Introduction
Astrocytomas are the most common type of primary
central nervous system neoplasm worldwide and have been classified
into four malignancy grades by the World Health Organisation (WHO)
(1). Recently it has become
evident that the growth of human astrocytic tumors is driven by
complex signaling networks (2,3).
Malfunctions in Wnt signaling are responsible for the development
of numerous types of cancer, and the present study proposes that
aberrant Wnt signaling is important in the development and invasion
of astrocytic brain tumors. Numerous novel findings, reported in
the last two years, demonstrate that Wnt signaling is as important
in glioma formation and invasiveness as other basic cellular
pathways (2–4). Our previous studies recognized the
involvement of Wnt signaling in astrocytomas (5,6) and
in particular showed that upregulation of transcription factors
associated with the pathway, T-cell factor 1 (TCF1) and lymphoid
enhancer-binding factor-1 (LEF1), is associated with higher
malignancy grades (7).
The canonical Wnt signaling cascade is controlling
events ranging from cell cycle regulation and embryonic cell fate
determination to cell motility (8,9). The
signaling pathway is activated by the binding of different Wnt
ligands to specific serpentine receptors, termed frizzleds (Fzs).
As a consequence, the β-catenin levels rise, and β-catenin
translocates to the nucleus where it binds to transcription factors
LEF/TCF (9,10). As a result of this binding, certain
target genes are activated, including c-Myc, N-myc, c-Jun and
cyclin D1, which reveals why constitutive activation of the Wnt
pathway is responsible for tumorigenesis. A critical step in the
inactivation of Wnt signaling involves the control of β-catenin
degradation. The signaling pathway is inactive when low β-catenin
expression levels are maintained (11).
Wnt signaling is regulated at various levels by a
large number of molecular effectors. The modulating molecules
function as either antagonists or agonists of the signaling, and
fine-tuning of their association is particularly important for cell
homeostasis and normal tissue functioning. Activation of Wnt
signaling is controlled by different antagonists, among which are
members of the secreted frizzled-related protein (SFRP) family.
SFRPs are a family of soluble proteins known for their ability to
inhibit the signaling pathway by binding to Wnt ligands and/or Fz
receptors. In humans, the SFRP family numbers five of its members,
of which SFRP3 is the orthologue of the founding member,
frizzled-related protein B (FrzB) (12–14).
SFRPs were the first Wnt antagonists identified and they are
structurally associated with Fz proteins. On the N-terminal these
proteins (length, ~300 amino acids) possess an Fz-like
cysteine-rich domain (CRD), which displays similar sequence
homology to the CRD domain on the extracellular aspect of the Fz
receptors. However, unlike the Fz receptors, the SFRPs do not
possess transmembrane or cytosolic domains. In front of the CRD
domain there is a sequence for a signal peptide. In addition to the
CRD region, SFRPs have a hydrophilic region on the C-terminal that
appears to confer heparin-binding properties (14–16).
Previous studies demonstrated aberrant expression of
SFRPs in different types of cancer (17,18).
Although SFRPs were initially considered to be tumor suppressors
that exerted an inhibitory role in Wnt signaling (as they have been
found to be downregulated in the majority of tumors investigated),
novel findings report that SFRP also stimulates and, thus,
activates the Wnt signaling pathway (13,19).
Furthermore, there are numerous reports regarding the
overexpression of SFRPs in cancer, therefore this behavior can no
longer be considered as inconsistent, but indicates that SFRPs
exert a dual role in deleterious Wnt signaling (18,19).
The molecular mechanisms and genetic landscapes of
human astrocytic brain tumors require further elucidation. As
malignant astrocytomas are characterized by diffuse infiltration of
the surrounding non-neoplastic tissue, an explanation regarding the
cell invasion processes is of particular importance. Therefore, the
aim of the present study was to investigate and compare the
expression intensity and localization of the SFRP3 protein within
different histopathological grades of astrocytic brain tumor. The
SFRP3 protein was evaluated, as there are various indications that
it is associated with gliomas. A previous study has reported a
downregulation of SFRP expression levels in various types of
cancer, indicating a loss of function (18). Furthermore, an SFRP family member
strongly promoted the growth of intracranial glioma xenografts in
nude mice and promoted glioma cell growth in vitro (20). In addition, novel findings
demonstrate SFRP3 as an important morphogen of mouse neurogenesis
(21–23). However, to the best of our
knowledge, the involvement of the SFRP3 protein has not been
investigated in astrocytoma patients.
Materials and methods
Tumor specimens
Fifty-five astrocytic brain tumor samples were
collected from the Hospital Centers, Sisters of Charity (Zagreb,
Croatia) and the University Hospital Center Zagreb (Zagreb,
Croatia) between May 2007 and October 2015. The tumors were
identified by magnetic resonance imaging in different cerebral
regions. During surgery, the tumors were removed using a
microneurosurgical technique. The patients had no family history of
brain tumors, and all tumors were analyzed by pathologists and
classified into four grades, according to WHO guidelines (1,24).
There were 10 pilocytic (grade I), 15 diffuse (grade II), and 11
anaplastic (grade III) astrocytomas and 19 glioblastomas (grade
IV). There were 28 male patients and 27 female patients. The age of
the patients ranged from 3- to 73-years-old (mean age, 43.38 years;
median age, 45.00 years). The mean age at diagnosis was 44.04 and
42.70 years for males and females, respectively. Ethical approval
was obtained from the Ethical Committees Medical School University
of Zagreb (Zagreb, Croatia), Hospital Center Sisters of Charity and
University Hospital Center (380-59-10106-14-55/147) and the
patients provided informed consent.
Immunohistochemistry
The samples were fixed in formalin, embedded in
paraffin (both Kemika, Zagreb, Croatia), sliced into 4-µm
thick sections and fixed onto capillary-gap microscope slides
(Dako, Glostrup, Denmark). The sections were immunostained using
streptavidin-horseradish peroxidase/3,3′-diaminobenzidine (using
EnVision™ REAL™ detection systems; K5007; Dako). Briefly, sections
were dewaxed by immersion in xylene (Kemika) twice for 5 min.
Subsequently, the sections were rehydrated in a descending ethanol
dilution series (Kemika) and rinsed in dH20 for 5 min.
Sections were then microwaved twice for 10 min at 700 W in
retrieval solution (S2369; Dako), cooled at room temperature for 15
min, and microwaved once for 4 min at 350 W to unmask the epitopes.
To block endogenous peroxidase activity, cells were fixed in
methanol (Kemika) with 3% H2O2. Non-specific
binding was blocked by incubating samples with Protein Block,
Serum-Free Ready-To-Use (Dako North America, Inc., Carpinteria, CA,
USA) for 30 min at 4°C. Next, the primary antibody, rabbit
polyclonal anti-human FRP3 (1:50; sc-13941; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) was applied for 30 min at
room temperature. Slides were subsequently washed three times in
phosphate-buffered saline (PBS; Amresco, Solon, OH, USA)/goat
serum, and the horseradish peroxidase-conjugated anti-rabbit/mouse
secondary LINK antibodies from the Dako kit (K5007) were applied
for 16 min at room temperature. Slides were washed a further three
times in PBS/goat serum and were incubated with substrate chromogen
solution (from the EnVision™ REAL™ kits) for 30 sec.
The sections were counterstained with Harris
hematoxylin (Dako). Healthy brain and glioblastoma negative
controls underwent the same staining procedure, but these samples
were not incubated with primary antibodies. The frontal cortex of a
healthy brain, and malignant melanoma and kidney tissue samples
served as positive controls. Immunohistochemical staining was
evaluated by assessing the staining intensity by three independent
observers who were blinded to the experimental procedures. The
staining intensity was scored as follows: No expression or very
weak expression, 0/+; moderate expression, ++; and strong
expression, +++. Two hundred cells in a hot spot, which is an area
containing the most characteristics of malignant tissue and most
active proliferative rate, of each sample were analyzed. The slides
were scanned using a digital scanner (NanoZoomer 2.0-RS; Hamamatsu
Photonics, Hamamatsu, Japan), and ImageJ software (National
Institutes of Health, Bethesda, Maryland, USA) was used to
determine the cell number and the intensity of SFRP3
expression.
Statistical analysis
All individuals were analyzed for the following
features: malignancy grade, gender, age, SFRP3 protein expression
intensities and localizations. Differences in the values of SFRP3
expression levels (weak, moderate or strong) and the number of
counted cells for each intensity were tested with analysis of
variance (ANOVA) following Leven's analysis of homogeneity of
variance (if significance of Leven's statistic was <0.05 the
non-parametric Mann-Whitney test was employed). ANOVA was used to
determine potential differences in the values of SFRP3 expression
with regard to different malignancy grades, cellular localization,
different age categories and gender. Student's t-test was used to
analyze differences in membranous location. All statistical
evaluations were performed using SPSS 14.0 (SPSS Inc., Chicago, IL,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels and localizations of
SFRP3 protein
The SFRP3 protein, a molecule that is considered to
have an antagonistic role in Wnt signaling, but has never been
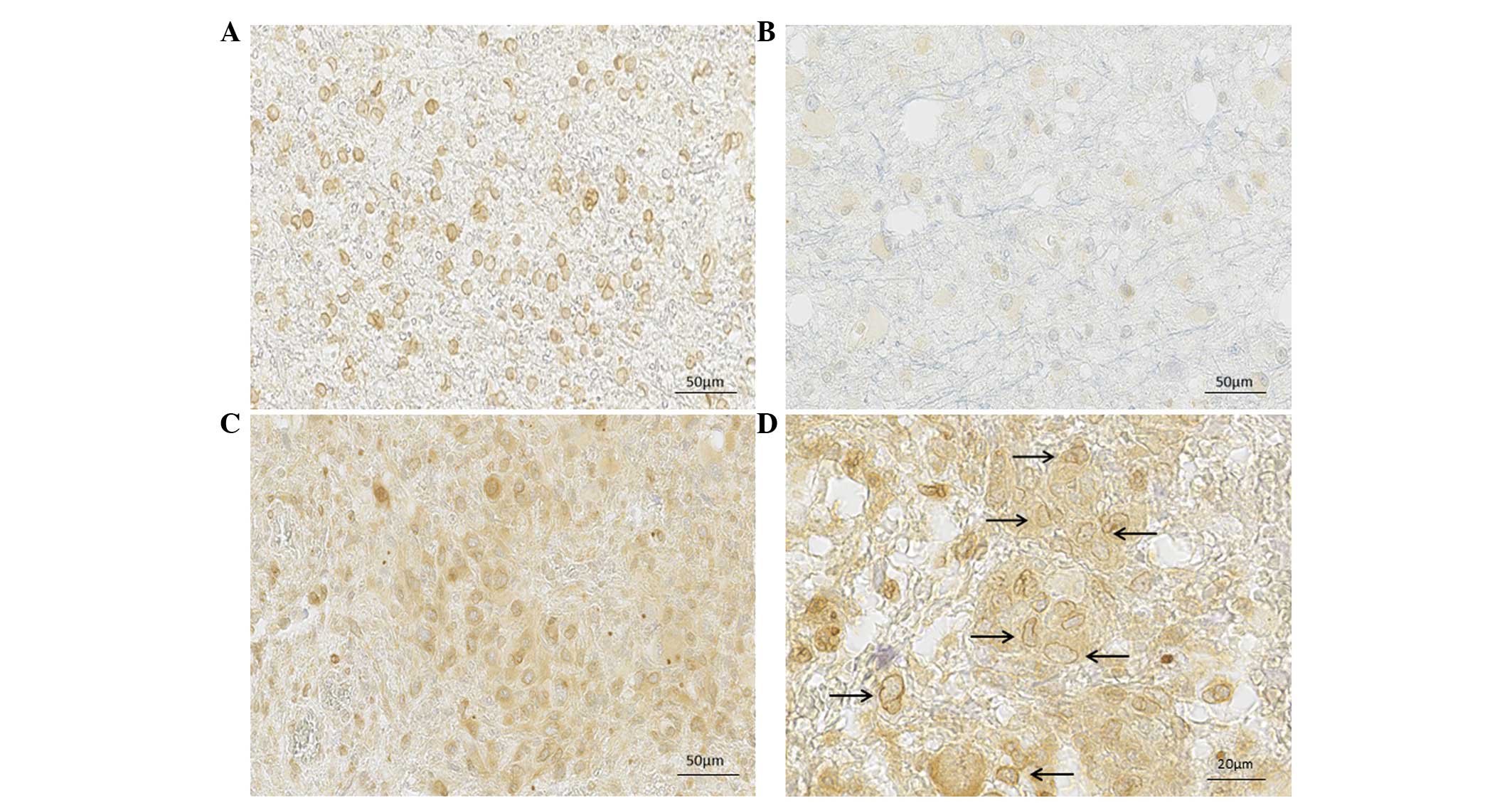
investigated in astrocytomas, was observed in the present study. On
a sample of 55 astrocytic brain tumors, the expression and
localization of SFRP3 was determined throughout different
malignancy grades. SFRP3 was positively stained and localized in
the nuclei and cytoplasm of tumor cells. Furthermore, different
expression patterns were observed in nuclear and cytoplasmic
localization, with varying staining intensities. Notably, specific
cellular compartments demonstrated different intensities and,
therefore, the separate staining intensities for cytoplasmic and
nuclear compartments were assessed.
From the total sample, the mean number of cells with
weak or no expression was 104; 25 and 61 cells (mean value)
demonstrated moderate nuclear and cytoplasmic expression,
respectively; and 18 and 41 cells (mean value) demonstrated strong
nuclear and cytoplasmic expression, respectively (Fig. 1).
Expression levels and localizations in
different malignancy grades
When the sample was divided according to malignancy
grade, the differences in expression levels and localizations were
significant. The levels of moderate and strong expression in the
cell nucleus were associated with reduced numbers of cells in
samples of higher malignancy grade tumors; whereas for cytoplasmic
staining, the number of cells with strong SFRP3 expression was
higher in astrocytoma grades III and IV when compared with samples
from lower grade tumors.
When comparing the percentage of counted cells
exhibiting low or no expression with a specific tumor grade, no
statistically significant difference was identified between low
nuclear or low cytoplasmic staining and the different grades
(F=0.815; P=0.491). However, when moderate and strong nuclear and
cytoplasmic expression levels were investigated, the differences
between expression levels and malignancy grade were statistically
significant. Moderate nuclear expression levels were statistically
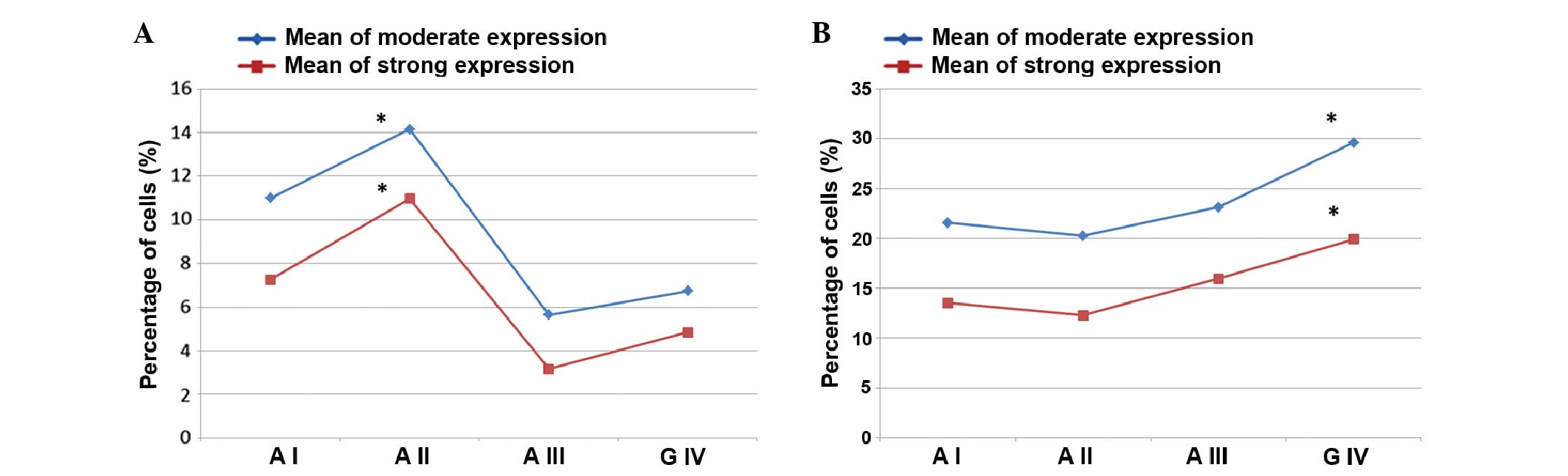
different (F=3.874; P=0.014), and grade I and II astrocytomas
demonstrated the highest expression values: Grade I, 10.99; grade
II, 14.14; grade III, 5.65; and grade IV, 6.73. Similarly, strong
nuclear expression levels were different according to the
malignancy grades (F=3.30; P=0.028) with grades I and II
astrocytomas showing greater values: Grade I, 7.26; grade II,
10.96; grade III, 3.17; and grade IV, 4.85. The association between
nuclear moderate and strong staining in the different malignancy
grades is presented in Fig.
2A.
Upon analysis of the cytoplasmic expression levels,
moderate cytoplasmic expression was identified to be significantly
different across the malignancy grades (F=2.319; P=0.086), while
strong cytoplasmic expression levels did not demonstrate a
statistically significant difference (F=1.708; P=0.177). The
association between cytoplasmic moderate and strong staining in the
different malignancy grades is presented in Fig. 2B.
SFRP3 expression levels and localizations
in the two groups
Samples were then grouped according to clinical
malignancy grades. First, the group of pooled astrocytoma II and
III was examined in comparison with the group of grade IV with the
highest malignancy grade. The nuclear expression levels between
those two groups were not statistically different for the moderate
and strong expression levels (Fig.
2A). However, a statistically significant difference was noted
for the cytoplasmic expression levels. Values of moderate
cytoplasmic expression were higher in grade IV (29.62) than in
astrocytoma II and III (21.47) and this difference was significant
(F=3.950; P=0.053). The difference between strong cytoplasmic
expression levels were also statistically significant between
astrocytoma II and III, and IV (F=3.959; P=0.05). Grade IV
glioblastomas demonstrated higher values (19.92) when compared with
astrocytoma grades II and III (13.84) (Fig. 2B).
The present study also aimed to investigate whether
there is difference between low and high grade astrocytomas.
Therefore, the sample was grouped into low grade (I and II) and
high grade (III and IV) groups. Strong statistical differences for
moderate and high nuclear expression levels were demonstrated
(Fig. 2A). For moderate nuclear
expression a significant difference was identified between the low
and high grade astrocytomas (F=10.573; P=0.002). The high grade
tumors had lower values of moderate nuclear expression (6.33) when
compared with the low grade tumors (12.88). Strong nuclear levels
were evaluated with the Mann-Whitney U test, which indicated that
high tumor grades were associated with significantly lower levels
of strong nuclear expression when compared with grade I and II
astrocytomas (P=0.018). Analysis of the low and high grade
astrocytoma groups using cytoplasmic staining showed that moderate
cytoplasmic expression levels were significantly higher (F=3.381;
P=0.072) in the astrocytoma III and glioblastoma groups than in the
astrocytoma I and II groups (Fig.
2B). Furthermore, strong cytoplasmic expression was
significantly higher (F=4.116; P=0.048) in the high grade tumor
group (A III and G IV) as compared with the low grade group (A I
and A II).
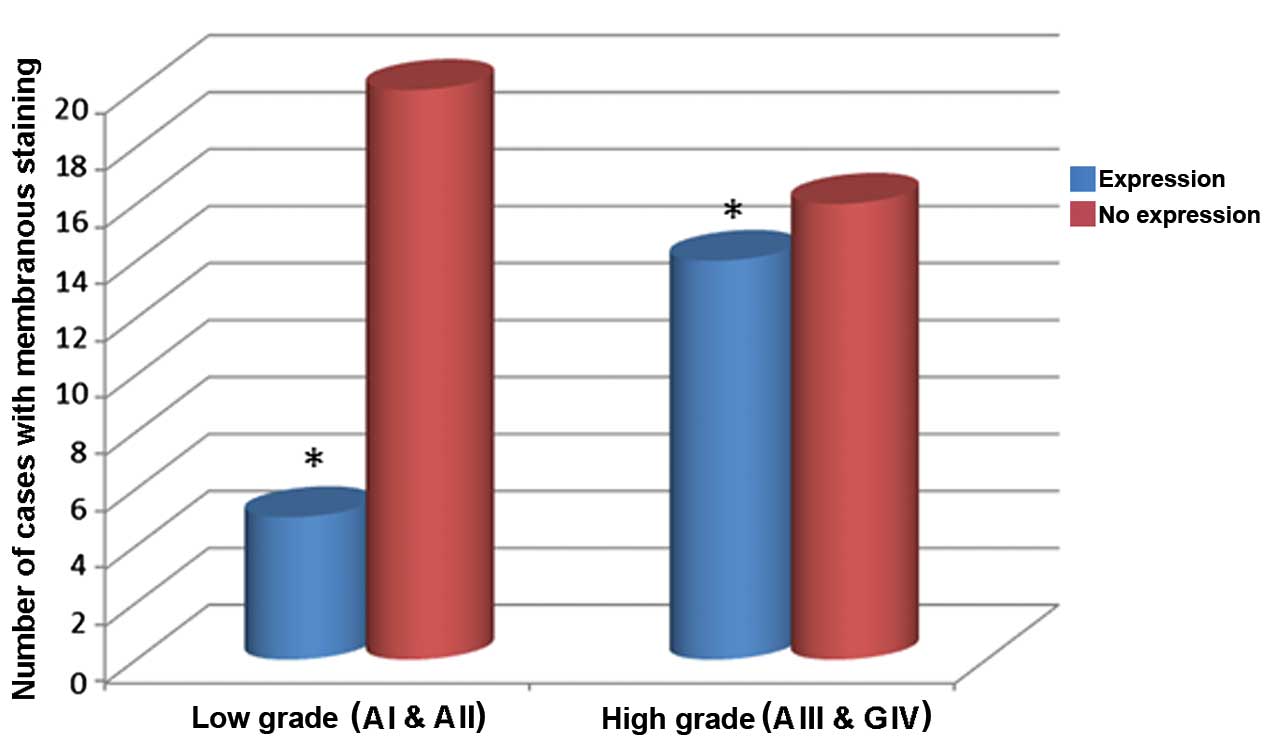
In addition, 34.5% of the samples demonstrated
membranous localization of the signal. Positive membranous staining
was observed in a relatively low number of cells per sample.
However, the association of the membranous localization and the
malignancy grades was investigated. It was demonstrated that lower
grade astrocytomas exhibited reduced membranous staining when
compared with higher grade astrocytomas, and that the difference
between grades was significant (P=0.036; Fig. 3).
SFRP3 expression and epidemiological
characteristics
The next step was to investigate the association
between epidemiological characteristics of the astrocytoma patients
and the SFRP3 expression levels. When the molecular findings were
analyzed with the demographic variables it was not possible to
demonstrate that the expressions levels and locations were
associated with the analyzed age categories. However, statistically
significant differences between moderate and strong nuclear
expression levels between genders were established. Female patients
with high grade astrocytomas (grades III and IV) showed higher
moderate and strong nuclear expression levels than male patients,
and this difference was significant (moderate: F=8.422, P=0.007;
strong: F=9.262, P=0.005). Notably, no difference in cytoplasmic
expression levels was identified between genders.
The results indicated that SFRP3 expression levels
in the nucleus decreased in the higher astrocytoma grades,
indicating the expected behavior as a tumor suppressor and an
antagonist of Wnt signaling, whereas SFRP3 expression levels in the
cytoplasm were increased in the high grade astrocytomas, compared
with low grade astrocytomas. This may indicate that SFRP3 also acts
as an agonist of Wnt signaling, promoting invasive behavior.
Discussion
SFRPs are a family of soluble proteins known for
their ability to negatively modulate the Wnt signaling cascade. It
has been found that this protein family is also involved in
different types of cancer (13,18).
The present study demonstrated different expression patterns and
staining intensities of the SFRP3 protein in a sample of astrocytic
brain tumors of different pathohistological grades. The number of
cells with low or no expression was not significantly different
between the different tumor grades. However, when moderate and
strong nuclear and cytoplasmic expression levels were investigated,
the differences between expression levels and malignancy grade were
statistically significant. Grade I and II astrocytomas demonstrated
significantly higher expression values in moderate and strong
nuclear expression. The analyses on cytoplasmic expression levels
showed that moderate cytoplasmic expression was significantly
differently distributed throughout the malignancy grades, whereas
high cytoplasmic expression levels were not identified to be
statistically different. However, when the sample was divided into
two groups, one that included astrocytoma II and III and the other
that included grade IV, statistical differences were observed for
the moderate and high cytoplasmic expression levels. Furthermore,
moderate and high cytoplasmic expression levels were significantly
higher in grade IV when compared with astrocytoma II and III. Thus,
demonstrating that SFRP3 may exert different effects; in the
nucleus, SFRP3 functions as a tumor suppressor, while its
cytoplasmic expression levels indicate oncogenic properties in
higher grade astrocytomas. There are numerous papers that support
SFRPs context-dependent dual role and are consistent with the
present findings (18,19). Hirata et al (17) investigated renal cell carcinoma and
identified that the expression level of SFRP3 protein was decreased
in primary renal cancer tissue samples when compared with normal
kidney tissue samples; however, the level was restored in
metastatic renal cancer tissues. In addition, the authors suggested
that there may be a change in SFRP3 function, from that of a tumor
suppressor to an oncogene, in renal cancer progression and
metastasis. The present results are consistent with these findings,
thus, it is hypothesized that the function of SFRP3 may alter
during astrocytoma progression and the observed increase of
cellular SFRP3 expression in glioblastoma in the current study may
induce aggressive behavior and invasion. Additional studies
regarding the dual role of SFRP in breast cancer demonstrate that
SFRPs were highly overexpressed and associated with tumor
progression (18,25). In addition, endometrial cancer
studies observed a dual role of SFRPs in Wnt signaling, with the
majority supporting the notion that SFRPs may inhibit Wnt
signaling. However, there are studies that have shown SFRP4
expression to be positively correlated with cancer malignancy
(26). In ovarian cancer, SFRP4
expression tends to be downregulated, however, there are studies
regarding the high expression of this protein in cancer tissue
samples (27). Huang et al
(28) demonstrated that there was
an association between SFRP4, and risk of rectal cancer and
early-stage colorectal cancer. In colorectal cancer patients, SFRP4
expression was significantly increased in the cancerous tissue
samples when compared with the non-cancerous colorectal mucosa. The
SFRP4 protein was upregulated in 45% of colorectal cancer tissue
samples when compared with the matched non-cancerous tissue
samples.
Astrocytomas are the most common type of brain tumor
in humans, and glioblastoma are particularly proliferative and
their invasive nature is correlated with particularly poor clinical
outcomes (29,30). In the present study SFRP3
expression was demonstrated to vary among different astrocytic
malignancy grades. As pilocytic astrocytomas are considered to be
clinically, biologically and histologically distinct from WHO grade
II-IV gliomas, they may be regarded as a benign reference. However,
according to the WHO classification and cellular characteristics
pilocytic astrocytomas are described as low grade astrocytic tumors
(grade I). Diffuse are also low grade (grade II), while anaplastic
astrocytoma (grade III) and glioblastoma multiforme (grade IV) are
classified as high grade astrocytomas. This classification was
useful, as the SFRP3 protein showed marked differences between the
low and high grade tumors. When the sample was split into low and
high grade tumor groups, statistical differences for moderate and
high nuclear expression levels were observed. High grade tumors
exhibited lower values of moderate and strong nuclear expression
when compared with low grade tumors. Cytoplasmic staining of high
and low grade tumor groups showed that moderate cytoplasmic
expression levels were significantly higher in the astrocytoma III
and IV group when compared with the astrocytoma I and II group.
Furthermore, strong cytoplasmic expression was identified to be
significantly higher in the high grade tumors compared with the low
grade tumors. Thus, in contrast to the normal antagonistic role, it
was demonstrated that high cytoplasmic expression levels may act as
a Wnt signaling activator and induce tumor invasion.
The function of SFRPs in tumor development also
indicated that SFRPs do not always act as Wnt antagonists. Tissue
culture experiments demonstrated that, at low concentrations, SFRP1
potentiates Wnt activity rather than inhibits it (31,32).
In combination with other observations, this finding has led to the
suggestion that SFRP1 has low- and high-affinity binding sites for
Wingless and Wnt ligands; binding to the high-affinity site
promotes Wnt signaling, whereas binding to the low-affinity site
inhibits it (16,31). Gradient formation of expressed
proteins must also be considered. Sun et al (23) identified that SFRP3 is expressed at
a gradient (i.e. at different levels) along the septo-temporal axis
of the dentate gyrus, which is established during postnatal
development. Therefore, gradients of SFRP3 expression may
contribute in various ways to cancer initiation and progression. It
remains unclear whether SFRPs antagonize Wnt signaling by
interacting with Wnt ligands via their N-terminal CRD or the
C-terminal domain (31,33). The conflicting data may result from
differential affinities among SFRPs and their Wnt partners, or the
use of different ligands. Thus, SFRPs may block Wnt signaling
either by interacting with Wnt proteins, to prevent them from
binding to Fz proteins, or by forming non-functional complexes with
Fz (16,34).
The findings of the present study regarding
membranous localization of SFRP3 and its significant prevalence in
high grade tumors is consistent with the findings of high
cytoplasmic expression levels in high grade tumors (17–19,25,26,28).
Therefore, it is proposed that events occurring at the cell surface
are also important and may influence tumorigenesis and downstream
cellular signaling. The observations from the current study of
SFRP3 protein expression in the cellular membrane do not elucidate
whether the SFRPs bind to Wnt or the receptor, however, do indicate
localization that could make it a possibility. Statistical
significance in moderate and strong nuclear expression levels
between the genders was demonstrated. Female patients with high
grade astrocytomas (grades III and IV) demonstrated higher nuclear
expression levels than male patients.
Our previous study found β-catenin to be upregulated
and transferred to the nucleus in astrocytic brain tumors (35). It was also demonstrated that the
transcription factors of the Wnt signaling pathway were upregulated
(7). Strong TCF1 and LEF1
expression levels were observed in 51.6 and 71.0% of glioblastomas,
respectively. Astrocytoma grade I showed almost opposite expression
levels, with weak or no expression of TCF1 and LEF1 in 63.2 and
68.2%, respectively. Additionally, statistical analysis confirmed
significant differences in protein expression levels and indicated
that LEF1 may serve as a potential diagnostic marker distinguishing
glioblastomas from astrocytomas (7). Wnt/β-catenin signaling is essential
for tumorigenesis, however its molecular mechanisms are not fully
understood. Zhang et al (36) demonstrated upregulation of
β-catenin in gliomas. Subsequently, it was discovered that Forkhead
Box m1 (Foxm1)/β-catenin interaction is required for glioma
formation, and represents a mechanism for canonical Wnt signaling
during tumorigenesis. The findings by Zhang et al (36) elucidate a mechanism for β-catenin
nuclear translocation via binding to the transcription factor,
Foxm1. Furthermore, this interaction is maintained in the nucleus,
where the two proteins form a complex with TCF transcription
factors on the promoters of Wnt/β-catenin target genes. There are
increasing studies investigating the role of the Wnt signaling
pathway in human astrocytomas, however very few have investigated
its role in progression. To the best of our knowledge, the role of
SFRP3 has not yet been investigated in astrocytomas. Kahlert et
al (37) demonstrated that Wnt
signaling enhances motility of glioblastoma cells in vitro
by activating molecules that promote the mesenchymal phenotype. In
addition, the authors identified that the distribution of the
nuclear β-catenin signal was predominantly within the invasive
front of the tumor, which indicates that Wnt signaling is important
in the regulation of malignant cell motility.
Studies regarding SFRP1 (4) have demonstrated a novel molecular
miR-328-dependent mechanism, which via SFRP1 inhibition and Wnt
activation contributes to the infiltrative glioma phenotype at
early stages of glioma progression. The authors showed that a low
SFRP1 expression level is a negative prognostic factor in gliomas.
Roth et al (20)
investigated SFRP1 and SFRP2 and demonstrated that the two proteins
are produced by the majority of malignant glioma cell lines. It was
found that SFRP2 promotes glioma cell growth in vivo and
that these SFRPs were important modulators of the pathophysiology
of malignant brain tumors.
The present results regarding SFRP3 expression
levels and localizations provide novel insights into Wnt signaling
changes in astrocytic brain tumors. Although it is easier to assign
a single dimension to a certain protein, it appears that the
majority of molecules possess numerous dimensions, and the current
findings on SFRP3 support this hypothesis. Although SFRP3, also
termed FrzB, has been associated with the inhibition of Wnt
signaling, there are numerous congruous reports indicating that it
activates Wnt signaling during progression and metastasis. Specific
spatio-temporal dynamic expression of SFRP3, similar to a
developmental gradient (22), is
occurring in astrocytoma progression and glioblastoma development.
Two distinct mechanisms participate in the loss of SFRP expression
in cancer: Allelic loss and epigenetic silencing (14). It has been shown that the majority
of SFRPs are epigenetically silenced via promoter hypermethylation
in numerous types of cancer (38,39).
Although SFRP3 possesses no CpG islands in the promoter region,
there have been no reports regarding the association between SFRP3
expression and epigenetic silencing; thus, it appears that its
downregulation is achieved by another molecular mechanism.
Astrocytic brain tumors and, in particular,
glioblastoma demonstrate great heterogeneity that has recently been
explained by a sub-population of glioblastoma cancer stem cells,
which are the predominant tumorigenic force (3). The Wnt signaling pathway is known to
critically regulate self-renewal and differentiation of neural
stem/progenitor cells (3,40,41).
Rheinbay et al (3)
demonstrated that achaete-scute family bHLH transcription factor 1,
a transcription factor essential for maintenance and in vivo
tumorigenicity of glioblastoma cancer stem cells, activates Wnt
signaling and that it is linked to the activation of LEF1. Rampazzo
et al (2) described that
Wnt activation promotes a marked differentiation of glioblastoma
cancer stem cells towards a less aggressive phenotype.
In conclusion, the present results indicate that the
decreasing SFRP3 expression level in the nucleus is positively
correlated with increasing astrocytoma grade; whereas the increase
in SFRP3 protein expression in the cytoplasm of higher grade
astrocytomas demonstrates the dual nature of SFRP3. In certain
cases, SFRP3 acts as an antagonist, while in other cases it serves
as an agonist of the Wnt signaling pathway. The findings suggest
that molecular changes in Wnt signaling are important in astrocytic
tumor etiology. The novel molecular features may provide resources
for future investigations regarding the pathogenesis mechanisms and
tumor biology, and may facilitate with developing effective
therapeutic strategies against this lethal type of cancer. SFRP3
may be adopted as a potential tool for combating Wnt driven
tumorigenesis.
Acknowledgments
The present study was supported by a grant from the
Croatian Science Foundation (grant no. 6625).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rampazzo E, Persano L, Pistollato F, Moro
E, Frasson C, Porazzi P, Della Puppa A, Bresolin S, Battilana G,
Indraccolo S, et al: Wnt activation promotes neuronal
differentiation of glioblastoma. Cell Death Dis. 4:e5002013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rheinbay E, Suvè ML, Gillespie SM,
Wakimoto H, Patel AP, Shahid M, Oksuz O, Rabkin D, Martuza RL,
Rivera MN, et al: An aberrant transcription factor network
essential for Wnt signaling and stem cell maintenance in
glioblastoma. Cell Rep. 3:1567–1579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delic S, Lottmann N, Stelzl A, Liesenberg
F, Wolter M, Götze S, Zapatka M, Shiio Y, Sabel MC, Felsberg J, et
al: MiR-328 promotes glioma cell invasion via SFRP1-dependent
Wnt-signaling activation. Neuro Oncol. 16:179–190. 2014. View Article : Google Scholar :
|
|
5
|
Nikuševa-Martić T, Beroš V, Pećina-Šlaus
N, Pećina HI and Bulić-Jakuš F: Genetic changes of CDH1, APC, and
CTNNB1 found in human brain tumors. Pathol Res Pract. 203:779–787.
2007. View Article : Google Scholar
|
|
6
|
Pećina-Šlaus N, Martić TN, Kokotović T,
Kusec V, Tomas D and Hrasćan R: AXIN-1 protein expression and
localization in glioblastoma. Coll Antropol. 35(Suppl 1): 101–106.
2011.
|
|
7
|
Pećina-Šlaus N, Kafka A, Tomas D, Marković
L, Okštajner PK, Sukser V and Krušlin B: Wnt signaling
transcription factors TCF-1 and LEF-1 are upregulated in malignant
astrocytic brain tumors. Histol Histopathol. 29:1557–1564.
2014.
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: Wnt signaling in cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
10
|
Fagotto F: Looking beyond the Wnt pathway
for the deep nature of β-catenin. EMBO Rep. 14:422–433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kafka A, Bašić-Kinda S and Pećina-Šlaus N:
The cellular story of dishevelleds. Croat Med J. 55:459–467. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoang B, Moos M Jr, Vukicevic S and Luyten
FP: Primary structure and tissue distribution of FRZB, a novel
protein related to Drosophila frizzled, suggest a role in skeletal
morphogenesis. J Biol Chem. 271:26131–26137. 1997.
|
|
13
|
Shi Y, He B, You L and Jablons DM: Roles
of secreted frizzled-related proteins in cancer. Acta Pharmacol
Sin. 28:1499–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bovolenta P, Esteve P, Ruiz JM, Cisneros E
and Lopez-Rios J: Beyond Wnt inhibition: New functions of secreted
Frizzled-related proteins in development and disease. J Cell Sci.
121:737–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones SE and Jomary C: Secreted
Frizzled-related proteins: Searching for relationships and
patterns. Bioessays. 24:811–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirata H, Hinoda Y, Ueno K, Majid S, Saini
S and Dahiya R: Role of secreted frizzled-related protein 3 in
human renal cell carcinoma. Cancer Res. 70:1896–1905. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surana R, Sikka S, Cai W, Shin EM, Warrier
SR, Tan HJ, Arfuso F, Fox SA, Dharmarajan AM and Kumar AP: Secreted
frizzled related proteins: Implications in cancer. Biochim Biophys
Acta. 1845:53–65. 2014.
|
|
19
|
Mii Y and Taira M: Secreted Wnt
'inhibitors' are not just inhibitors: Regulation of extracellular
Wnt by secreted Frizzled-related proteins. Dev Growth Differ.
53:911–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roth W, Wild-Bode C, Platten M, Grimmel C,
Melkonyan HS, Dichgans J and Weller M: Secreted Frizzled-related
proteins inhibit motility and promote growth of human malignant
glioma cells. Oncogene. 19:4210–4220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrison-Uy SJ and Pleasure SJ: Wnt
signaling and forebrain development. Cold Spring Harb Perspect
Biol. 4:a0080942012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Huang H, Chen Y, Liu Y, Zhang Z,
Ma Q and Qiu M: Dynamic expression of secreted Frizzled-related
protein 3 (sFRP3) in the developing mouse spinal cord and dorsal
root ganglia. Neuroscience. 248:594–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Bonaguidi MA, Jun H, Guo JU, Sun
GJ, Will B, Yang Z, Jang MH, Song H, Ming GL and Christian KM: A
septo-remporal molecular gradient of sfrp3 in the dentate gyrus
differentially regulates quiescent adult hippocampal neural stem
activation. Mol Brain. 8:522015. View Article : Google Scholar
|
|
24
|
Pazanin L: Histopatholoy of glial tumors.
Medicina fluminensis. 47:157–166. 2011.In Croatian.
|
|
25
|
Lee JL, Chang CJ, Wu SY, Sargan DR and Lin
CT: Secreted frizzled-related protein 2 (SFRP2) is highly expressed
in canine mammary gland tumors but not in normal mammary glands.
Breast Cancer Res Treat. 84:139–149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abu-Jawdeh G, Comella N, Tomita Y, Brown
LF, Tognazzi K, Sokol SY and Kocher O: Differential expression of
frpHE: A novel human stromal protein of the secreted frizzled gene
family, during the endometrial cycle and malignancy. Lab Investig.
79:439–447. 1999.PubMed/NCBI
|
|
27
|
Drake J, Shearwood AM, White J, Friis R,
Zeps N, Charles A and Dharmarajan A: Expression of secreted
frizzled-related protein 4 (SFRP4) in primary serous ovarian
tumours. Eur J Gynaecol Oncol. 30:133–141. 2009.PubMed/NCBI
|
|
28
|
Huang D, Yu B, Deng Y, Sheng W, Peng Z,
Qin W and Du X: SFRP4 was overexpressed in colorectal carcinoma. J
Cancer Res Clin Oncol. 136:395–401. 2010. View Article : Google Scholar
|
|
29
|
Paw I, Carpenter RC, Watabe K, Debinski W
and Lo HW: Mechanisms regulating glioma invasion. Cancer Lett.
362:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D
and Chen J: The challenges and the promise of molecular targeted
therapy in malignant gliomas. Neoplasia. 17:239–255. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uren A, Reichsman F, Anest V, Taylor WG,
Muraiso K, Bottaro DP, Cumberledge S and Rubin JS: Secreted
frizzled-related protein-1 binds directly to Wingless and is a
biphasic modulator of Wnt signaling. J Biol Chem. 275:4374–4382.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xaviera CP, Melikovaa M, Chumana Y, Üren
A, Baljinnyama B and Rubina JS: Secreted Frizzled-related protein
potentiation versus inhibition of Wnt3a/β-catenin signaling. Cell
Signal. 26:94–101. 2014. View Article : Google Scholar
|
|
33
|
Lin K, Wang S, Julius MA, Kitajewski J,
Moos M Jr and Luyten FP: The cysteine-rich frizzled domain of
Frzb-1 is required and sufficient for modulation of Wnt signaling.
Proc Natl Acad Sci USA. 94:11196–11200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bafico A, Gazit A, Pramila T, Finch PW,
Yaniv A and Aaronson SA: Interaction of frizzled related protein
(FRP) with Wnt ligands and the frizzled receptor suggests
alternative mechanisms for FRP inhibition of Wnt signaling. J Biol
Chem. 274:16180–16187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nikuševa Martić T, Pećina-Šlaus N, Kušec
V, Kokotović T, Mušinović H, Tomas D and Zeljko M: Changes of
AXIN-1 and beta-catenin in neuroepithelial brain tumors. Pathol
Oncol Res. 16:75–79. 2010. View Article : Google Scholar
|
|
36
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kahlert UD, Maciaczyk D, Doostkam S, Orr
BA, Simons B, Bogiel T, Reithmeier T, Prinz M, Schubert J,
Niedermann G, et al: Activation of canonical WNT/β-catenin
signaling enhances in vitro motility of glioblastoma cells by
activation of ZEB1 and other activators of
epithelial-to-mesenchymal transition. Cancer Lett. 325:42–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng YY, Yu J, Wong JP, Man EP, To KF,
Jin VX, Li J, Tao Q, Sung JJ, Chan JK and Leung WK: Frequent
epigenetic inactivation of secreted frizzled-related protein 2
(SFRP2) by promoter methylation in human gastric cancer. Br J
Cancer. 97:895–901. 2007.PubMed/NCBI
|
|
39
|
Schiefer L, Visweswaran M, Perumal V,
Arfuso F, Groth D, Newsholme P, Warrier S and Dharmarajan A:
Epigenetic regulation of the secreted frizzled-related protein
family in human glioblastoma multiforme. Cancer Gene Ther.
21:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reya T and Clevers H: Wnt signaling in
stem cells and cancer. Nature. 434:834–850. 2005. View Article : Google Scholar
|