoride membranes (0.22 mm; Bio-Rad Laboratories, Inc., Munich,
Germany). The membrane was incubated with anti-NF-κB-p65 (sc-372;
1,2,000), rabbit polyclonal anti-phosphorylated (p)-p38MAPK
(sc-101758), anti-p38MAPK (sc-728), rabbit polyclonal anti-iNOS
(sc-651; all 1:1,000) or β-actin (1:2,000; sc-130656; all Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies
overnight at 4°C. Membranes were then incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody
(sc-2370; 1:2,000; Santa Cruz Biotechnology, Inc.) and enhanced
chemiluminescence kit (GE Healthcare Life Sciences, Chalfont, UK).
The relative protein expression was determined using a Gel Dox 1000
fluorescent image analysis system (Bio-Rad Laboratories, Inc.).
Caspase-3 activity
Protein was extracted from intestinal tissue
samples, as described for western blotting, and quantified using
the BCA assay kit, according to the manufacturer's protocol. Equal
concentrations of protein were incubated with Ac-DEVD-pNA (Beyotime
Institute of Biotechnology) at 37°C for 2 h in the dark, as
previously described (25), and
the absorbance at 405 nm was measured using a Varioskan Flash
spectral scanning multimode reader.
Statistical analysis
Data from the individual groups were presented as
the mean ± standard deviation and were analyzed using analysis of
variance followed by Tukey-Kramer multiple comparisons test.
Statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL< USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Glycyrrhizic acid alters the expression
of inflammatory factors in rat enteritis
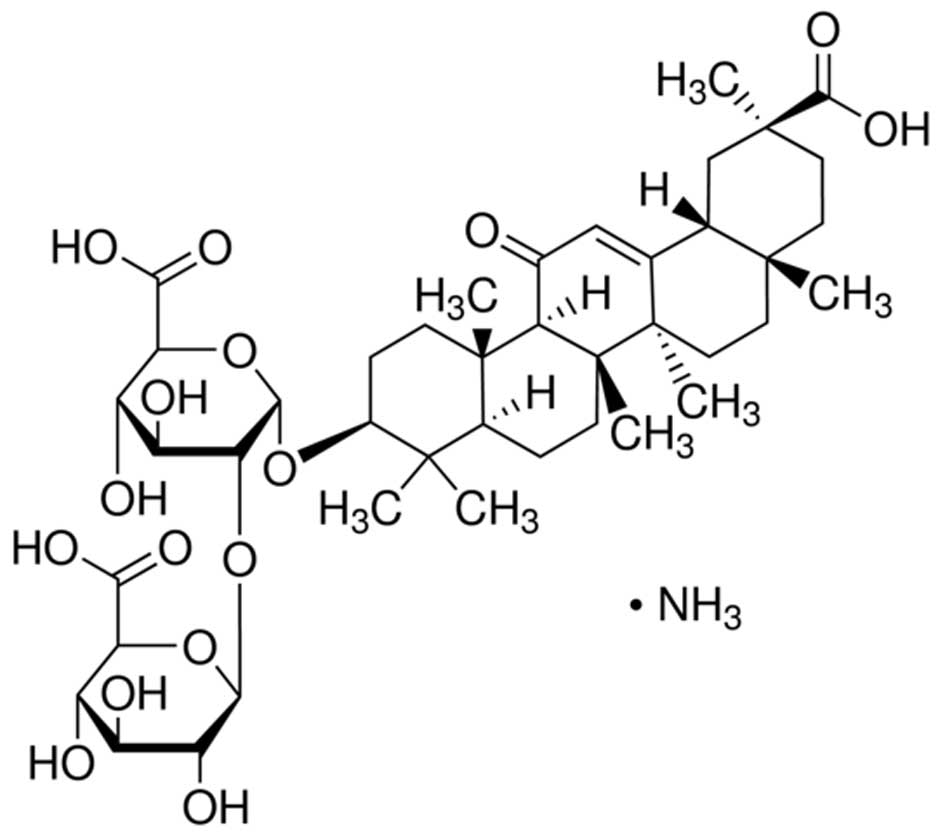
The chemical structure of glycyrrhizic acid (95.0%;
Sigma-Aldrich, St. Louis, MO, USA) is presented in Fig. 1. The current study examined the
effect of glycyrrhizic acid on the levels of several inflammatory
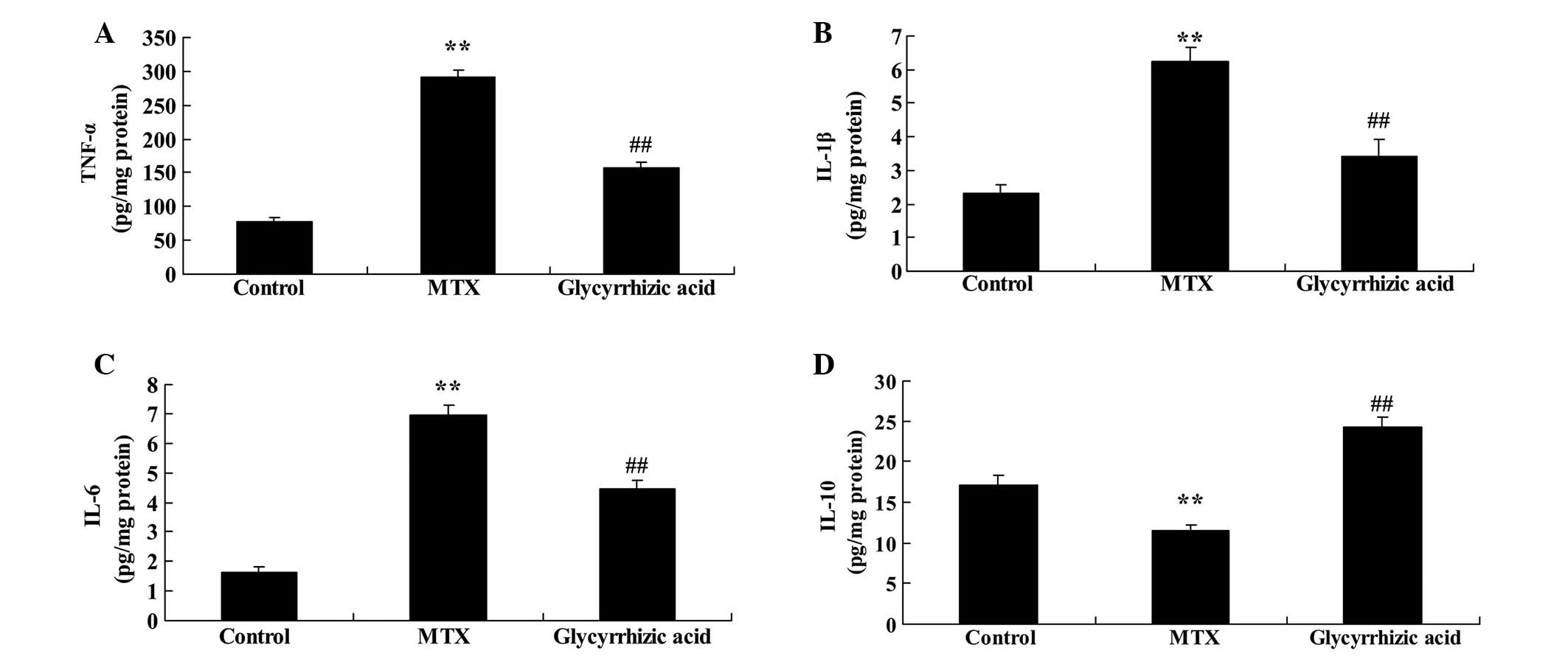
factors in a rat model of enteritis. The protein concentration
levels of TNF-α, IL-1β and IL-6 were significantly increased in
rats with MTX-induced enteritis compared with the control group
(P<0.01; Fig. 2A–C).
Pretreatment with glycyrrhizic acid significantly alleviated the
increase of TNF-α, IL-1β and IL-6 resulting from MTX-induced
enteritis (P<0.01; Fig. 2A–C).
Conversely, IL-10 levels were significantly reduced in rats with
MTX-induced enteritis compared with the control group (P<0.01;
Fig. 2D), and glycyrrhizic acid
treatment significantly raised the IL-10 levels compared with the
MTX group (P<0.01; Fig.
2D).
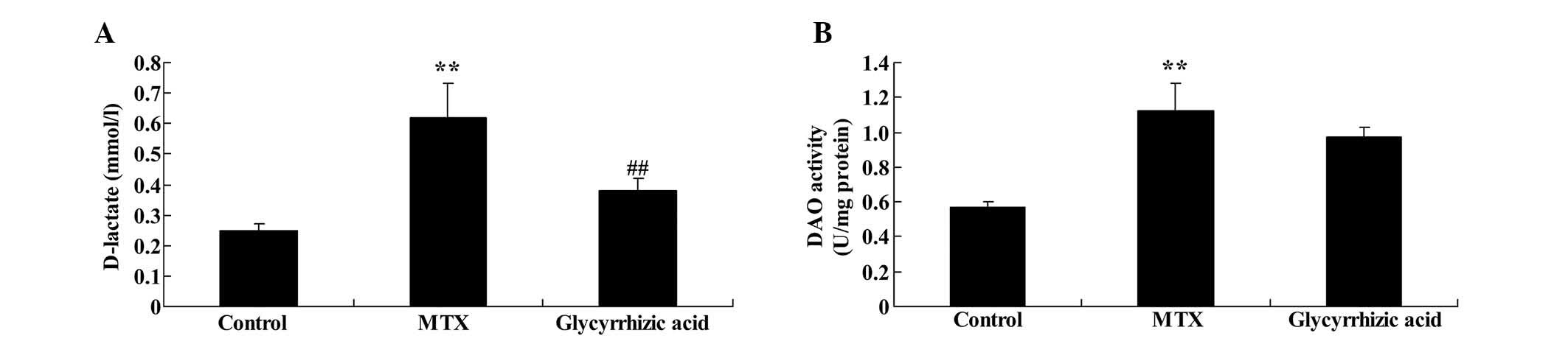
D-lactate concentration levels and DAO
activity are increased by glycyrrhizic acid in rat enteritis
The current study probed whether glycyrrhizic acid
prevents D-lactate and DAO changes that occur in rat enteritis. The
D-lactate concentration and DAO activity were significantly
increased in MTX-induced enteritis compared with control group
(P<0.01; Fig. 3). Furthermore,
treatment with glycyrrhizic acid significantly reduced the
D-lactate concentration levels compared with the MTX group
(P<0.01), however, glycyrrhizic acid did not inhibit the
increased DAO activity in MTX-induced enteritis (Fig. 3B).
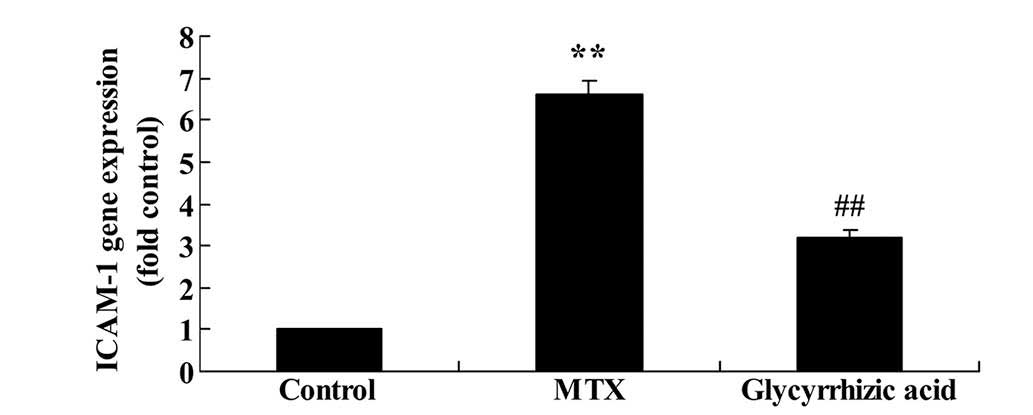
ICAM-1 expression is reduced by
glycyrrhizic acid in rat enteritis
As presented in Fig.
4, ICAM-1 mRNA expression was significantly higher in
MTX-induced enteritis compared with expression in control rats
(P<0.01). However, administration of glycyrrhizic acid
significantly reduced the ICAM-1 gene expression compared with
MTX-induced enteritis rats (P<0.01; Fig. 4).
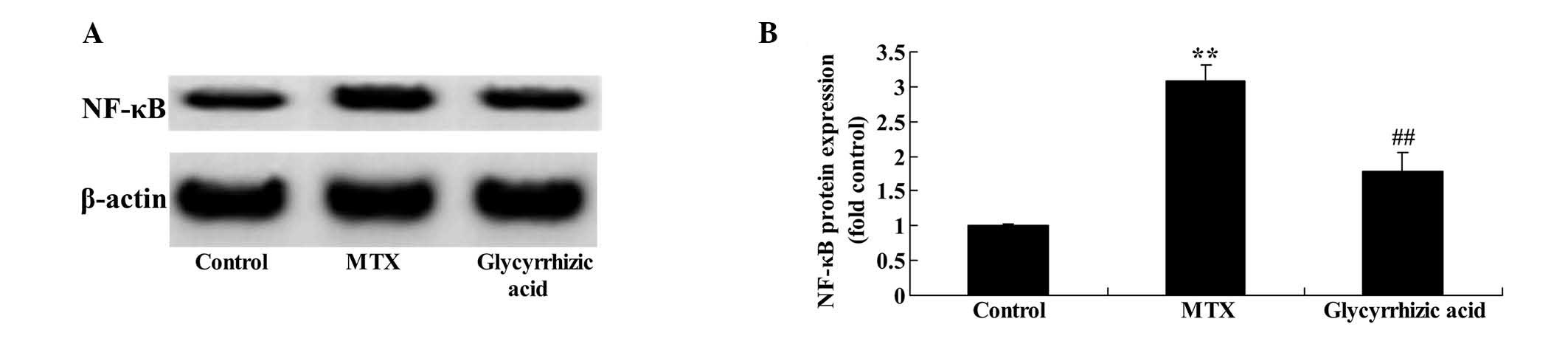
Increase of NF-κB-p65 protein expression
in enteritis is prevented by glycyrrhizic acid in rat
enteritis
As shown in Fig. 5,
MTX-induced enteritis resulted in significantly increased NF-κB-p65
expression compared with the control group (P<0.01). As
expected, glycyrrhizic acid significantly suppressed the
MTX-induced increase in NF-κB-p65 expression (P<0.01; Fig. 5).
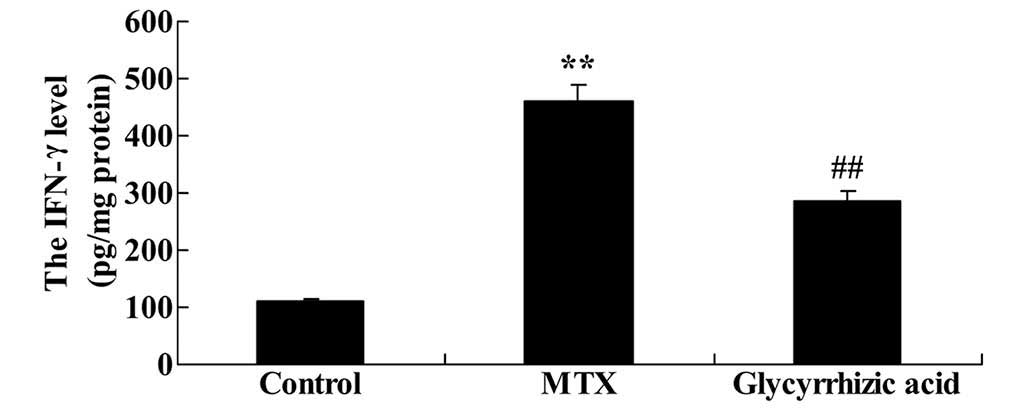
Glycyrrhizic acid prevents increase in
IFN-γ concentration levels in rat enteritis
There was a significant increase IFN-γ levels in
rats with MTX-induced enteritis compared with the control rats
(P<0.01; Fig. 6). As presented
in Fig. 6, glycyrrhizic acid
treatment prevented the promotion of IFN-γ levels observed in the
MTX group (P<0.01).
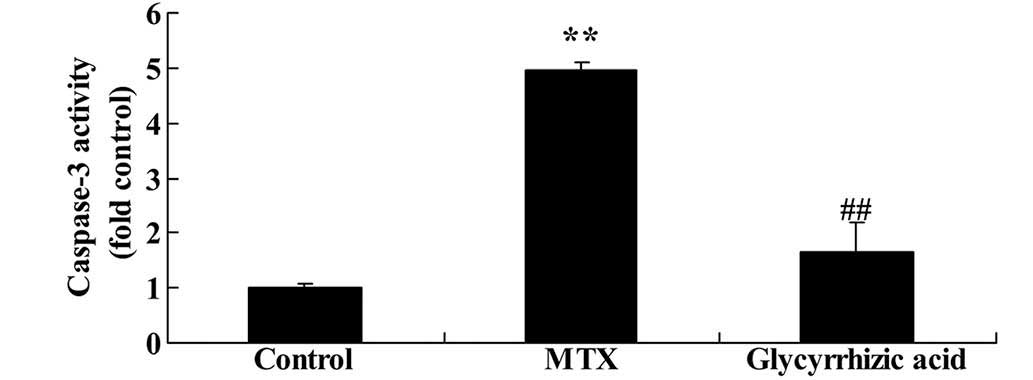
Glycyrrhizic acid reduces caspase-3
activity in rat enteritis
To understand the underlying mechanism of
glycyrrhizic-mediated anti-apoptosis, the present study examined
whether glycyrrhizic acid could inhibit caspase-3 activity in an
MTX-induced enteritis model. As presented in Fig. 7, rats in the MTX group exhibited
significantly increased caspase-3 activity compared with control
group (P<0.01). However, the elevation of caspase-3 activity was
significantly inhibited by treatment with glycyrrhizic acid
compared with the MTX group (P<0.01; Fig. 7).
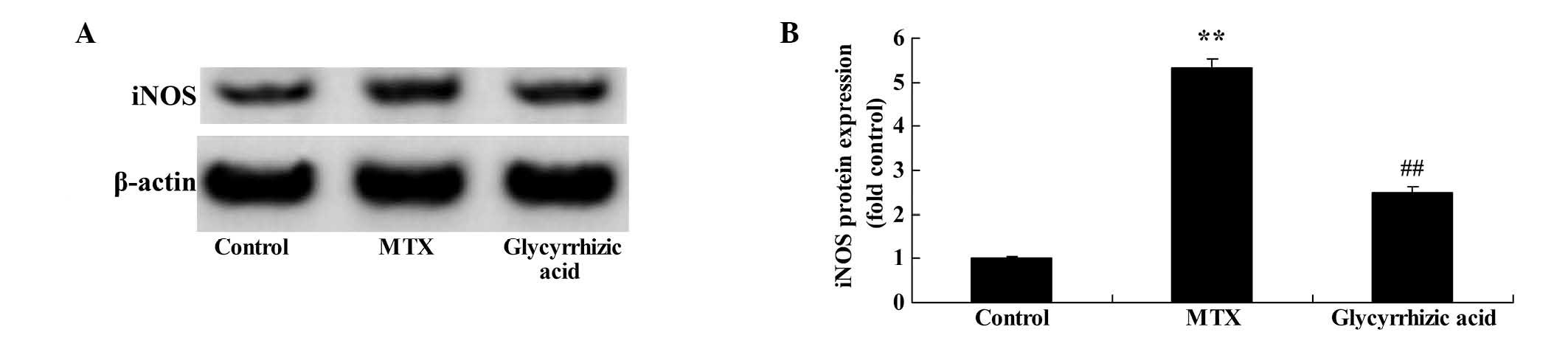
Glycyrrhizic acid prevents iNOS protein
expression in rat enteritis
To examine the underlying mechanisms of
glycyrrhizic-mediated changes in nitric oxide (NO) levels, the
current study examined whether glycyrrhizic acid inhibits iNOS
protein expression in the MTX-induced enteritis rat model. As
presented in Fig. 8, the iNOS
protein expression was significantly elevated in MTX-induced
enteritis compared with the control group (P<0.01). However,
treatment with glycyrrhizic acid reduced the increase of iNOS
protein expression, as compared with the MTX-induced enteritis rats
(Fig. 8).
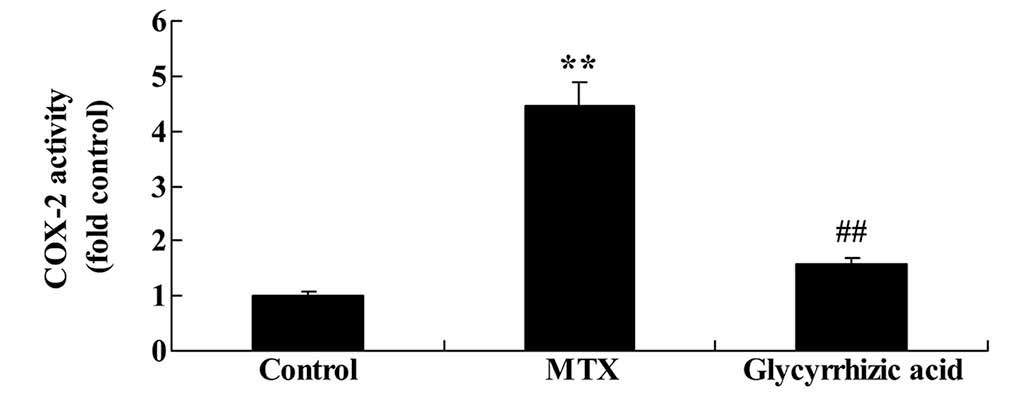
Glycyrrhizic acid inhibits COX-2 activity
in rat enteritis
To investigate the mechanism by which the
anti-oxidant effects of glycyrrhizic acid are mediated, the present
study examined the influence of glycyrrhizic acid on COX-2
activity. Compared with the control group, the COX-2 activity was
significantly increased in rats with MTX-induced enteritis
(P<0.01; Fig. 9). This increase
was significantly attenuated by glycyrrhizic acid treatment
compared with the MTX group (P<0.01; Fig. 9).
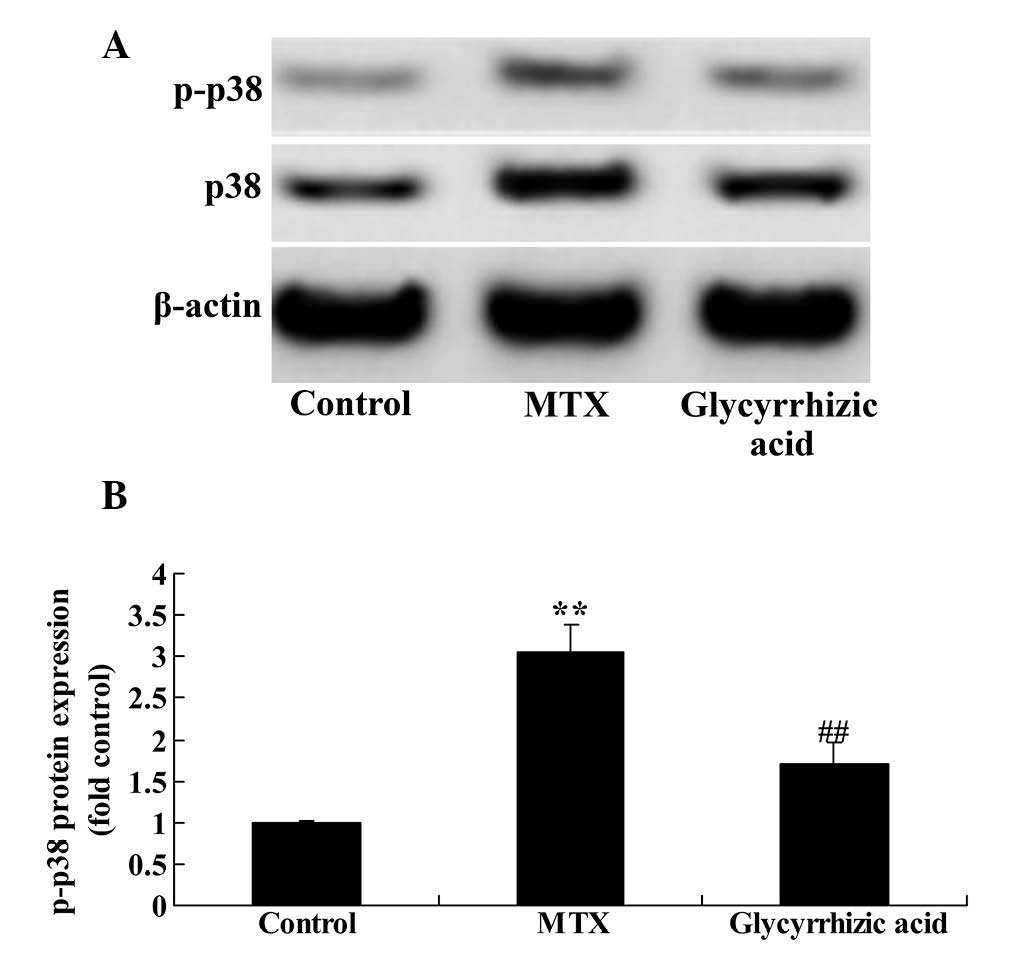
Glycyrrhizic acid prevents p38MAPK
expression in rat enteritis
To examine the effects of glycyrrhizic acid on the
MAPK signaling pathway, the protein expression levels of total and
p-p38MAPK, a key mediator of MAPK signaling, were measured using
western blotting. The present study observed that the p38MAPK
protein expression was significantly increased in MTX-induced
enteritis compared with the control group (P<0.01; Fig. 10). However, treatment with
glycyrrhizic acid significantly attenuated the increase in p38MAPK
protein expression observed in the MTX group (P<0.01; Fig. 10).
Discussion
IMB dysfunction is closely associated with the
paroxysm of enteritis. IMB dysfunction, induced by psychological
stress, intestinal infection, mechanical injury and other factors,
can increase intestinal mucosa permeability (1,2).
Increased mucosa permeability contributes to the translocation of
bacteria and antigens from the enteric cavity to the lamina propria
mucosae, resulting in activation of immune cells and an abnormal
mucosa immune response (26).
Additionally, damaging factors are present during the enteritis
paroxysm, which can further injure the IMB and aggravate the
abnormal mucosa immune response (26). Drugs for the treatment of enteritis
can reduce intestinal mucosal inflammation and regulate IMB
function (27). IMB function is
one of the therapeutic targets in the treatment of enteritis and is
an important area of investigation within enteritis pathophysiology
research (26,27). The current study observed that
pretreatment with glycyrrhizic acid significantly suppressed the
concentration levels of various inflammatory mediators that had
been increased in MTX-induced enteritis, including TNF-α, IL-1β,
IL-6 and D-lactate levels, whereas, IL-10 levels were reduced in
enteritis and increased by glycyrrhizic acid. Bhattacharjee et
al (28) demonstrated that
glycyrrhizic acid suppresses inflammatory responses and protects
against Leishmania parasites within the host. Orazizadeh
et al (29) observed that
glycyrrhizic acid protects against nanoparticle-induced
hepatotoxicity through anti-inflammatory mechanisms, and suggested
that glycyrrhizic acid may be a novel candidate for research and
development of enteritis therapeutics.
ICAM-1 is expressed on the membrane of endothelial
cells, white blood cells (WBC) and enterocytes of intestinal
tissues, acting as a receptor for β2-integrin present on WBCs.
Therefore, ICAM-1 is important in leukocyte movement and
aggregation during enteritis (30). Enteritis also induces the
expression of adhesion molecules, including ICAM-1 (31). During the acute phase of enteritis,
expression of ICAM-1 on the surface of activated vascular
endothelial cells is markedly increased, while the mortality of
ICAM-1-deficient mice is markedly reduced (30). Lower mortality, and reduced
inflammatory cell infiltration and injury of the intestinal tract
in ICAM-1-deficient mice indicates that ICAM-1 is important in the
development of enteritis (31).
The present data demonstrated that administration of glycyrrhizic
acid significantly reduces ICAM-1 mRNA expression in MTX-induced
enteritis rat. In addition, Wang et al (21) suggested that glycyrrhizic acid
attenuates glycative stress in the kidneys of diabetic mice through
inhibition of monocyte chemotactic protein-1 and ICAM-1. Together,
these results suggest that the anti-inflammatory activity of
glycyrrhizic acid during enteritis may be partially mediated
through inhibition of ICAM-1.
IFNs are a group of broad-spectrum anti-viral
glycoproteins secreted by cells upon attack by viruses. IFN-γ,
which is secreted by lymphocytes, is involved in the expression of
histocompatibility antigen and immune adjustment (32). It is understood that the
interaction between IFN-γ and TNF-α can change the structure of
intestinal epithelial cells and the barrier function, leading to
increased intestine permeability. Increased expression of IFN-γ is
commonly observed in enteritis (4,32).
In agreement with this, the present study demonstrated that
glycyrrhizic acid could reduced MTX-induced NF-κB-p65 and IFN-γ
protein expression in rat enteritis. Feng et al (33) demonstrated that glycyrrhizic acid
protects against advanced glycation end-product (AGE)-induced
endothelial dysfunction via inhibition of the receptor for
AGE/NF-κB signaling pathway. Wu et al (34) reported that glycyrrhizic acid
significantly reduced inflammatory via IFN-γ. This indicates that
inhibition of the IFN-γ signaling pathway may be associated with
the anti-inflammatory effects of glycyrrhizic acid in
enteritis.
Enteritis affects intestinal mucosa
microcirculation, inflammatory cell infiltration, blood
hypercoagulability, microthrombus and inflammatory polyp formation,
leading to hypoxia and ischemia of the intestinal mucosa
microcirculation, and a severe imbalance of cell factors, including
the IL and TNF-α superfamilies, colony stimulating factor,
chemokines and growth factors (35). iNOS levels are increased during
enteritis and are positively associated with the disease activity
index, which reflects the degree of inflammation. iNOS catalyzes
the production of NO, which is associated with the pathophysiology
of inflammatory diseases and cancer (5,32).
The results of the current study demonstrated that glycyrrhizic
acid significantly inhibits MTX-induced caspase-3 activity and iNOS
expression in enteritis. This suggests that glycyrrhizic acid has
anti-apoptotic effects and suppresses iNOS expression during
enteritis.
COX-2 is an inducible enzyme that is expressed at
low levels in the majority of tissues under normal conditions
(36). When induced by
pro-infammatory cytokines, several cell types, including
endothelial cells, vascular smooth muscle cells, mononuclear
macrophages and fibroblasts, rapidly increase the expression of
COX-2 to 8 to 10-fold the normal level (37). Increased COX-2 leads to the
production and accumulation of prostaglandin inflammatory factors,
promoting inflammatory responses and tissue damage (36). Overexpression of COX-2 promotes
cell proliferation, inhibits apoptosis and inhibits the immune
response, leading to dysregulation of the balance between
proliferation and apoptosis (36,37).
The present study demonstrated that glycyrrhizic acid significantly
reduces MTX-induced COX-2 activity in enteritis. Bhattacharjee
et al (28) also
demonstrated that glycyrrhizic acid suppressed COX-2 during L.
donovani infection. Additionally, Cherng et al (38) reported that glycyrrhizic acid
inhibited NF-κB and COX-2 expression, prevented DNA damage and
facilitated DNA repair.
As an important member of the MAPK signaling
pathway, p38MAPK is widely expressed in a variety of tissues
(39). Under normal physiological
conditions, p38MAPK typically exhibits low activity and is
activated upon stimulation by growth factors, lipopolysaccharides
and stress (40). Previous
research has demonstrated that when cells are stimulated by the
aforementioned factors, p38MAPK regulates the inflammatory response
via the production of pro-infammatory cytokines, including TNF-α,
IL-1, IL-6 and IL-8, as well as anti-inflammatory cytokines, such
as IL-10 (40,41). As a result, p38MAPK influences the
balance between pro- and anti-inflammatory cytokines, thereby
influencing processes that cause enteritis (42). The current study demonstrated that
glycyrrhizic acid significantly downregulates the protein
expression levels of p38MAPK in MTX-induced enteritis.
Additionally, several other previous studies demonstrated that
glycyrrhizic acid attenuates glycative stress in the kidneys of
diabetic mice through suppression of NF-κB and p-p38MAPK (21,43).
These data support the hypothesis that the anti-inflammatory
effects of glycyrrhizic acid may be associated with p38MAPK
signaling.
In conclusion, the results of the current study
demonstrate that glycyrrhizic acid exerts a protective effect
during MTX-induced enteritis and may be useful as a therapeutic
agent for digestive tract diseases. Furthermore, the protective
effect of glycyrrhizic acid is associated with anti-inflammatory
and anti-apoptotic pathways, suppression of ICAM-1, IFN-γ, iNOS and
COX-2 expression, and downregulation of the p38MAPK pathway.
Therefore, glycyrrhizic acid may be a novel therapeutic approach
for the treatment of enteritis.
References
|
1
|
Takagi T, Naito Y, Okada H, Takaoka M,
Oya-Ito T, Yamada S, Hirai Y, Mizushima K, Yoshida N, Kamada K, et
al: Hemopexin is upregulated in rat intestinal mucosa injured by
indomethacin. J Gastroenterol Hepatol. 27(Suppl 3): 70–75. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim H, Song MJ, Brian H and Choi K: A
comparative analysis of ethnomedicinal practices for treating
gastrointestinal disorders used by communities living in three
national parks (Korea). Evid Based Complement Alternat Med.
2014:1080372014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoidis CN, Misiakos EP, Patapis P,
Fotiadis CI and Spyropoulos BG: Potential benefits of pro- and
prebiotics on intestinal mucosal immunity and intestinal barrier in
short bowel syndrome. Nutr Res Rev. 24:21–30. 2011. View Article : Google Scholar
|
|
4
|
Vandenbroucke RE, Dejonckheere E, Van
Hauwermeiren F, Lodens S, De Rycke R, Van Wonterghem E, Staes A,
Gevaert K, López-Otin C and Libert C: Matrix metalloproteinase 13
modulates intestinal epithelial barrier integrity in inflammatory
diseases by activating TNF. EMBO Mol Med. 5:932–948. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XP, Zhang J, Song QL and Chen HQ:
Mechanism of acute pancreatitis complicated with injury of
intestinal mucosa barrier. J Zhejiang Univ Sci B. 8:888–895. 2007.
View Article : Google Scholar
|
|
6
|
Lin JE, Snook AE, Li P, Stoecker BA, Kim
GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S and
Waldman SA: GUCY2C opposes systemic genotoxic tumorigenesis by
regulating AKT-dependent intestinal barrier integrity. PLoS One.
7:e316862012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vetrano S, Ploplis VA, Sala E,
Sandoval-Cooper M, Donahue DL, Correale C, Arena V, Spinelli A,
Repici A, Malesci A, et al: Unexpected role of anticoagulant
protein C in controlling epithelial barrier integrity and
intestinal inflammation. Proc Natl Acad Sci USA. 108:19830–19835.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talukder JR, Boyd B, Griffin A, Jaima A
and Rajendran VM: Inflammatory cytokine TNF-α inhibits
Na(+)-glutamine cotransport in intestinal epithelial cells. Can J
Physiol Pharmacol. 91:275–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao X, Wang W, Wei S and Li W: Review of
pharmacological effects of Glycyrrhiza radix and its bioactive
compounds. Zhongguo Zhong Yao Za Zhi. 34:2695–2700. 2009.In
Chinese.
|
|
10
|
Asl MN and Hosseinzadeh H: Review of
pharmacological effects of Glycyrrhiza sp and its bioactive
compounds. Phytother Res. 22:709–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isbrucker RA and Burdock GA: Risk and
safety assessment on the consumption of Licorice root (Glycyrrhiza
sp), its extract and powder as a food ingredient, with emphasis on
the pharmacology and toxicology of glycyrrhizin. Regul Toxicol
Pharmacol. 46:167–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin EM, Zhou HY, Guo LY, Kim JA, Lee SH,
Merfort I, Kang SS, Kim HS, Kim S and Kim YS: Anti-inflammatory
effects of glycyrol isolated from Glycyrrhiza uralensis in
LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol.
8:1524–1532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franceschelli S, Pesce M, Vinciguerra I,
Ferrone A, Riccioni G, Patruno A, Grilli A, Felaco M and Speranza
L: Licocalchone-C extracted from Glycyrrhiza glabra inhibits
lipopolysaccharide-interferon-γ inflammation by improving
antioxidant conditions and regulating inducible nitric oxide
synthase expression. Molecules. 16:5720–5734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Zhou M, Ying X, Cheng B, Han Y, Nie
Y, Hou Y and Bai G: Identification of nuclear factor-kappaB
inhibitors in the folk herb Rhizoma Menispermi via
bioactivity-based ultra-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry
analysis. BMC Complement Altern Med. 14:3562014. View Article : Google Scholar
|
|
15
|
Mi Wi S, Park J, Shim JH, Chun E and Lee
KY: Ubiquitination of ECSIT is crucial for the activation of
p65/p50 NF-kappaBs in Toll-like receptor 4 signaling. Mol Biol
Cell. 26:151–160. 2015. View Article : Google Scholar :
|
|
16
|
Chen R, Li M, Zhang Y, Zhou Q and Shu HB:
The E3 ubiquitin ligase MARCH8 negatively regulates IL-1β-induced
NF-κB activation by targeting the IL1RAP coreceptor for
ubiquitination and degradation. Proc Natl Acad Sci USA.
109:14128–14133. 2012. View Article : Google Scholar
|
|
17
|
Yin H, Liu Z, Li F, Ni M, Wang B, Qiao Y,
Xu X, Zhang M, Zhang J, Lu H and Zhang Y: Ginsenoside-Rg1 enhances
angio-genesis and ameliorates ventricular remodeling in a rat model
of myocardial infarction. J Mol Med (Berl). 89:363–375. 2011.
View Article : Google Scholar
|
|
18
|
Park KR, Nam D, Yun HM, Lee SG, Jang HJ,
Sethi G, Cho SK and Ahn KS: β-Caryophyllene oxide inhibits growth
and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1
pathways and ROS-mediated MAPKs activation. Cancer Lett.
312:178–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim CK, Choi YK, Lee H, Ha KS, Won MH,
Kwon YG and Kim YM: The farnesyltransferase inhibitor LB42708
suppresses vascular endothelial growth factor-induced angiogenesis
by inhibiting ras-dependent mitogen-activated protein kinase and
phosphatidylinositol 3-kinase/Akt signal pathways. Mol Pharmacol.
78:142–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Institute of Laboratory Animal Resources
(US); Committee on Care Use of Laboratory Animals National
Institutes of Health (US); Division of Research Resources: Guide
for the care and use of laboratory animals. 8th edition. National
Academies Press; Washington, DC: 2011
|
|
21
|
Wang ZH, Hsieh CH, Liu WH and Yin MC:
Glycyrrhizic acid attenuated glycative stress in kidney of diabetic
mice through enhancing glyoxalase pathway. Mol Nutr Food Res.
58:1426–1435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan R, Khan AQ, Lateef A, Rehman MU,
Tahir M, Ali F, Hamiza OO and Sultana S: Glycyrrhizic acid
suppresses the development of precancerous lesions via regulating
the hyper-proliferation, inflammation, angiogenesis and apoptosis
in the colon of Wistar rats. PLoS One. 8:e560202013. View Article : Google Scholar
|
|
23
|
Wang ZX, Huang CY, Hua YP, Huang WQ, Deng
LH and Liu KX: Dexmedetomidine reduces intestinal and hepatic
injury after hepatectomy with inflow occlusion under general
anaesthesia: A randomized controlled trial. Br J Anaesth.
112:1055–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Best SM, Wolfinbarger JB and Bloom ME:
Caspase activation is required for permissive replication of
Aleutian mink disease parvovirus in vitro. Virology. 292:224–234.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cipriani S, Mencarelli A, Chini MG,
Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A and Fiorucci
S: The bile acid receptor GPBAR-1 (TGR5) modulates integrity of
intestinal barrier and immune response to experimental colitis.
PLoS One. 6:e256372011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reynolds JM, Martinez GJ, Nallaparaju KC,
Chang SH, Wang YH and Dong C: Cutting edge: Regulation of
intestinal inflammation and barrier function by IL-17C. J Immunol.
189:4226–4230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhattacharjee S, Bhattacharjee A, Majumder
S, Majumdar SB and Majumdar S: Glycyrrhizic acid suppresses
Cox-2-mediated anti-inflammatory responses during Leishmania
donovani infection. J Antimicrob Chemother. 67:1905–1914. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orazizadeh M, Fakhredini F, Mansouri E and
Khorsandi L: Effect of glycyrrhizic acid on titanium dioxide
nanoparticles-induced hepatotoxicity in rats. Chem Biol Interact.
220:214–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lord JD, Chen J and Kozarek RA: A case of
fatal idiopathic enteritis and multiple opportunistic infections
associated with dendritic cell deficiencies. J Gastrointestin Liver
Dis. 22:87–91. 2013.PubMed/NCBI
|
|
31
|
Miner P, Wedel M, Bane B and Bradley J: An
enema formulation of alicaforsen, an antisense inhibitor of
intercellular adhesion molecule-1, in the treatment of chronic,
unremitting pouchitis. Aliment Pharmacol Ther. 19:281–286. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang CF, Wu TC, Wu CC, Lee CC, Lo WT,
Hwang KS, Hsu ML and Peng HJ: Sublingual vaccination with sonicated
Salmonella proteins and mucosal adjuvant induces mucosal and
systemic immunity and protects mice from lethal enteritis. APMIS.
119:468–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Q, Tang Y, Zhang J, Hu X, Wang Q and
Huang J: Therapeutic effects of glycyrrhizic acid on asthma airway
inflammation in mice and its mechanism. Zhonghua Yi Xue Za Zhi.
94:3338–3344. 2014.In Chinese.
|
|
35
|
Jorge E, Vergara P and Martin MT: Ileal
inducible nitric oxide synthase mRNA expression in response to
stress is modified in Sprague-Dawley rats exposed to a previous
intestinal inflammation. Stress. 15:62–73. 2012. View Article : Google Scholar
|
|
36
|
Li J, Feng G, Liu J, Rong R, Luo F, Guo L,
Zhu T, Wang G and Chu Y: Renal cell carcinoma may evade the immune
system by converting CD4+Foxp3− T cells into
CD4+CD25+Foxp3+ regulatory T
cells: Role of tumor COX-2-derived PGE2. Mol Med Rep. 3:959–963.
2010.
|
|
37
|
Sun L, Liu J, Cui D, Li J, Yu Y, Ma L and
Hu L: Anti-inflammatory function of Withangulatin A by targeted
inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J Cell
Biochem. 109:532–541. 2010.
|
|
38
|
Cherng JM, Tsai KD, Yu YW and Lin JC:
Molecular mechanisms underlying chemopreventive activities of
glycyrrhizic acid against UVB-radiation-induced carcinogenesis in
SKH-1 hairless mouse epidermis. Radiat Res. 176:177–186. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Fu Y, Xue S, Ai A, Chen H, Lyu Q
and Kuang Y: The M2 polarization of macrophage induced by
fractalkine in the endometriotic milieu enhances invasiveness of
endometrial stromal cells. Int J Clin Exp Pathol. 7:194–203.
2014.PubMed/NCBI
|
|
40
|
Zhang X, Li C, Li J, Xu Y, Guan S and Zhao
M: Protective effects of protocatechuic acid on acute lung injury
induced by lipopolysaccharide in mice via p38MAPK and NF-κB signal
pathways. Int Immunopharmacol. 26:229–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bredeson S, Papaconstantinou J, Deford JH,
Kechichian T, Syed A, Saade GR and Menon R: HMGB1 promotes a
p38MAPK associated non-infectious inflammatory response pathway in
human fetal membranes. PLoS One. 9:e1137992014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akiho H, Tokita Y, Nakamura K, Satoh K,
Nishiyama M, Tsuchiya N, Tsuchiya K, Ohbuchi K, Iwakura Y, Ihara E,
et al: Involvement of interleukin-17A-induced hypercontractility of
intestinal smooth muscle cells in persistent gut motor dysfunction.
PLoS One. 9:e929602014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wojcik M, Zieleniak A, Zurawska-Klis M,
Cypryk K and Wozniak LA: Increased expression of immune-related
genes in leukocytes of patients with diagnosed gestational diabetes
mellitus (GDM). Exp Biol Med (Maywood). 2015.
|