Introduction
Leukemia is a malignant neoplasm (unregulated
proliferation of immature blood cells) and originates in the bone
marrow and/or mutant hematopoietic stem cells (1). The neoplastic cells move into the
blood, accumulating in large numbers causing the clinical disease
in patients (2). Leukemias,
including acute myeloid, B-lymphoblastic, T-lymphoblastic and
leukemias of ambiguous lineage, are well documented in patients
(3). Thus far, the primary
treatment for patients with leukemia is chemotherapy, which induces
a complete remission and is consolidated with further cycles of
treatment (4). However,
chemotherapy has relatively low efficacy and high toxicity in
patients with leukemia (4).
Therefore, the treatment of leukemia remains a therapeutic
challenge, and the identification and development of novel
biomarkers is required.
Isothiocyanates are present in a variety of
cruciferous vegetables and have biological activity, including
anticarcinogenic activity (5–8).
Sulforaphane (SFN) is an isothiocyanate and effectively inhibits
chemically-induced tumors in rodents (9,10) by
inducing the phase II detoxification enzymes (11). A previous study demonstrated the
cell cycle specificity of SFN mediated apoptosis in Jurkat
T-leukemia cells (12). Another
previous study indicated that the generation of cellular reactive
oxygen species (ROS) serves a pivotal role in the initiation of
SFN-triggered apoptosis in the U937 cells (13). In addition, SFN induces
cytodifferentiation in granulocytic and macrophagic lineage, and
resulted in a significant increase in the apoptotic cell fraction
(14). A previous study
demonstrated that SFN suppresses the tumor necrosis
factor-α-mediated activation of nuclear factor-κB and induces
apoptosis through the activation of ROS-dependent caspase-3 in
human leukemia cells (15).
Furthermore, SFN acts as an adjunctive agent to improve the
therapeutic response in high-risk patients with acute lymphoblastic
leukemia and activated Akt signaling (16). A previous study demonstrated that
SFN may be a novel therapeutic agent, protecting against
malignancies, and further research on SFN is required in a wider
population of patients with leukemia (17).
A previous study indicated that natural compounds
and crude extract of natural plants promotes immune responses in
leukemia mice (18–20). To the best of our knowledge, no
previous study has demonstrated that SFN may have an effect on the
immune responses in leukemia mice in vivo. Therefore, in the
present study, the effect of SFN on the immune responses in
leukemia BALB/c mice in vivo was investigated. The results
of the present study demonstrated that SFN promoted immune
responses, including increasing the macrophage phagocytosis and
natural killer (NK) cell activities in leukemia BALB/c mice.
Materials and methods
Materials and reagents
SFN and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Tissue culture plastics
were obtained from BD Biosciences (San Jose, CA, USA). Gibco
RPMI-1640 medium, fetal bovine serum (FBS), L-glutamine and
penicillin/streptomycin were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Antibodies against CD3, CD19,
CD11b and Mac-3 were obtained from BD Biosciences (BD Pharmingen;
San Diego, CA, USA). SFN was dissolved in DMSO at 1% stock solution
and was maintained at −20°C in a 50 ml tube, in the dark.
Murine WEHI-3 leukemia cells
Murine WEHI-3 myelomonocytic leukemia cells were
obtained from the Food Industry Research and Development Institute
(Hsinchu, Taiwan, ROC). The cells were maintained in RPMI-1640
medium, supplemented with 10% FBS, 100 units/ml penicillin, 100
μg/ml streptomycin and 2 mM L-glutamine at 37°C with 5%
CO2 in a humidified incubator, as previously described
(18–20).
Male BALB/c mice and SFN treatment
Male BALB/c mice (n=50; age, 4-weeks-old; weight,
~22–25 g) were obtained from the National Laboratory Animal Center
(Taipei, Taiwan, ROC). The mice were maintained on 12 h light/dark
cycles at 25°C in the animal center of China Medical University
(Taichung, Taiwan, ROC). Animal experiments were reviewed and
approved by the Institutional Animal Care and Use Committee of
China Medical University (approval ID: 101-238-C). All animals were
cared for according to the institutional animal ethical guidelines
of the China Medical University, as previously described (18). The mice were randomly divided into
five groups (n=10/group), and for groups II–IV, the mice were
peritoneally inoculated with 1×106 WEHI-3 leukemia cells
to generate a leukemia model. The groups were divided and separated
as follows: Group I, mice were fed a normal diet as control; group
II, mice were fed a normal diet as positive control; group III–V,
mice were treated with 285, 570 or 1,140 mg/kg SFN in olive oil,
respectively. SFN in olive oil was administered by gavage for 20
days and the body weight was recorded. At the end of treatment, all
mice were weighed and sacrificed by euthanasia with CO2,
as previously described (18–20).
Immunofluorescence staining for surface
markers in isolated white blood cells
Following treatment, mice from each group were
individually weighted and blood samples were collected. The liver
and spleen were individually collected and splenocytes were
isolated to measure the activity of NK cells, as previously
described (18). Cell markers in
isolated leukocytes were measured. Briefly, 1 ml blood was
collected, lysed with 1X Pharm Lyse lysing buffer (BD Biosciences)
to destroy red blood cells, according to the manufacturer's
protocol, and the leukocytes were collected. Isolated leukocytes
were stained with fluorescein isothiocyanate (FITC)-conjugated
monoclonal hamster anti-mouse CD3 (cat. no. 553062; 1:10),
phycoerythrin PE-conjugated monoclonal rat anti-mouse CD19 (cat.
no. 553786; 1:20), FITC-conjugated monoclonal rat anti-mouse CD11b
(cat. no. 553310; 1:20) and PE-conjugated rat monoclonal anti-mouse
Mac-3 (cat. no. 553324; 1:20) primary antibodies for 30 min at room
temperature, and were subsequently washed three times with
phosphate-buffered saline. Following washing, the leukocytes were
stained with secondary antibody and the percentage of cell markers
was determined using flow cytometer (FACS Calibur; BD Biosciences),
the data was analyzed using Cell Quest Pro (version 5.2.1; BD
Biosciences), as previously described (18–20).
Measurement of phagocytotic
macrophages
Following treatment, macrophages were isolated from
the peripheral blood mononuclear cells (PBMC) and the peritoneum,
as previously described (18–20),
and phagocytosis was determined using the PHAGOTEST kit (Orpegen
Pharma GmbH, Heidelberg, Germany). Briefly, isolated macrophages
were placed in plates and 50 μl Escherichia coli-FITC
was added to the cells, according to the manufacturer's protocol.
The samples were subsequently analyzed for phagocytosis by flow
cytometry and were quantified using the Becton Dickinson CellQuest
software (BD Biosciences), as previously described (18–20).
Measurement of NK cell cytotoxic
activity
Following treatment, splenocytes were isolated and
placed into a 96-well plate (1×105 cells/well), with 1
ml RPMI-1640 medium. YAC-1 cells (2.5×107 cells) and
PKH-67/Dil.C buffer (Sigma-Aldrich) were added to the cells,
according to the manufacturer's protocol, and were mixed thoroughly
for 2 min at 25°C. PBS (2 ml) was added to the wells for 1 min,
followed by 4 ml medium for 10 min. Following the incubation, the
cells were centrifuged for 2 min at 25°C and 290 × g, and the NK
cell cytotoxic activity was measured using flow cytometry, as
previously described (18–20).
Measurement of T and B cell
proliferation
Isolated splenocytes were seeded into 96-well plates
(1×105 cells/well) with 100 μl RPMI-1640 medium.
To measure T cell proliferation, concanavalin A (Con A; 5
μg/ml) was added to the cells for a 3-day stimulation. To
measure B cell proliferation, lipopolysaccharide (LPS; 5
μg/ml) was added to the cells for a 5-day stimulation.
Following this stimulation, proliferation was determined using the
CellTiter 96 AQueous One Solution Cell Proliferation Assay kit
(Promega Corporation, Madison, WI, USA), as previously described
(18–20).
Statistical analysis
The data are expressed as the mean ± standard
deviation. All experiments were repeated a minimum of three times.
The association between the control and SFN-treated groups was
analyzed using the Student's t-test in Sigmaplot (version 12.0;
Systat Software, Inc., San Jose, CA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
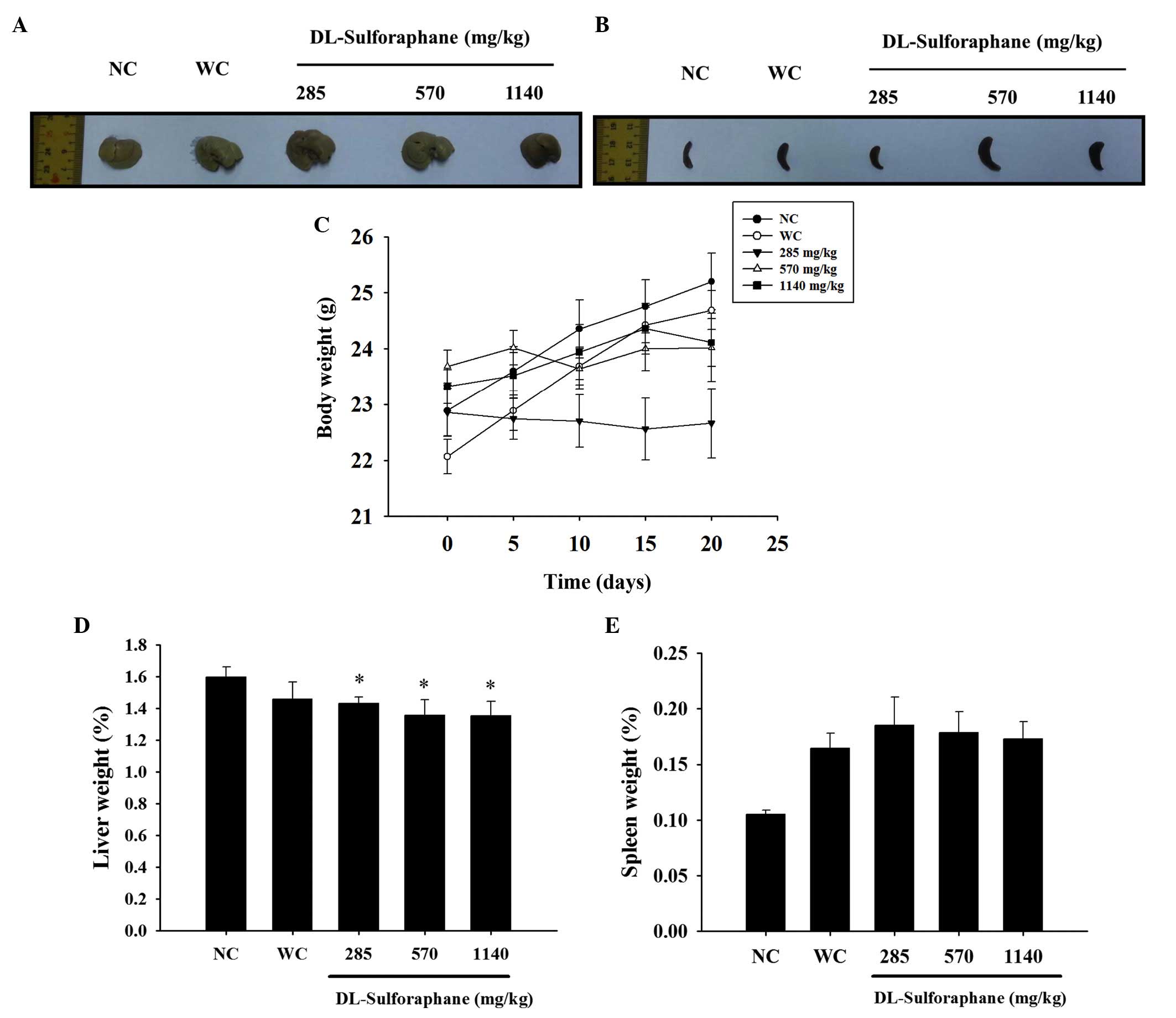
SFN treatment influences body, liver and
spleen weights in leukemic mice
In accordance with the in vivo protocol,
normal and WEHI-3 cells generating leukemic mice were divided into
five groups: Normal control, untreated; leukemic positive control,
untreated; and leukemic mice treated with SFN at various doses for
20 days. The representative liver and spleen images are
demonstrated in Fig. 1A and B, and
the body, liver and spleen weights are shown in Fig. 1C–E, respectively. These results
indicated that SFN had no significant effect on the body and spleen
weights (Fig. 1C and E), with the
exception that a low dose (285 mg/kg of SFN) decreased the body
weight when compared with the untreated leukemic mice, as shown in
Fig. 1B.
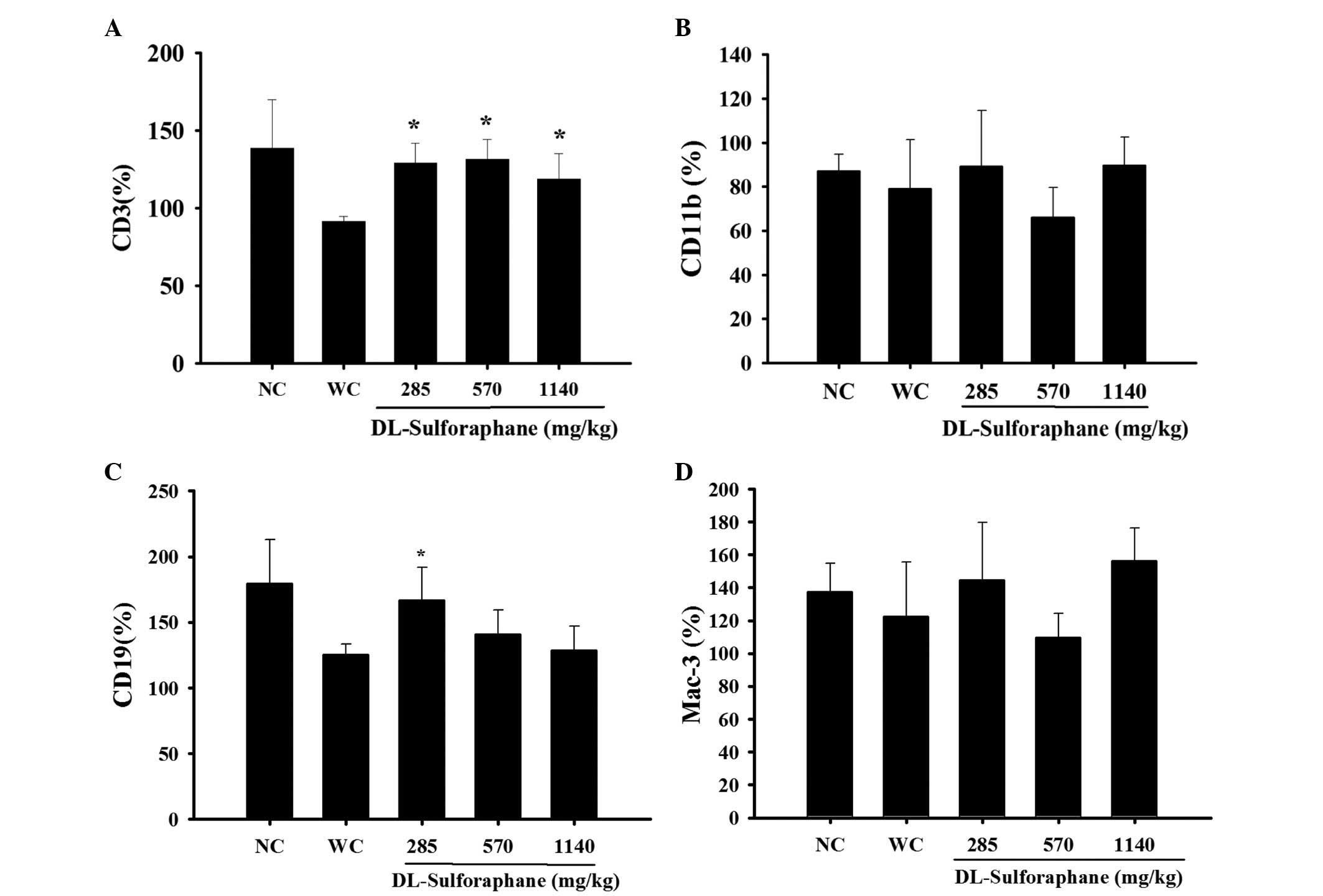
SFN treatment influences cell markers in
the white blood cells of leukemic mice
Blood samples were collected to measure the levels
CD3, CD19, CD11b and Mac-3 cell markers using flow cytometry. As
demonstrated in Fig. 2A, SFN (285,
570 or 1,140 mg/kg) treatment significantly promoted the percentage
levels of CD3 compared with the control groups (P<0.05).
Furthermore, SFN (285 mg/kg) treatment significantly promoted the
percentage of levels CD119 (P<0.05; Fig. 2B), however had no significant
effect on the levels of CD11b (Fig.
2C) and Mac-3 (Fig. 2D),
compared with the control groups.
SFN treatment effects the phagocytotic
activity of macrophages from the PBMC and peritoneal cavity of
leukemic mice
Cells were isolated from PBMC and peritoneal cavity
following treatment to determine the levels of phagocytosis using
flow cytometry. As demonstrated in Fig. 3A, 285 mg/kg SFN treatment
significantly increased the phagocytotic activity of macrophages
from PBMC compared with the WEHI-3 control group (P<0.05). In
addition, 285, 570 and 1,140 mg/kg SFN treatment significantly
decreased the phagocytotic activity of macrophages from the
peritoneal cavity compared with the normal control group
(P<0.05; Fig. 3B).
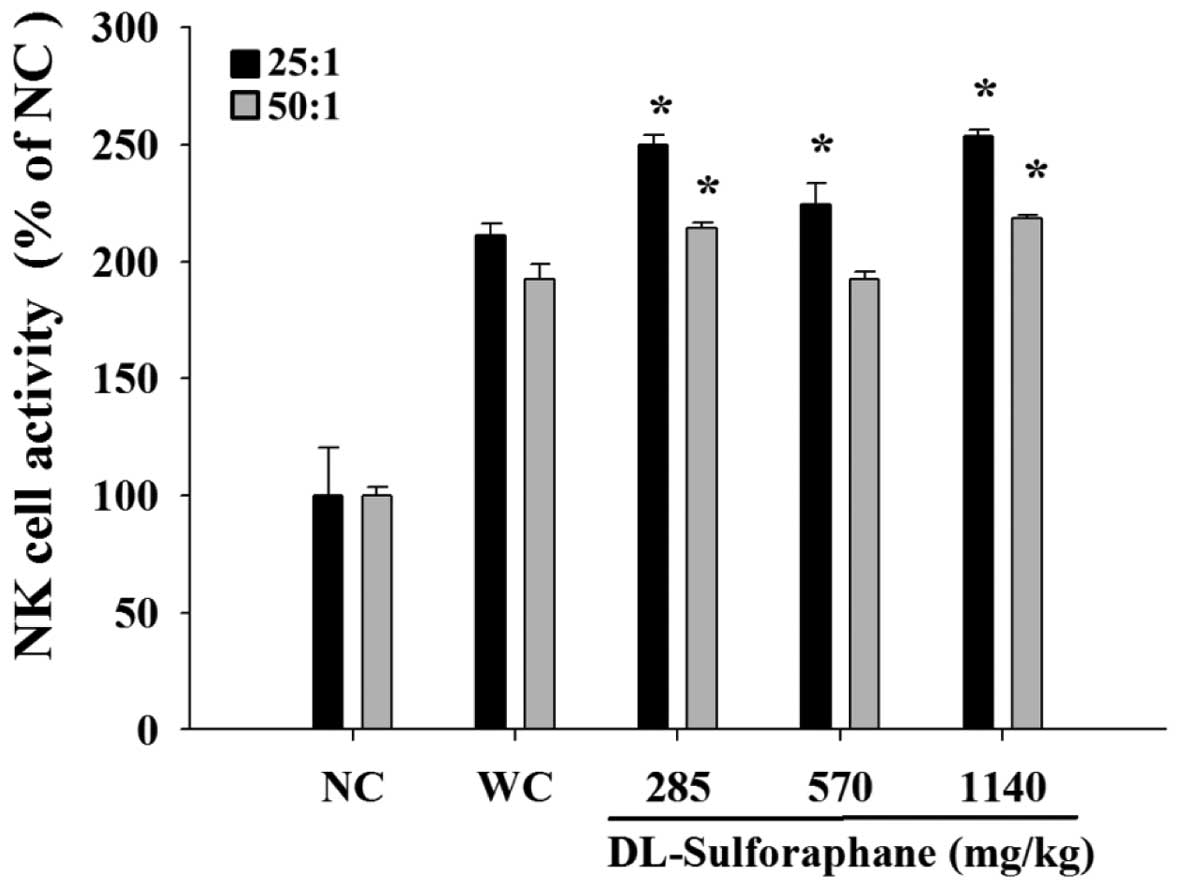
SNF treatment influences the cytotoxic
activity of NK cells from leukemic mice
Splenocytes were isolated from the leukemic mice and
were used at different ratios as NK effector cells in cytolytic
assays against YAC-1 target cells. The NK activity was measured by
flow cytometry and the results indicated that YAC-1 cells were
killed by NK cells following treatment with 285, 570 or 1,140 mg/kg
SFN, when compared with the untreated leukemic mice (Fig. 4).
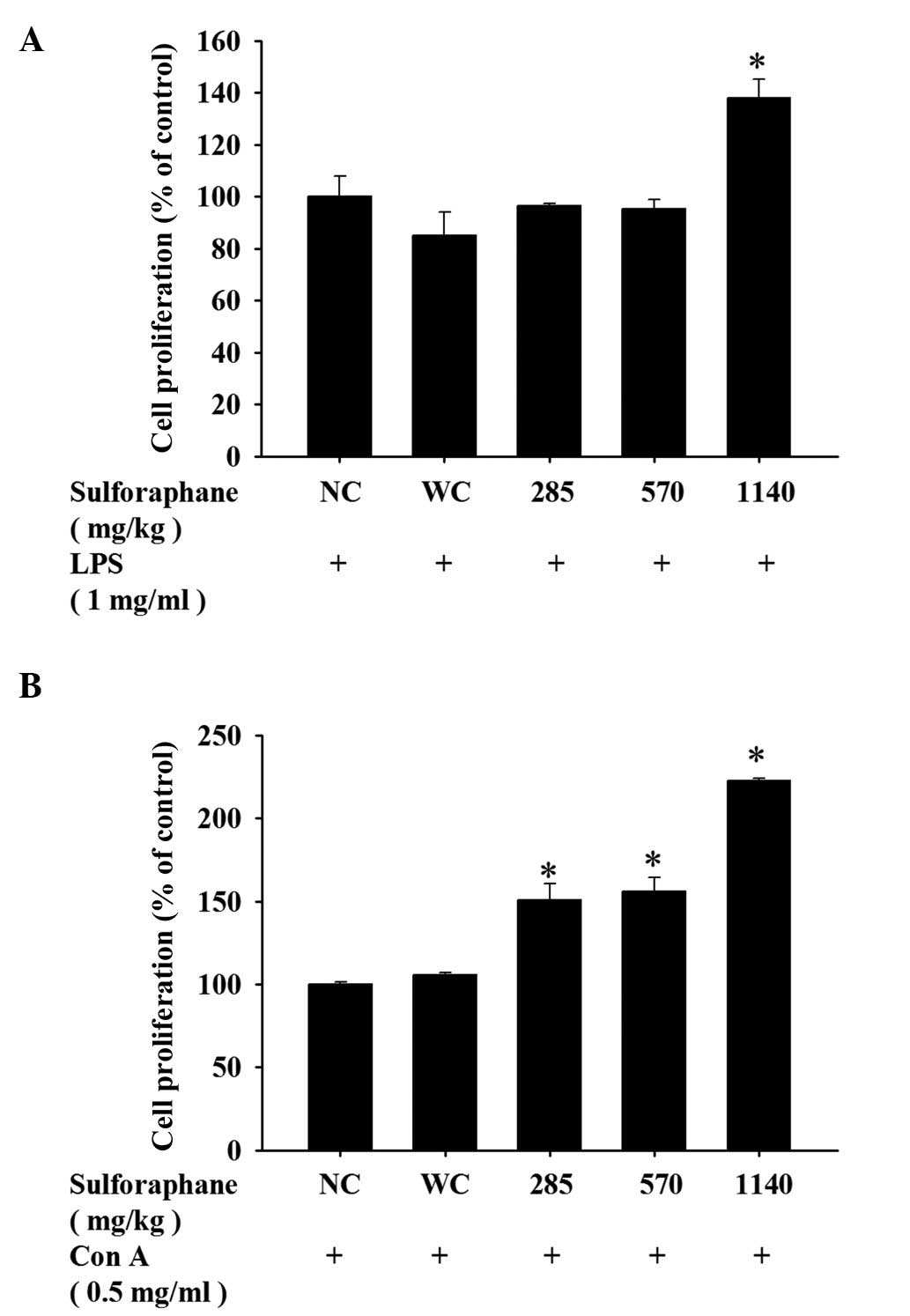
SFN treatment has an effect on B and T
cell proliferation in leukemic mice
To assess any differences in the proliferative
capacity of T and B cells following SFN treatment, isolated cells
from the spleen of each mouse were cultured and stimulated with Con
A and LPS, respectively. As demonstrated in Fig. 5A, treatment with 1,140 mg/kg SFN
led to a marked increase of B cell proliferation compared with the
control groups. However, treatment with 285, 570 or 1,140 mg/kg SFN
resulted in a marked increase of T cell proliferation compared with
the control groups (Fig. 5B).
Discussion
In the present study, murine WEHI-3 cells were
utilized to generate leukemic mice. The mice were randomly divided
into different groups for oral SFN treatment at various
concentrations. Following treatment, blood samples were collected
from the mice for cell marker analysis and measurement of
phagocytotic activity in macrophages. In addition, splenocytes were
isolated to assess the NK cell activity, and the proliferation of T
and B cells. The results of the present study indicated that SFN
treatment had no significant effect on body and spleen weights
(Fig. 1C and E), however 280 mg/kg
SFN resulted in reduced body weights (Fig. 1C) when compared with the control
groups. Furthermore, SFN increased T and B cell markers (Fig. 2A and C), however, had no
significant effect on the cell markers of monocytes or macrophages
(Fig. 2B and D). SFN treatment
significantly increased the phagocytotic activity of macrophages
from PBMCs (Fig. 3A) and the
peritoneal cavity (Fig. 3B).
Furthermore, SFN treatment at all concentrations increased T cell
proliferation (Fig. 5B) compared
with the control groups, however only 1,140 mg/kg SFN treatment
resulted in an increase in B cell proliferation (Fig. 5A).
In order to protect against invading foreign
antigens, numerous white blood cells interact to produce immune
responses in humans (1).
Clinically, numerous plant-derived bioactive compounds have been
used to treat patients with cancer, including paclitaxel from
Taxus brevifolia and camptothectin from Camptotheca
acuminata (21–24). A previous study demonstrated that
SFN induces cell cycle arrest and induces apoptosis, suggesting
that it may be a novel therapeutic against leukemia malignancies
(17). The antileukemic effect of
SFN was demonstrated in blasts from pediatric patients with acute
lymphoblastic leukemia (16),
however, no sufficient and reliable data exist in literature with
regards to the effect of SFN on the immune responses of
WEHI-3-induced leukemic mice in vivo. Therefore, in the
present study, the effects of SFN treatment on the immune responses
of leukemic mice were investigated in vivo.
SFN treatment had no significant effect on the liver
and spleen weights, however, 280 mg/kg treatment significant
decreased the body weight of leukemic mice when compared with
untreated leukemia mice. The present study demonstrated that SFN
promoted a cellular population based on increased cell marker
levels, including CD3 (T cells) and CD19 (B cells), however, had no
significant effect on CD11 (monocytes) populations in leukemic
mice. Furthermore, SFN treatment resulted in an increase in the
phagocytotic activity of macrophages from PBMC (Fig. 3A) and peritoneal cavity (Fig. 3B), therefore, the function of SFN
on the Mac-3 marker and macrophage function requires further
research.
As demonstrated in Fig.
4, YAC-1 cells were killed by NK cells following SFN treatment
in a dose-dependent manner. SFN treatment (285, 570 or 1,140 mg/kg)
resulted in increased T cell proliferation following Con A
stimulation (Fig. 5B), however
only treatment with 1,140 mg/kg SFN resulted in increased B cell
proliferation following LPS stimulation (Fig. 5A). Previous studies demonstrated
that macrophages serve important roles in innate immunity (25,26),
stimulating of NK cell cytotoxicity which may lead the increased
immune responses (27). Previous
studies demonstrated that cells, including macrophages, T and B
cells, interact with each other resulting in an immune response
(25,26). For example, activated T cells
release cytokines promoting the function and activity of
macrophages (25,26). Further research is required to
assess the immune responses.
In conclusion, the present study suggested that SFN
treatment modulated the immune responses through increasing the T
and B cell markers population, T and B cells proliferation,
phagocytotic activity of macrophages and increase of NK cell
cytotoxicity in leukemic mice in vivo. Whether SFN has a
direct antileukemic effect in immune modulations requires further
investigation.
Acknowledgments
The present study was supported by a grant from the
China Medical University in Taichung, Taiwan (grant no.
CMU102-ASIA-20).
References
|
1
|
Alabsi AM, Ali R, Ideris A, Omar AR, Bejo
MH, Yusoff K and Ali AM: Anti-leukemic activity of Newcastle
disease virus strains AF2240 and V4-UPM in murine myelomonocytic
leukemia in vivo. Leuk Res. 36:634–645. 2012. View Article : Google Scholar
|
|
2
|
Dameshek W: Chronic lymphocytic leukemia -
an accumulative disease of immunolgically incompetent lymphocytes.
Blood. 29(Suppl): 566–584. 1967.
|
|
3
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A, et al: The 2008 revision of the
World Health Organization (WHO) classification of myeloid neoplasms
and acute leukemia: Rationale and important changes. Blood.
114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marshall GM, Dalla Pozza L, Sutton R, Ng
A, de Groot-Kruseman HA, van der Velden VH, Venn NC, van den Berg
H, de Bont ES, Maarten Egeler R, et al: High-risk childhood acute
lymphoblastic leukemia in first remission treated with novel
intensive chemotherapy and allogeneic transplantation. Leukemia.
27:1497–1503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conaway CC, Wang CX, Pittman B, Yang YM,
Schwartz JE, Tian D, McIntee EJ, Hecht SS and Chung FL: Phenethyl
isothiocyanate and sulforaphane and their N-acetylcysteine
conjugates inhibit malignant progression of lung adenomas induced
by tobacco carcinogens in A/J mice. Cancer Res. 65:8548–8557. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fimognari C, Nüsse M, Berti F, Iori R,
Cantelli-Forti G and Hrelia P: Isothiocyanates as novel cytotoxic
and cytostatic agents: Molecular pathway on human transformed and
non-transformed cells. Biochem Pharmacol. 68:1133–1138. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et
al: Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu C, Shen G, Yuan X, Kim JH,
Gopalkrishnan A, Keum YS, Nair S and Kong AN: ERK and JNK signaling
pathways are involved in the regulation of activator protein 1 and
cell death elicited by three isothiocyanates in human prostate
cancer PC-3 cells. Carcinogenesis. 27:437–445. 2006. View Article : Google Scholar
|
|
9
|
Fahey JW, Haristoy X, Dolan PM, Kensler
TW, Scholtus I, Stephenson KK, Talalay P and Lozniewski A:
Sulforaphane inhibits extracellular, intracellular, and
antibiotic-resistant strains of Helicobacter pylori and prevents
benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA.
99:7610–7615. 2002. View Article : Google Scholar
|
|
10
|
Zhang Y, Kensler TW, Cho CG, Posner GH and
Talalay P: Anticarcinogenic activities of sulforaphane and
structurally related synthetic norbornyl isothiocyanates. Proc Natl
Acad Sci USA. 91:3147–3150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keck AS and Finley JW: Cruciferous
vegetables: Cancer protective mechanisms of glucosinolate
hydrolysis products and selenium. Integr Cancer Ther. 3:5–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fimognari C, Lenzi M, Sciuscio D,
Cantelli-Forti G and Hrelia P: Cell-cycle specificity of
sulforaphane-mediated apoptosis in Jurkat T-leukemia cells. In
Vivo. 21:377–380. 2007.PubMed/NCBI
|
|
13
|
Choi WY, Choi BT, Lee WH and Choi YH:
Sulforaphane generates reactive oxygen species leading to
mitochondrial perturbation for apoptosis in human leukemia U937
cells. Biomed Pharmacother. 62:637–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fimognari C, Lenzi M, Cantelli-Forti G and
Hrelia P: Induction of differentiation in human promyelocytic cells
by the isothiocyanate sulforaphane. In Vivo. 22:317–320.
2008.PubMed/NCBI
|
|
15
|
Moon DO, Kim MO, Kang SH, Choi YH and Kim
GY: Sulforaphane suppresses TNF-alpha-mediated activation of
NF-kappaB and induces apoptosis through activation of reactive
oxygen species-dependent caspase-3. Cancer Lett. 274:132–142. 2009.
View Article : Google Scholar
|
|
16
|
Suppipat K, Park CS, Shen Y, Zhu X and
Lacorazza HD: Sulforaphane induces cell cycle arrest and apoptosis
in acute lymphoblastic leukemia cells. PLoS One. 7:e512512012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fimognari C, Turrini E, Sestili P,
Calcabrini C, Carulli G, Fontanelli G, Rousseau M, Cantelli-Forti G
and Hrelia P: Antileukemic activity of sulforaphane in primary
blasts from patients affected by myelo- and lympho-proliferative
disorders and in hypoxic conditions. PLoS One. 9:e1019912014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu HF, Tung WL, Yang JS, Huang FM, Lee CS,
Huang YP, Liao WY, Chen YL and Chung JG: In vitro suppression of
growth of murine WEHI-3 leukemia cells and in vivo promotion of
phagocytosis in a leukemia mice model by indole-3-carbinol. J Agric
Food Chem. 60:7634–7643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu FS, Yang JS, Yu CS, Chiang JH, Lu CC,
Chung HK, Yu CC, Wu CC, Ho HC and Chung JG: Safrole suppresses
murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates
macrophage phagocytosis and natural killer cell cytotoxicity in
leukemic mice. Environ Toxicol. 28:601–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin JJ, Lu KW, Ma YS, Tang NY, Wu PP, Wu
CC, Lu HF, Lin JG and Chung JG: Alpha-phellandrene, a natural
active monoterpene, influences a murine WEHI-3 leukemia model in
vivo by enhancing macrophague phagocytosis and natural killer cell
activity. In Vivo. 28:583–588. 2014.PubMed/NCBI
|
|
21
|
Hattori Y, Satouchi M, Shimada T, Urata Y,
Yoneda T, Mori M, Nishimura T, Sunadome H, Kumagai T, Imamura F, et
al: A phase 2 study of bevacizumab in combination with carboplatin
and paclitaxel in patients with non-squamous non-small-cell lung
cancer harboring mutations of epidermal growth factor receptor
(EGFR) after failing first-line EGFR-tyrosine kinase inhibitors
(HANSHIN Oncology Group 0109). Lung Cancer. 87:136–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Kim J, Chang E, Choi W, Lee K, Yoon
H, Jung S, Park M, Yoon J and Kim S: A Phase II Trial of
Neoadjuvant Chemotherapy with Genexol (Paclitaxel) and Epirubicin
for Locally Advanced Breast Cancer. J Breast Cancer. 17:344–349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otake A, Yoshino K, Ueda Y, Sawada K,
Mabuchi S, Kimura T, Kobayashi E, Isobe A, Egawa-Takata T,
Matsuzaki S, et al: Usefulness of duloxetine for Paclitaxel-induced
peripheral neuropathy treatment in gynecological cancer patients.
Anticancer Res. 35:359–363. 2015.PubMed/NCBI
|
|
24
|
Schmid D, Jarvis GE, Fay F, Small DM,
Greene MK, Majkut J, Spence S, McLaughlin KM, McCloskey KD,
Johnston PG, et al: Nanoencapsulation of ABT-737 and camptothecin
enhances their clinical potential through synergistic antitumor
effects and reduction of systemic toxicity. Cell Death Dis.
5:e14542014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie S, Chen M, Yan B, He X, Chen X and Li
D: Identification of a role for the PI3K/AKT/mTOR signaling pathway
in innate immune cells. PLoS One. 9:e944962014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guleria I and Pollard JW: The trophoblast
is a component of the innate immune system during pregnancy. Nat
Med. 6:589–593. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Z, Sinzger C, Reichel JJ, Just M and
Mertens T: Natural killer cells can inhibit the transmission of
human cytomegalovirus in cell culture using mechanisms from innate
and adaptive immune response. J Virol. 89:2906–2917. 2015.
View Article : Google Scholar
|