Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
malignant diseases in adults, and is the most common type of kidney
cancer. The incidence of this cancer has increased over several
years, contributing to a steady increase in mortality rate in
developing and developed countries (1–3).
Cigarette smoking (4,5), obesity (6,7),
hypertension (8) and certain
environmental factors (9) are
well-known risk factors for RCC. However, the majority of patients
exhibit no identifiable risk factor, and the underlying pathogenic
mechanisms of risk factors remain obscure. Roughly 1/3 patients
with RCC are diagnosed at the late phase of the disease, missing
the opportunity of surgical management. Almost all RCC pathological
types are resistent to chemotherapeutics and radiation therapy. At
present, immunotherapy is the major treatment for the late phase
disease, however, the response rate is <20% (10). Although sorafenib and sunitinib
have shown better response in metastatic RCC, the overall survival
remains markedly poor (11,12).
Consequently, novel antitumor agents and methods with high
efficiency are urgently required.
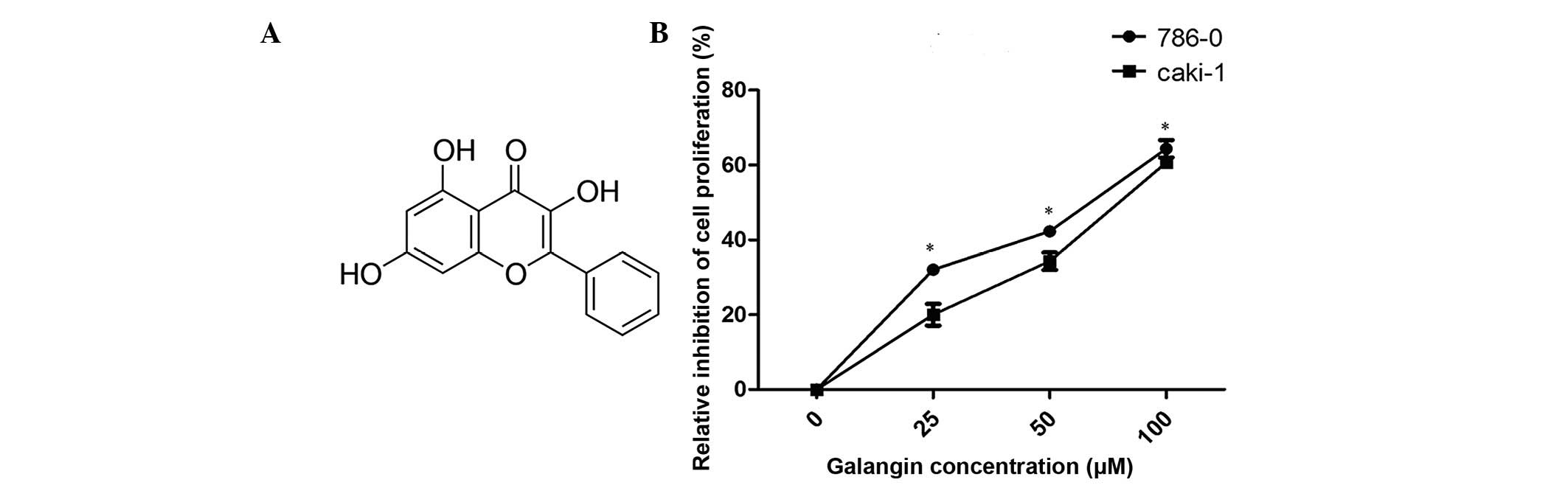
Galangin (3,5,7-trihydroxyflavone; Fig. 1A) is a naturally active flavonoid
from the root of Alpinia officinarum Hance, which has been
used as a herbal medicine in Asian cultures for a variety of
symptoms for centuries (13,14).
Several previous studies have demonstrated that galangin has
anticancer effects against several cancer types. Galangin induces
apoptosis in gastric cancer cells via the regulation of ubiquitin
carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase
(15). Galangin also inhibits the
growth and metastasis of B16F10 melanoma cells (16). However, little is understood about
its influence on RCC.
The present study investigated the effect of
galangin on RCC and demonstrated that galangin inhibited RCC cell
proliferation, cell invasion and induced apoptosis in vitro.
It was also revealed that galangin inhibited RCC cell invasion by
suppressing the epithelial-mesenchymal transition (EMT) and induced
apoptosis, accompanied by the production of reactive oxygen species
(ROS).
Materials and methods
Cell culture
The human RCC cell lines, Caki-1 and 786-0, were
obtained from the Chinese Academy of Sciences Cell Bank (Shanghai,
China). The cells were cultured in McCoy's 5A medium and RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
respectively, containing 100 mg/ml penicillin and 100 mg/ml
streptomycin (Beyotime Institute of Biotechnology, Shanghai,
China), and supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
with 5% CO2.
Agents and chemicals
All chemicals and reagents used in the present study
were molecular biology grade. Galangin was purchased from
Sigma-Aldrich (St. Louis, MO, USA), at a purity of 98%, and was
dissolved in dimethyl sulfoxide (DMSO). Malondialdehyde (MDA),
total antioxidant capacity (T-AOC) and superoxide dismutase (SOD)
assay kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The production of ROS was measured
using dichlorofluorescein-diacetate (DCFH-DA; Molecular Probes;
Sigma-Aldrich). The primary antibodies for monoclonal rabbit
anti-human SOD (1:1,000; cat. no. 2770), monoclonal rabbit
anti-human catalase (1:1,000; cat. no. 12980), monoclonal rabbit
anti-human E-cadherin (1:1,000; cat. no. 3195), monoclonal rabbit
anti-human N-cadherin (1:1,000; cat. no. 13116), monoclonal rabbit
anti-human vimentin (1:1,000; cat. no. 5741) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell proliferation assay
The Caki-1 and 786-0 cell lines were incubated with
different concentrations of galangin (0 µM, 25 µM, 50
µM, 100 µM). Following a 24-h incubation the cells
were seeded into 96-well plates at a density of 2×103
cells/well. Cell proliferation was determined using a Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology) following the
manufacturer's protocol. Absorbance was detected at the wavelength
of 450 nm using a spectophotometer (Multiskan FC; Thermo Fisher
Scientific, Inc.) Three wells were measured for cell viability per
group.
Cell invasion assays
For the invasion assays, 5×104 cells in
200 ml serum-free medium were placed in the upper chamber of the
Transwell (pore size, 8 mm; BD Biosciences, Franklin Lakes, NJ,
USA) coated with Matrigel (BD Biosciences), according to the
manufacturer's protocol. Medium containing 20% FBS was added to the
lower chamber. Following incubation for 24 h at 37°C, the cells
remaining on the upper membrane were removed and those on the lower
surface of the membrane were fixed in 95% ethanol and stained with
crystal violet (Beyotime Institute of Biotechnology). A total of 10
random fields were counted. All of the experiments were performed
in triplicate.
Wound healing assay
The 786-0 and Caki-1 cells were grown to confluent
monolayers, which were serum starved for 12 h. A 1 ml pipette tip
was drawn across the center of the well to produce a clean wound
area and the wounded cell layer was washed with fresh medium to
remove loose cells. Immediately following wounding and an
incubation for 24 h at 37°C in the presence or absence of 100
µM galangin, images of the wound healing process were
captured digitally (magnification, ×200). The gap distance was
normalized against the control level and was compared between 0 and
24 h. The mean values were obtained from at least three separate
experiments.
Analysis of apoptosis
The RCC cells were seeded into 6-well plates
overnight and were subsequently treated with different
concentrations of galangin for 48 h. The cells were collected by
trypsinization (Gibco; Thermo Fisher Scientific, Inc.) and were
washed at least twice with cold phosphate-buffered saline (PBS).
The cells were resuspended in 1X binding buffer (Beyotime Institute
of Biotechnology) at a concentration of 1×106 cells/ml.
A total of 5 µl annexin V-fluorescein isothiocyanate reagent
and 10 µl propidium iodide were added to the cell
suspension, and were incubated for 15 min at room temperature in
the dark. The stained cells were analyzed by flow cytometry
(Becton-Dickinson, San Jose, CA, USA). The data was analysed using
FlowJo software (version 7.6.1; FlowJo LLC, OR, USA)
Detection of ROS
For measurement of intracellular ROS levels, the
DCFH-DA method was used. The cells were harvested following
treatment with Galangin and were subsequently washed once with
ice-cold PBS. The cells were treated with DCFH-DA (at a final
concentration of 10 mol/l in serum-free medium). Following
incubation for 20 min at 37°C in the dark, the cells were washed
twice with PBS. Intracellular ROS accumulation was measured by flow
cytometry. The median fluorescence intensity values were
calculated. All experiments were performed in triplicate.
SOD, T-AOC and MDA determination
The cells were seeded at 70% confluence into 6-well
plates. After 24 h incubation, the cells were treated with
different concentrations of galangin for 48 h. Following treatment,
the cells were detached by trypsinization, collected by
centrifugation (878 × g, for 10 min at 4°C) and resuspended in PBS.
The suspensions were used immediately for SOD, T-AOC and MDA
assays, according to the manufacturer's protocol.
Western blot analysis
The cells were lysed in radioimmunoprecipitation
buffer (Nanjing KeyGen BioTech Co., Ltd., Nanjing, China),
supplemented with protease inhibitors at 4°C for 1 h. The protein
samples were collected from cell lysates. The protein content of
the supernatants was determined with a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). The protein samples (40
µg per lane) were electrophoresed in 10% SDS-PAGE gels
(Beyotime Institute of Biotechnology) and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following transfer, the membranes were blocked for 2 h at
room temperature with 5% non-fat milk. The membranes were
subsequently incubated with primary antibodies at 4°C overnight.
The membranes were washed three times with Tris-buffered saline [20
mM Tris-HCl (pH 7.6), 137 mM NaCl], containing 0.01% Tween-20, and
were subsequently incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:1,000; cat. no. A0208;
Beyotime Institute of Biotechnology) at room temperature for 2 h.
Following three washes with TBST the blots were detected using
chemiluminescence using a microplate photometer (Multiskan FC;
Thermo Fisher Scientific, Inc.), they were visualized Gel Doc XR+
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein
levels were determined by normalizing against the levels of GAPDH
using a monoclonal rabbit anti-human antibody (1:1,000; cat. no.
5174; Cell Signaling Technology, Inc.).
Statistical analysis
The data are presented as the mean ± standard
deviation from at least three independent experiments. Statistical
calculations were performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis was performed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Galangin inhibits the proliferation of
RCC cells
The antiproliferative effects of galangin on RCC
cells were measured using a CCK-8 assay (Fig. 1B). Galangin had a significant
inhibitory effect on 786-0 and Caki-1 cell growth in a
dose-dependent manner. The cell viabilities of the two cell lines,
786-0 and Caki-1, at 100 µM concentrations were 64.1 and
59.2%, respectively (Fig. 1B).
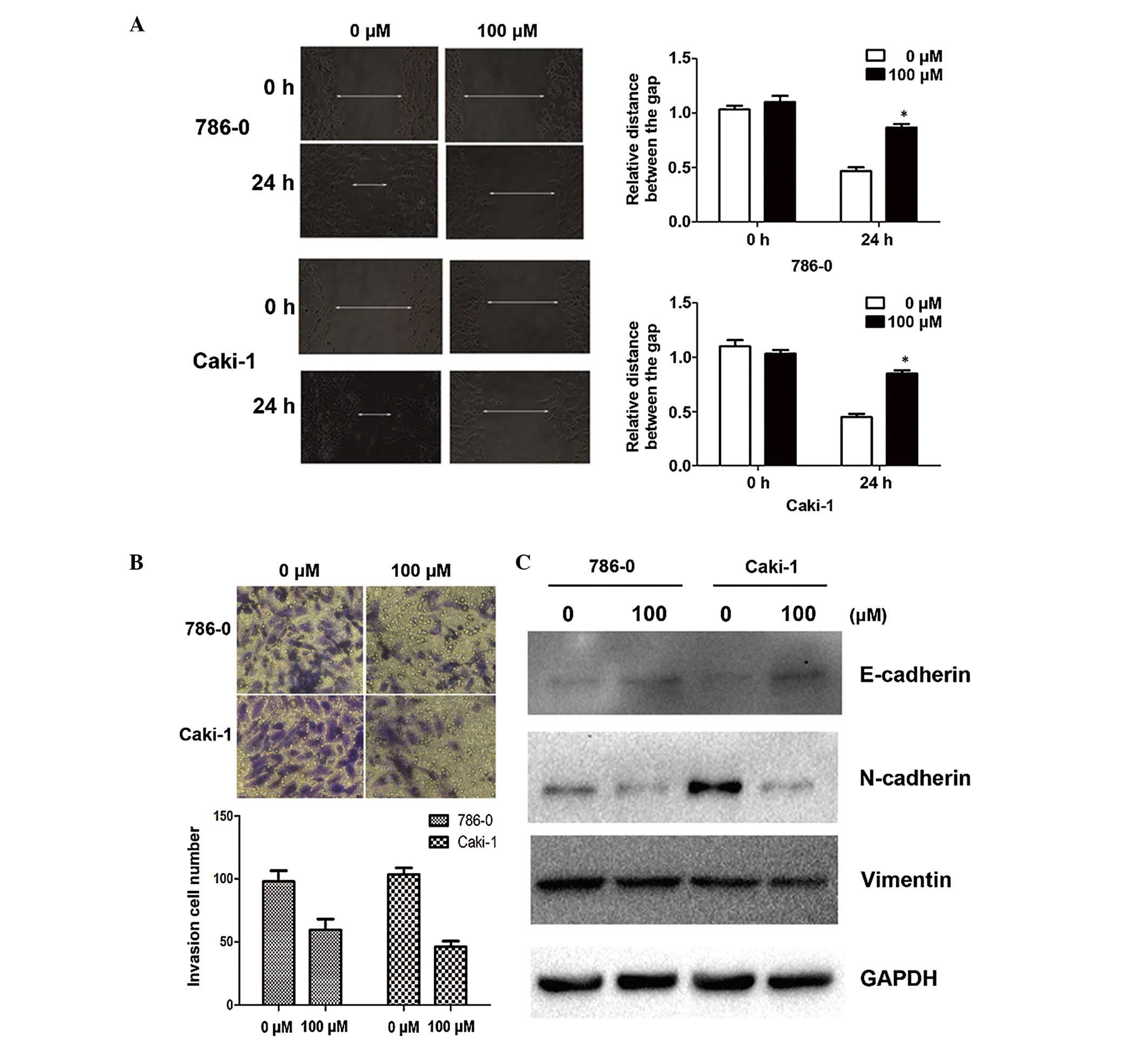
Galangin suppresses cell migration and
invasion in vitro by inhibiting the EMT
Since cell adhesion is one of the essential steps
involved in cancer metastasis, the present study investigated the
effect of galangin on cancer cell migration and invasion. As shown
by the wound healing assay, 100 µM galangin significantly
slowed the rate by which the cells migrated to the wounded area
compared with the control group at 24 h (Fig. 2A). Furthermore, using an invasion
assay, a marked reduction in the number of invasive cells was
observed when the cells were treated with galangin at a
concentration of 100µM for 24 h (Fig. 2B). Alterations in epithelial and
mesenchymal markers, including N-cadherin, E-cadherin and vimentin,
were determined by immunoblotting. The results demonstrated a
reduction in the expression levels of N-cadherin and vimentin, and
an increase in the expression of E-cadherin (Fig. 2C).
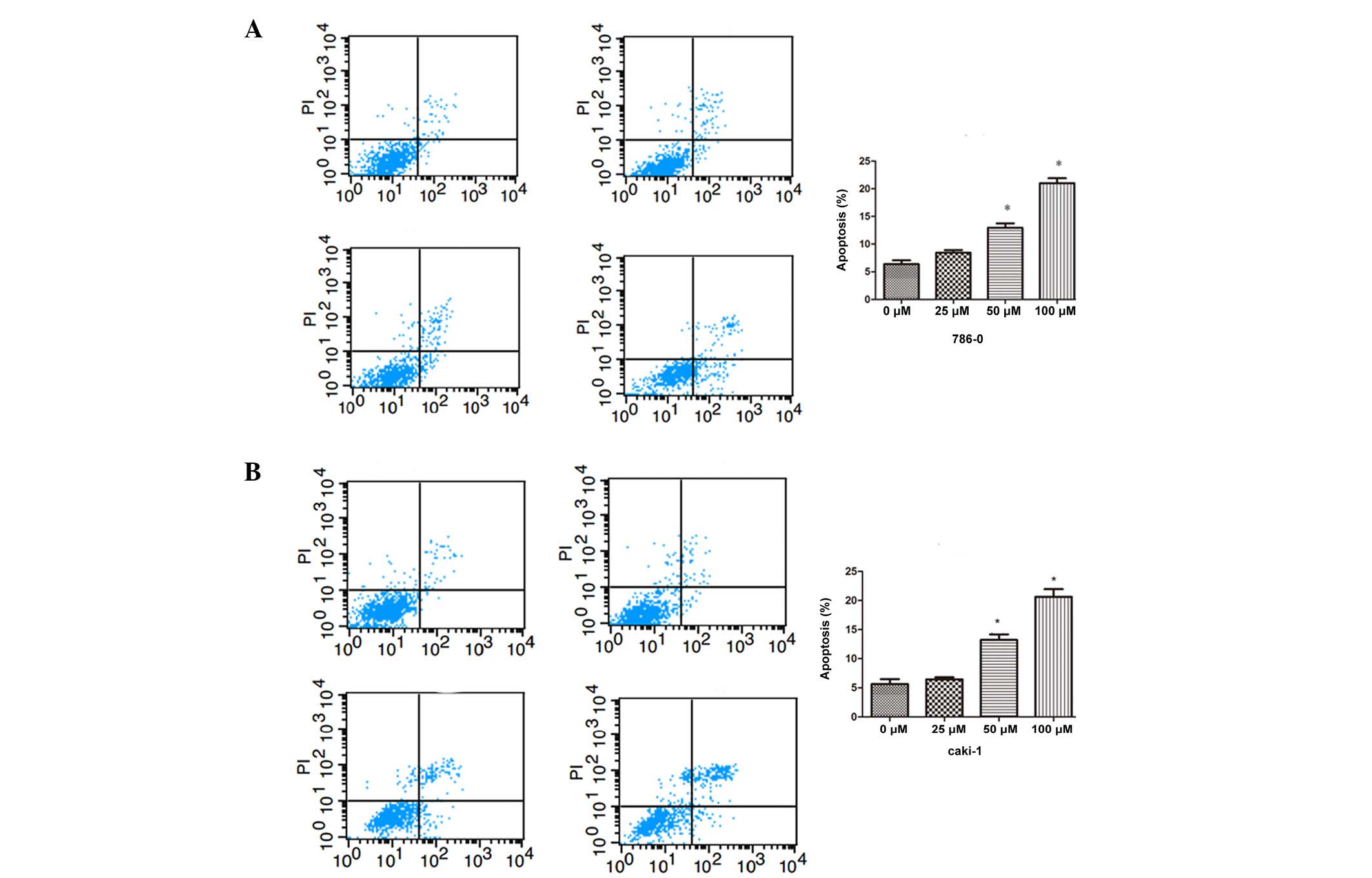
Effect of galangin on RCC cell
apoptosis
To assess the antitumor effect of different
concentrations of galangin in 786-0 and Caki-1 cells, apoptosis was
assessed by flow cytometry. As shown in Fig. 3, the number of apoptotic cells were
significantly increased compared with the control group in a
dose-dependent manner, in both the 786-0 and Caki-1 cells.
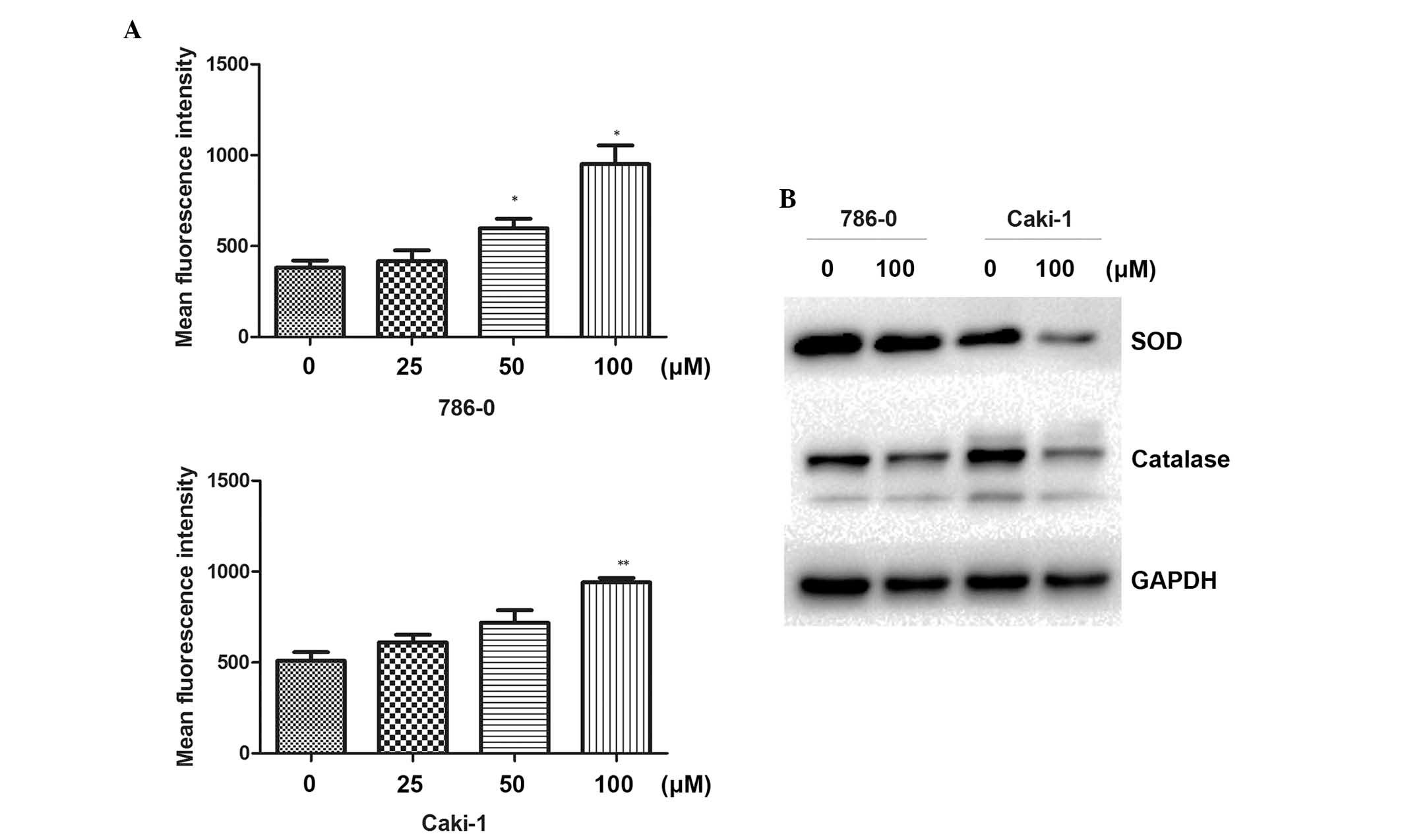
Increase of intracellular ROS following
galangin treatment
Following treatment of the cells with different
concentrations of galangin for 48 h, the DCFH-DA mean fluorescence
intensity was markedly increased and positively correlated with
galangin concentration (Fig. 4A).
The result revealed that galangin enhanced intracellular ROS levels
in the RCC cells. To understand the molecular mechanisms involved
in the ROS induced by galangin, the effect of galangin on the
expression of ROS-associated proteins was next investigated. As
shown in Fig. 4B, the galangin
treatment group significantly downregulated the levels of SOD and
catalase. These data demonstrated that the antitumor apoptotic
effect of galangin was associated with intracellular ROS in RCC
cells.
SOD, T-AOC and MDA activity
The activity of the antioxidant enzyme, SOD, was
markedly decreased in the galangin treatment group in both 786-0
and Caki-1 cells. The activity of T-AOC was significantly reduced
compared with the control group in the RCC cell lines. MDA, the
stable metabolite of lipid peroxidation products, was markedly
increased following exposure to 100 µM galangin (Table I).
 | Table IActivity of SOD, T-AOC and MDA at
different concentrations of galangin in 786-0 and Caki-1 cells. |
Table I
Activity of SOD, T-AOC and MDA at
different concentrations of galangin in 786-0 and Caki-1 cells.
| Galangin
(µM) | SOD (U/mg) | T-AOC (nmol/mg) | MDA (nmol/mg) |
|---|
| 786-0 | | | |
| 0 | 35.85±2.10 | 3.20±0.21 | 7.31±0.18 |
| 25 | 35.22±1.79 | 2.81±0.17 | 8.45±0.49 |
| 50 | 29.12±1.99a | 2.30±0.25a | 8.20±1.04 |
| 100 | 25.70±2.07b | 1.65±0.38b | 13.68±0.50c |
| Caki-1 | | | |
| 0 | 31.2±1.04 | 1.88±0.19 | 3.76±0.43 |
| 25 | 32.1±1.05 | 1.65±0.16 | 4.08±.28 |
| 50 | 28.11±1.80 | 1.46±0.26 | 5.87±0.58b |
| 100 | 25.18±1.05b | 0.97±0.21b | 7.01±0.41c |
Discussion
Numerous previous studies have concentrated on the
effects of flavonoids in cancer treatment. Flavonoids are regarded
as possible chemopreventive agents against various cancer types,
which are generally non-toxic and reveal a diverse range of
beneficial biological activities (17). It has been recognized as a
promising cancer chemopreventive agent (17). However, no study has investigated
the influence of galangin on RCC. The aim of the present study was
to assess the effect of galangin on 786-0 and Caki-1 cells, and to
gain preliminary insight into the underlying molecular
mechanism.
In the present study, the anticancer activities of
galangin against human 786-0 and Caki-1 RCC cells were assessed.
Firstly, it was determined that galangin inhibited RCC cell
proliferation in a dose-dependent manner. It was also revealed that
galangin inhibited RCC invasion by suppressing the EMT and induced
apoptosis, accompanied by the production of ROS.
The EMT is the differentiation switch to change
epithelial polarized cells into motile mesenchymal cells, which is
vital in embryonic development, fibrotic diseases, and invasion and
metastasis of human cancer. The EMT allows cells to obtain
fibroblast-like properties and reduces intercellular adhesion and
increases motility (18–20). In addition, the EMT is dysregulated
in cancer cells and is characterized by the acquisition of a
mesenchymal phenotype, leading to increased motility, allowing the
tumor cells to metastasize (21).
The EMT is regulated by a variety of signaling pathways, including
tumor growth factor-β, epidermal growth factor and hepatocyte
growth factor (22). Decreased
expression of E-cadherin is considered an important step in the
progression of tumor metastasis and is a fundamental event in the
EMT (23). In the present study,
exposure of 786-0 and Caki-1 cells to galangin resulted in
increased expression of E-cadherin and decreased expression levels
of N-cadherin and vimentin. These results suggested that galangin
suppressed the EMT in 786-0 and Caki-1 cells. The present study
used wound healing and invasion assays to evaluate the migration
and invasion of the cells. Treatment with100 µM
significantly decreased the migratory and invasive capabilities of
the RCC cells in vitro. Taken together, this data revealed
that galangin inhibited tumor cell invasion and migration, which
may modulate the EMT process.
The present study has also revealed that different
concentrations of galangin may induce apoptosis of RCC cells. The
effect of galangin on increasing the intracellular ROS level was
noted. Accumulation of ROS is an important contributing factor for
the apoptosis of various types of cancer cell (24,25).
ROS, including O2−, H2O2 and
hydroxyl radical, which are the side products of normal metabolism
or environmental stress, can cause mitogenic to proliferative
effects at low concentrations, and induce cell damage and cell
death when ROS generation exceeds the cellular antioxidant defenses
(26). In the present study, the
intracellular ROS levels in 786-0 and Caki-1 cells treated with
galangin were revealed to be increased at a high concentrations.
However, previous reports demonstrate that flavonoids have certain
antioxidant properties (27,28).
To further confirm the present result, the expression levels of
T-AOC, SOD and MDA were determined. MDA, a biomarker of ROS damage,
was significantly increased following treatment. By contrast, the
production of the antioxidant enzymes, T-AOC and SOD, were
significantly decreased in the 786-0 and Caki-1 cells. Western
blotting also demonstrated that treatment with 100 µM
galangin suppressed the activity of two antioxidant enzymes, SOD
and catalase. These results suggested that the proapoptotic effects
of galangin may be mediated by the production of intracellular ROS
at a large concentration.
Surgery has been the mainstay treatment for early
stage RCC tumors. For advanced RCC, traditional chemotherapeutic
agents are generally considered to be inefficient. Therefore, novel
therapeutic approaches against RCC are necessary. The present
research demonstrated that galangin exerted an antiproliferative
property, inhibited cell invasion and induced apoptosis in RCC,
which suggested that galangin may be a novel approach for the
treatment of RCC.
In conclusion, galangin exerted an antiproliferative
property in a dose-dependent manner. In addition, galangin induced
cell apoptosis by increasing the intracellular concentration of ROS
at a large dose and inhibited cell invasion by suppressing the EMT.
Therefore, combining galangin with other drugs may increase the
therapeutic potential. Further studies are required to determine
the influence of galangin in the progression of RCC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81270685).
References
|
1
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hollingsworth JM, Miller DC, Daignault S
and Hollenbeck BK: Five-year survival after surgical treatment for
kidney cancer: A population-based competing risk analysis. Cancer.
109:1763–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hunt JD, van der Hel OL, McMillan GP,
Boffetta P and Brennan P: Renal cell carcinoma in relation to
cigarette smoking: Meta-analysis of 24 studies. Int J Cancer.
114:101–108. 2005. View Article : Google Scholar
|
|
5
|
Yuan JM, Castelao JE, Gago-Dominguez M, Yu
MC and Ross RK: Tobacco use in relation to renal cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 7:429–433. 1998.PubMed/NCBI
|
|
6
|
Bjørge Tand Tretli S and Engeland A:
Relation of height and body mass index to renal cell carcinoma in
two million Norwegian men and women. Am J Epidemiol. 160:1168–1176.
2004. View Article : Google Scholar
|
|
7
|
van Dijk BA, Schouten LJ, Kiemeney LA,
Goldbohm RA and van den Brandt PA: Relation of height, body mass,
energy intake and physical activity to risk of renal cell
carcinoma: Results from the Netherlands cohort study. Am J
Epidemiol. 160:1159–1167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McLaughlin JK, Chow WH, Mandel JS,
Mellemgaard A, McCredie M, Lindblad P, Schlehofer B, Pommer W, Niwa
S and Adami HO: International renal-cell cancer study. VIII. Role
of diuretics, other anti-hypertensive medications and hypertension.
Int J Cancer. 63:216–221. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCredie M, Pommer W, McLaughlin JK,
Stewart JH, Lindblad P, Mandel JS, Mellemgaard A, Schlehofer B and
Niwa S: International renal-cell cancer study. II. Analgesics. Int
J Cancer. 60:345–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDermott DF: Immunotherapy of metastatic
renal cell carcinoma. Cancer. 115(Suppl 10): 2298–2305. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu
KD, Chen XY, Wen M and Liu HM: Galangin induces apoptosis of
hepatocellular carcinoma cells via the mitochondrial pathway. World
J Gastroenterol. 16:3377–3384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capasso R and Mascolo N: Inhibitory effect
of the plant flavonoid galangin on rat vas deferens in vitro. Life
Sci. 72:2993–3001. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DA, Jeon YK and Nam MJ: Galangin
induces apoptosis in gastric cancer cells via regulation of
ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione
S-transferase P. Food Chem Toxicol. 50:684–688. 2012. View Article : Google Scholar
|
|
16
|
Zhang W, Tang B, Huang Q and Hua Z:
Galangin inhibits tumor growth and metastasis of B16F10 melanoma. J
Cell Biochem. 114:152–161. 2013. View Article : Google Scholar
|
|
17
|
Heo MY, Sohn SJ and Au WW:
Anti-genotoxicity of galangin as a cancer chemopreventive agent
candidate. Mutat Res. 488:135–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Wang DM, Wang CM, Hu Y, Liu AH,
Zhang YL, Sun B and Song JG: Insulin receptor substrate-1
suppresses transforming growth factor-beta1-mediated
epithelial-mesenchymal transition. Cancer Res. 69:7180–7187. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan F, Samuel S, Evans KW, Lu J, Xia L,
Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA and Ellis LM:
Overexpression of snail induces epithelial-mesenchymal transition
and a cancer stem cell-like phenotype in human colorectal cancer
cells. Cancer Med. 1:5–16. 2012. View
Article : Google Scholar
|
|
21
|
Saito RA, Watabe T, Horiguchi K, Kohyama
T, Saitoh M, Nagase T and Miyazono K: Thyroid transcription
factor-1 inhibits transforming growth factor-beta-mediated
epithelial-to-mesenchymal transition in lung adenocarcinoma cells.
Cancer Res. 69:2783–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Najjar N, Chatila M, Moukadem H,
Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R and
Gali-Muhtasib H: Reactive oxygen species mediate
thymoquinone-induced apoptosis and activate ERK and JNK signaling.
Apoptosis. 15:183–195. 2010. View Article : Google Scholar
|
|
25
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems and apoptosis. Free Radic Biol Med.
48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamaley IA and Klyubin IV: Roles of
reactive oxygen species: Signaling and regulation of cellular
functions. Int Rev Cytol. 188:203–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: an updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–31. 2015. View
Article : Google Scholar
|
|
28
|
Agati G, Azzarello E, Pollastri S and
Tattini M: Flavonoids as antioxidants in plants: location and
functional significance. Plant Sci. 2012 Nov;196:67–76. View Article : Google Scholar : PubMed/NCBI
|