Introduction
Macrophages, which are critical effector cells,
contribute to the innate immune response against infection.
Macrophages are considered the most efficient pathogen scavengers,
and are the main source of proinflammatory mediators and cytokines,
including nitric oxide (NO), interleukin (IL)-6 and tumor necrosis
factor (TNF)-α. Proinflammatory mediators are essential for
inducing inflammation at the site of infection and for fighting
pathogenic infections (1,2). However, the excessive production of
proinflammatory cytokines has severe consequences, including tissue
damage and septic shock (3,4).
Therefore, suppressing the synthesis or release of proinflammatory
cytokines and mediators is a potential therapeutic strategy for the
treatment of septic shock-like diseases associated with
inappropriately amplified inflammation.
Several intracellular signaling pathways, including
the mitogen-activated protein kinase (MAPK) and nuclear factor-κB
(NF-κB) pathways, are activated in lipopolysaccharide (LPS)-induced
macrophages, and regulate inflammatory actions and immune
responses. In macrophages, the MAPK signaling pathway is one of the
most extensively investigated intracellular signaling cascades
involved in the LPS-induced inflammatory response (5–8). The
MAPK pathway is comprised of at least three signaling components:
Extracellular signal-regulated kinases 1/2 (ERK 1/2); c-Jun
N-terminal kinase (JNK); and p38 MAPK, all of which have been
demonstrated to induce the release of immune-related cytotoxic
factors and proinflammatory cytokines (9–11).
Furthermore, NF-κB is important for controlling innate and adaptive
immunity, and for regulating the expression of various genes during
the inflammatory response (12).
Activated NF-κB translocates into the nucleus of the cell and
interacts with κB-binding sites in the promoter regions of target
genes, in order to regulate the transcription of proinflammatory
genes, including inducible nitric oxide synthase (iNOS), TNF-α and
IL-6 (13,14).
LPS-stimulated NO production and iNOS expression
have previously been reported to be inhibited by 16α,
17α-epoxypregnenolone-20-oxime (EPREGO) in BV2 microglial cells via
downregulation of the JNK signaling pathway (15). These results indicated that EPREGO
may be associated with neuroinflammation. EPREGO is an organic
compound derived from 16-dehydropregnenolone-3-acetate (commonly
known as 16-DPA); however, the mechanisms underlying the
anti-inflammatory activity of EPREGO in macrophages remain unclear.
The present study aimed to investigate the anti-inflammatory
effects of EPREGO on LPS-stimulated macrophages.
Materials and methods
Chemicals
LPS (from Escherichia coli serotype 0111:B4)
and inhibitors of p38 MAPK (SB203580), ERK (PD98059) and JNK
(SP600125) were all purchased from Calbiochem (San Diego, CA, USA).
EPREGO, a steroid oxime syntheses product derived from
16-dehydropregnenolone-3-acetate, was obtained as previously
reported (15).
Cell culture
The RAW264.7 macrophages, immortalized mice
macrophage cells (Shanghai BOGO Industrial Co., Ltd., Shanghai,
China), was propagated in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) and (100 U/ml penicillin-100
μg/ml streptomycin (HyClone, GE Healthcare Life Sciences).
Exponentially growing RAW264.7 cells maintained in DMEM at 37°C and
5.0% CO2 were pretreated with 10 μg/ml EPREGO,
followed by 1 μg/ml LPS for the indicated times (0, 1, 3, 6,
12 and 24 h).
Biochemical assay for the production of
NO
NO production was assessed based on the accumulation
of nitrite in the medium using a colorimetric reaction with Griess
reagent. Culture supernatants were collected and mixed with an
equal volume of Griess reagent [0.1% N-(1-naphthyl)
ethyl-enediamine dihydrochloride, 0.1% sulfanilamide and 2.5%
H3PO4]. Absorbance was measured at 540 nm
using a UV MAX kinetic microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Cell cytotoxicity analysis
RAW 264.7 cells were seeded into a 96-well dish
(1.6×106 cells/ml) and exposed to 1, 5 or 10
μg/ml EPREGO without LPS for 24 h. A solution of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) was subsequently added to each
well and the cells were incubated for 2 h at 37°C in an atmosphere
containing 5% CO2. Subsequently, the supernatant was
removed and formazan was solubilized with dimethyl sulfoxide.
Absorbance was measured at 540 nm using a UV MAX kinetic microplate
reader (Molecular Devices, LLC).
Western blotting
Protein lysates (30 μg) were extracted with
protein lysis buffers (20 mM HEPES-OH, pH 7.0; 50 mM NaCl, 10%
glycerol and 0.5% Triton X-100) and incubated with 0.5 μg/ml
leupeptin; 0.7 μg/ml pepstatin A; 0.1 mM
4-(2-aminoethyl)-benzenesulfony fluoride and 2 μg/ml
apro-tinin for 30 min at 4°C. The proteins were then denatured for
5 min and separated using 12% sodium dodecyl
sulfate-poly-acrylamide gel electrophoresis and were transferred
onto nitrocellulose membranes (EMD Millipore, Billerica, MA, USA).
The membranes were blocked with 5% skimmed milk for 30 min, at room
temperature. They were then incubated with polyclonal rabbit
anti-iNOS (cat. no. 06-573, EMD Millipore, Billerica, MA, USA),
polyclonal rabbit anti-IκB-α (cat. no. sc-371), mouse monoclonal
anti-p-JNK (cat. no. sc-6254), anti-JNK (cat. no. sc-7345), mouse
monoclonal anti-p-P38 (cat. no. sc-7973), mouse monoclonal anti-P38
(cat. no. sc-7972), mouse monoclonal anti-p-ERK (cat. no. sc-7383),
and mouse monoclonal anti-ERK (cat. no. sc-514302) (all obtained
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and rabbit
anti-glyceraldehyde 3-phosphate dehydrogenase (cat. no. PLA0125,
Sigma-Aldrich) primary antibodies (dilution, 1:5,000) at 4°C
overnight. The membranes were washed five times with Tris-buffered
saline (TBS) containing Tween [10 mM Tris-HCl (pH 7.5), 150 mM NaCl
and 0.2% Tween-20] and were subsequently incubated with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. SAB3700878,
1:10,000) or anti-mouse immunoglobulin G (cat. no. SAB3701105,
1:10,000) (Sigma-Aldrich) for 1 h at room temperature. Following
the removal of excess antibodies by washing with TBS, specific
binding was detected using a chemiluminescence detection system (GE
Healthcare Life Sciences, Chalfont, UK) according to the
manufacturer's protocol.
Cytokine assays
The concentration of TNF-α and IL-6 in the cell
culture supernatant was measured using enzyme-linked immunosorbent
assay (ELISA) kits for TNF-α and IL-6 (eBioscience, Inc., San
Diego, CA, USA). RAW264.7 cells (2.5×105 cells) were
plated into a 48-well cell culture plate and incubated with various
concentrations of EPREGO (1, 5 and 10 μg/ml) and 1
μg/ml LPS for 24 h. The culture supernatant was collected
and assayed according to the manufacturer's protocols, in order to
determine the concentration of TNF-α and IL-6 that had been
released from the cells.
Measurement of phagocytosis and ROS by
flow cytometry
Macrophage phagocytosis was analyzed with flow
cytometry, according to a previously reported method (16). Briefly, Alexa 488-conjugated E.
coli (Ec-A) BioParticles (Invit-rogen; Thermo Fisher
Scientific, Inc.) were sonicated, added to the culture medium
without serum at the final time of LPS treatment and incubated at
37°C for 15 min. Following incubation, the cells were washed with
phosphate-buffered saline (PBS) three times and were resuspended in
500 μl PBS. The internalized fluorescence was immediately
determined from 10,000 cells using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). To determine ROS levels,
RAW264.7 cells were incubated with 10 mM CM-H2DCFDA
(Invitrogen; Thermo Fisher Scientific, Inc.), a fluorescence-based
ROS indicator, at 37°C for 15 min at the final time of various
treatments, and the DCFDA fluorescence intensity from 10,000 cells
was measured using FACScan (BD Biosciences). The results were
analyzed using WinMDI (Version 2.9, BD Biosciences) software.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Differences between experimental groups were analyzed
by one-way analysis of variance and a Tukey test. GraphPad Prism
software version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA)
was used to analyze results. P<0.01 was considered to indicate a
statistically significant difference.
Results
EPREGO inhibits the production of
proinflammatory cytokines in LPS-treated RAW264.7 cells
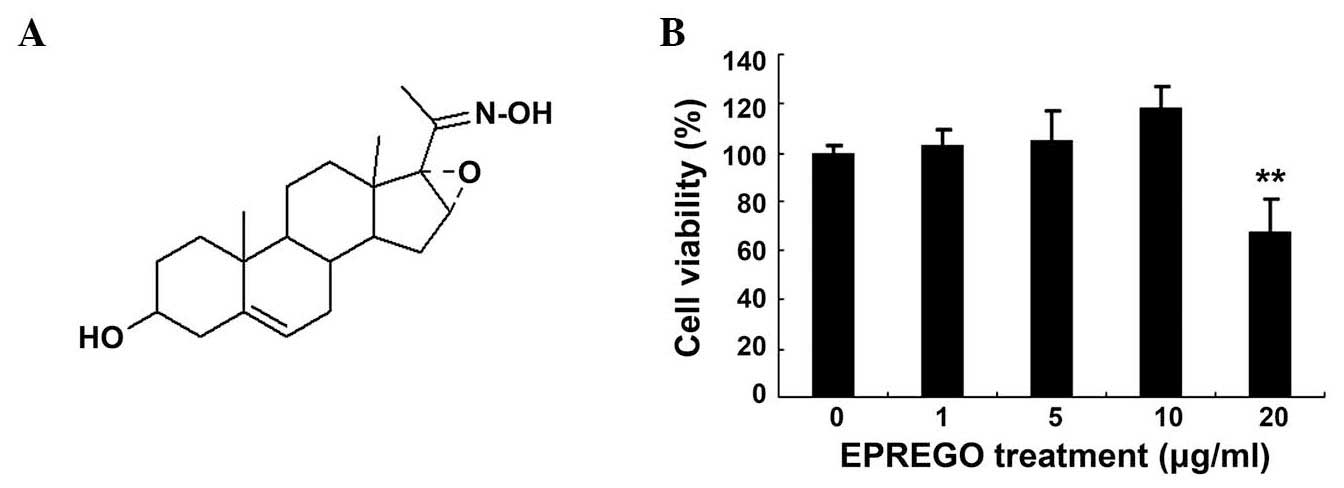
The chemical structure of EPREGO is presented in
Fig. 1A. EPREGO is a steroid oxime
that has previously been reported to exert an inhibitory effect on
LPS-stimulated NO production in BV2 microglia cells (15). To examine the effects of EPREGO on
macrophage cell viability, RAW264.7 cells were pretreated with
various concentrations of EPREGO for 24 h, then stimulated with LPS
for 12 h. Cell viability was detected using an MTT assay. As
demonstrated in Fig. 1B, cell
viability was not affected up to a concentration of 10
μg/ml, whereas, a higher concentration of EPREGO (20
μg/ml) significantly reduced the viability of RAW264.7 cells
compared with untreated cells (P=0.008). Therefore, in subsequent
studies 10 μg/ml was used as the highest treatment
concentration. To evaluate whether EPREGO has anti-inflammatory
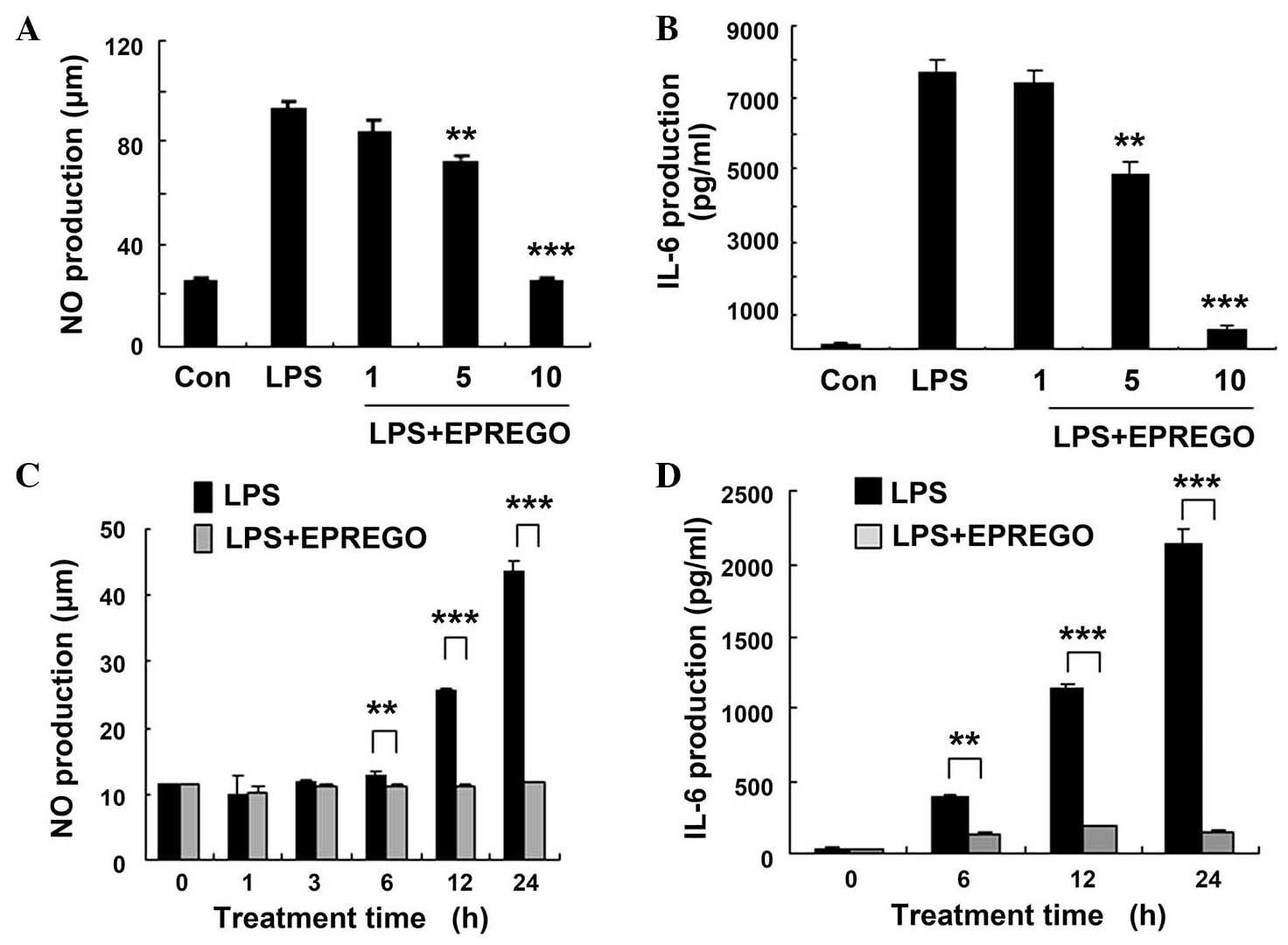
properties, the NO, IL-6 and TNF-α levels in RAW264.7 cell culture
supernatants were examined by ELISA following stimulation with LPS.
Compared with LPS-stimulated cells, treatment with EPREGO dose- and
time-dependently decreased the production of NO (P=0.009, P=0.0003
and P=0.0006 for 6, 12 and 24 h, respectively) and IL-6 (P=0.009,
P=0.0003 and P=0.0006 for 6, 12 and 24 h, respectively) in
LPS-treated RAW264.7 cells (Fig.
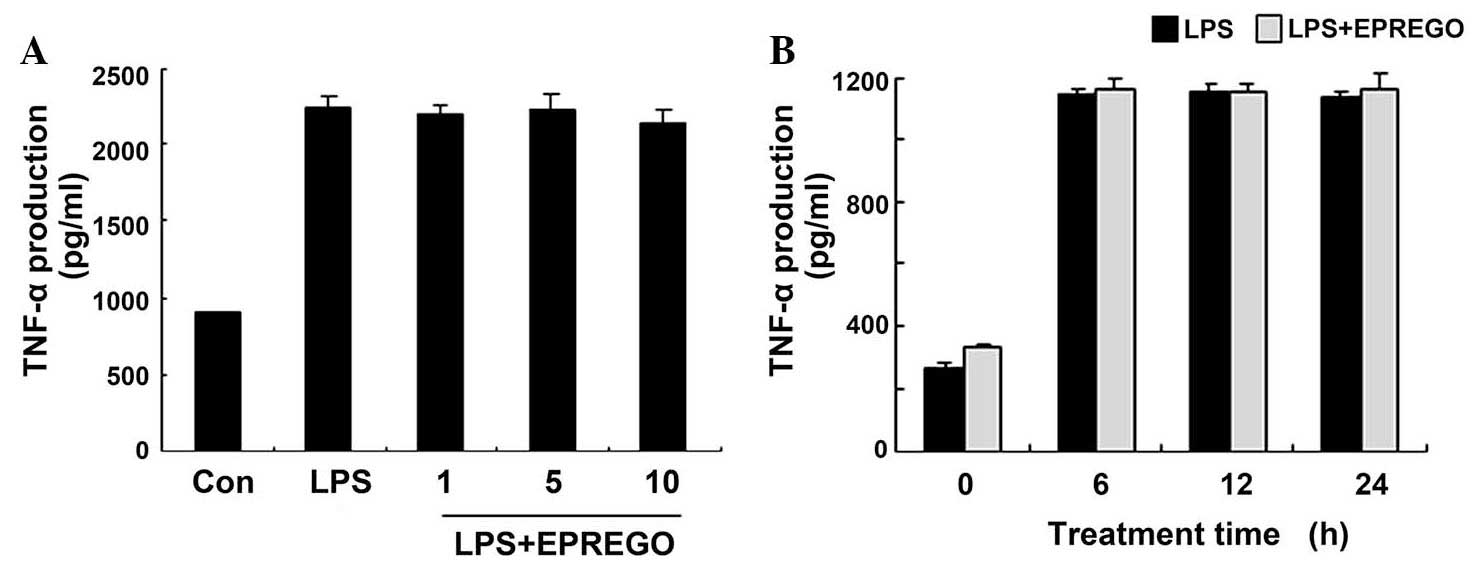
2), but did not markedly alter TNF-α secretion (Fig. 3). Furthermore, the results of
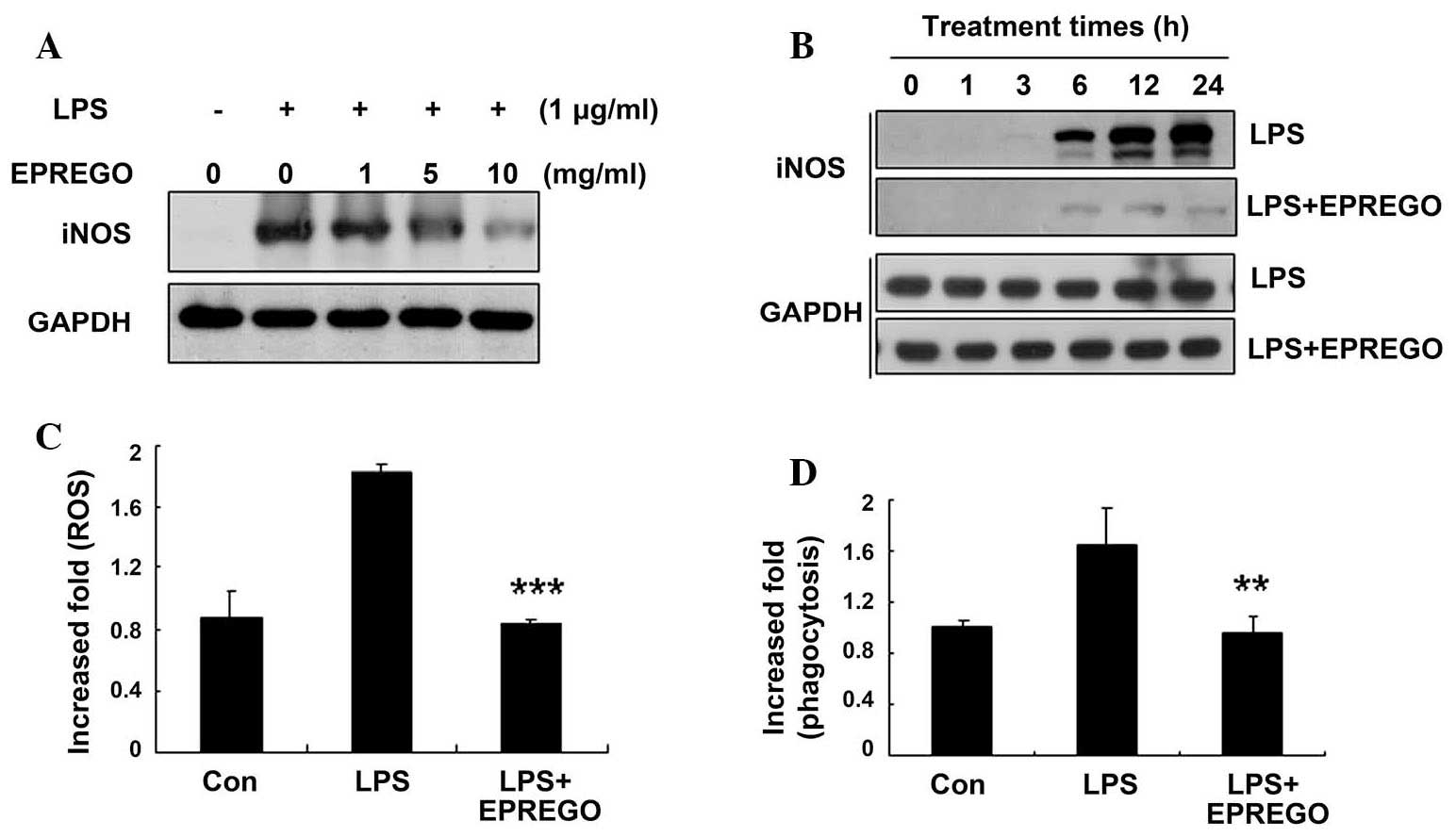
western blotting experiments demonstrated that, compared with LPS
stimulation, EPREGO inhibited iNOS (an enzyme involved in NO
production) protein expression in a dose- and time-dependent manner
(Fig. 4A and B), resulting in
decreased NO production.
EPREGO decreases cellular ROS levels and
macrophage phagocytosis in LPS-stimulated RAW264.7 cells
To investigate whether EPREGO affects the ROS levels
and phagocytic capacity of LPS-stimulated macrophages, RAW264.7
cells were pretreated with EPREGO (10 μg/ml) for 30 min,
followed by treatment with LPS (1 μg/ml) for 24 h. Cellular
ROS levels and phagocytosis were examined using flow cytometry. LPS
increased cellular ROS levels, which were significantly decreased
by treatment with EPREGO, almost to basal levels compared with
LPS-treated cells (Fig. 4C,
P=0.00011). Similarly, treatment with EPREGO significantly
decreased phagocytosis compared with LPS-stimulated RAW264.7 cells
(Fig. 4D, P=0.008). Together these
results (Figs. 2Figure 3–4) demonstrate that EPREGO may exert
anti-inflammatory effects in RAW264.7 cells.
EPREGO exhibits anti-inflammatory effects
by inhibiting MAPK signaling pathways
The MAPK and NF-κB signaling pathways are crucial
mediators of proinflammatory cytokine production in macrophages
(9–12). To determine the mechanism
underlying the anti-inflammatory activities of EPREGO in RAW264.7
cells, the MAPK pathways were investigated by detecting the
phosphorylation levels of ERK, JNK and p38. RAW264.7 cells were
pretreated with EPREGO (10 μg/ml) for 30 min, followed by
treatment with LPS (1 μg/ml) for the indicated times, and
MAPK phosphorylation was subsequently examined using western
blotting. The results demonstrated that treatment with LPS
upregulated ERK, p38 and JNK phosphorylation compared with in the
untreated cells, whereas phosphorylation was markedly downregulated
by EPREGO treatment compared with LPS-treated cells (Fig. 5A–C). Furthermore, the inhibitory
effect of EPREGO on the NF-κB signaling pathway was investigated by
examining IκBα degradation. IκBα degradation in RAW264.7 cells was
markedly inhibited by EPREGO treatment compared with LPS-stimulated
cells (Fig. 5D). To determine
whether the MAPK signaling pathways were associated with the
anti-inflammatory effects of EPREGO on LPS-stimulated macrophage
inflammation, the effects of pharmaceutical MAPK inhibitors were
assessed. The results demonstrated that treatment with SB203580
(p38 inhibitor), PD98059 (ERK inhibitor) and SP600125 (JNK
inhibitor), as well as EPREGO, significantly decreased NO (P=0.008,
P=0.0003, P=0.004 and P=0.0002 for SB203580, PD98059, SP600125 and
EPREGO, respectively) and IL-6 (P=0.0003, P=0.0007, P=0.0002 and
P=0.0003 for SB203580, PD98059, SP600125 and EPREGO, respectively)
production in RAW264.7 cells compared with LPS treatment (Fig. 6A and B), whereas TNF-α secretion
was not markedly altered (Fig.
6C). These results indicate that MAPK signaling pathways are
associated with the inhibitory effects of EPREGO on NO and IL-6
production in LPS-treated RAW264.7 cells.
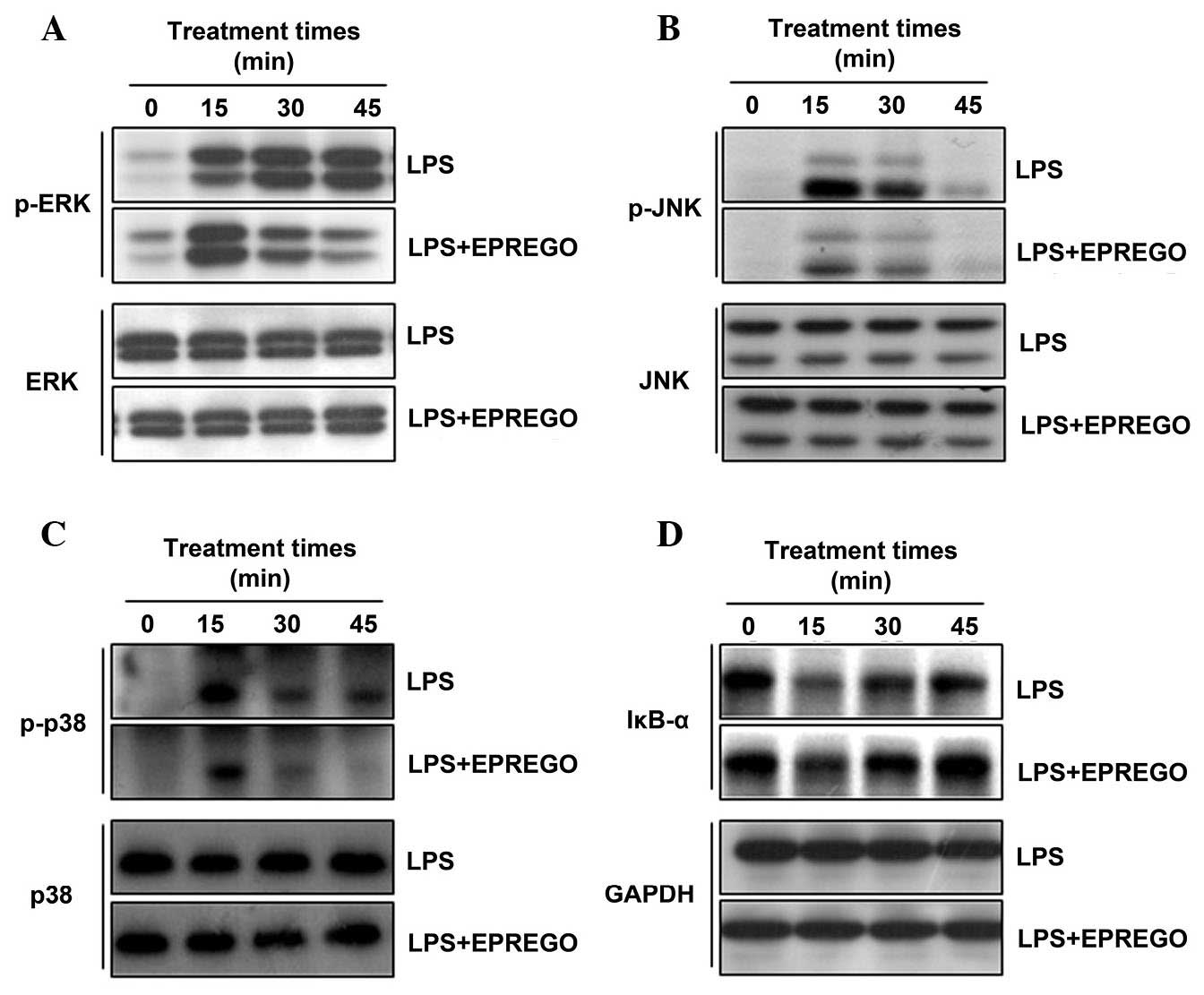 | Figure 5Effects of EPREGO on LPS-stimulated
mitogen-activated protein kinase phosphorylation and IκBα
degradation. RAW264.7 cells were pretreated with EPREGO (10
μg/ml) for 30 min, followed by treatment with LPS (1
μg/ml) for the indicated durations. The phosphorylation
levels of (A) ERK, (B) JNK and (C) p38, and (D) IκBα degradation
were detected using western blotting. The experiments were
conducted on three different samples. LPS, lipopolysaccharide; p,
phosphorylated; ERK, extracellular signal-regulated kinase; EPREGO,
16α, 17α-epoxypregnenolone-20-oxime; IκBα, inhibitor of κB α; JNK,
c-Jun N-terminal kinase; IκBα, inhibitor of κB α; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
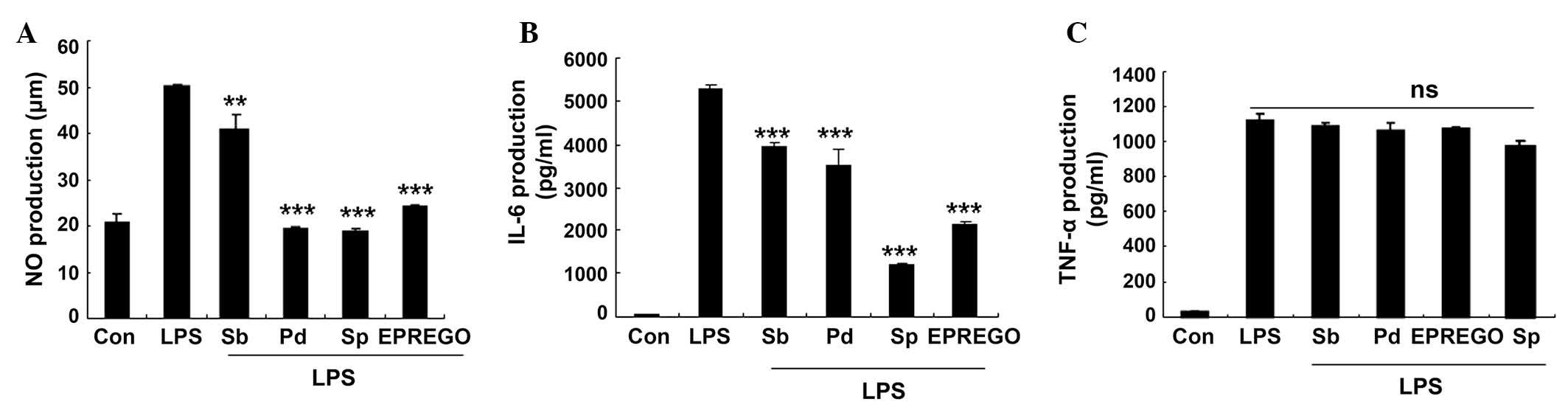 | Figure 6Mitogen-acitivated protein kinase
signaling pathway inhibitors reduced LPS-stimulated NO and IL-6
secretion. RAW264.7 cells were pretreated with SB203580 (p38
inhibitor), PD98059 (extracellular signal-regulated kinase
inhibitor) and SP600125 (c-Jun N-terminal kinase inhibitor) for 30
min followed by LPS (1 μg/ml) for 24 h. The levels of (A)
NO, (B) IL-6 and (C) TNF-α were measured using Griess reagents and
enzyme-linked immunosorbent assays. Data are presented as the mean
± standard error of the mean of three different samples.
**P<0.01 and ***P<0.001 vs.
LPS-stimulated cells. NO, nitric oxide; LPS, lipopolysaccharide;
Sb, SB203580; Pd, PD98059; Sp, SP600125; EPREGO, 16α,
17α-epoxypregnenolone-20-oxime; IL-6, interleukin-6; TNF-α; tumor
necrosis factor-α; ns, not significant. |
Discussion
Inflammation is the host response to infection and
injury; however, if uncontrolled, inflammatory mediators become
involved in the pathogenesis of various inflammatory disorders
(17). Proinflammatory cytokines
that are typically released by macrophages are critical for
initiating and sustaining the inflammatory response.
Following processing,
16-dehydropregnenolone-3-acetate, a major intermediary for hormone-
and steroid-associated drugs (18,19),
exhibits anti-inflammatory, anti-toxin, anti-shock and
anti-allergenic effects. EPREGO, which is derived from
16-dehydropregnenolone-3-acetate, was previously reported to
inhibit NO production in LPS-stimulated BV2 microglia cells
(15); however, to the best of our
knowledge, its potential anti-inflammatory properties have not been
investigated in macrophages. The present study investigated the
effects of EPREGO on proinflammatory cytokine production in
LPS-stimulated RAW264.7 macrophages. Treatment with EPREGO
significantly decreased LPS-stimulated NO and IL-6 production
(Fig. 2), and iNOS protein
expression, but did not alter TNF-α production (Fig. 3) in RAW264.7 cells. Furthermore,
EPREGO reduced the LPS-induced cellular ROS levels and phagocytosis
of RAW264.7 cells (Fig. 4). These
observations are consistent with previous reports (15), indicating that EPREGO exerts
anti-inflammatory activity in RAW264.7 cells.
Mechanistic analysis within the present study
demonstrated that EPREGO may significantly downregulate
LPS-stimulated phosphorylation of ERK, JNK and p38 (Fig. 5A–C), thus indicating that the
inhibitory activity of EPREGO on the production of NO and IL-6 in
RAW264.7 cells is associated with the MAPK signaling pathway. Upon
LPS recognition of complex proteins, including LPS binding protein,
cluster of differentiation 14, lymphocyte antigen 96 and Toll-like
receptor (TLR) 4, a series of TLR-mediated signal pathways activate
downstream IκB kinase (IKK) and MAPK pathways (20). The NF-κB and MAPK pathways are the
major intracellular signaling pathways activated by LPS binding to
TLR4 on the cell membrane (5,21).
MAPKs are a family of serine/threonine protein kinases responsible
for most cellular responses to cytokines, which are crucial for
regulating the production of inflammatory mediators (22–25).
As previously reported, EPREGO inhibits NO production and iNOS
expression by selectively downregulating JNK phosphorylation
(15). The present study
demonstrated that treatment with EPREGO inhibits the
phosphorylation of JNK, ERK and p38 in RAW264.7 cells (Fig. 5A–C). The differential effects of
EPREGO on MAPK (ERK, JNK and p38) signaling pathways between
microglia and macrophages may be due to the different
characteristics of the two cell types, and the mechanism for this
regulation should be investigated further. In addition, treatment
of RAW264.7 cells with pharmaceutical inhibitors for ERK (PD98059),
JNK (SP600125) and p38 (SB203580) inhibited the production of NO
and IL-6 (Fig. 6A and B). These
results suggested that EPREGO may alter the MAPK signaling cascade,
resulting in decreased NO and IL-6 production.
NF-κB is a major transcription factor that regulates
the expression of genes responsible for the innate and adaptive
immune responses. The inappropriate regulation of NF-κB has been
associated with cancer, and inflammatory and autoimmune diseases.
NF-κB has been demonstrated to be important for the inflammatory
response and the expression of inflammatory cytokines, including
NO, TNF-α and IL-6. NF-κB activation is induced by the dissociation
of IκBα/β, which are phosphorylated by IKK. The level of IκBα and
IκBβ degradation is commonly used as an indicator of NF-κB
activation (26). In the present
study, EPREGO was demonstrated to inhibit IκBα degradation in
LPS-stimulated RAW264.7 cells (Fig.
4D), which indicated that EPREGO potentially suppresses the
NF-κB signaling pathway, thus, reducing inflammatory
response-mediated NO and IL-6 production. These results are
consistent with previous reports demonstrating that the NF-κB
signaling pathway is essential for LPS-stimulated cytokine
production (27–30) in macrophages.
In the present study, although EPREGO inhibited IκBα
degradation and the phosphorylation of MAPKs (Fig. 5), it had no effect on TNF-α
production (Fig. 3), thus
suggesting that LPS-induced TNF-α secretion is influenced by other
signaling pathways. Indeed, it was previously reported that the
Janus kinase (JAK)/signal transducer and activator of transcription
(STAT) signaling pathway is involved in proinflammatory cytokine
and ROS production (31–33); therefore, it is hypothesized that
TNF-α production may be regulated by the JAK/STAT signaling
pathway, and an analysis of the inhibitory effect of EPREGO on
LPS-stimulated JAK/STAT signaling is currently being
investigated.
In conclusion, the results of the present study
indicated that EPREGO attenuates the production of NO and IL-6 in
LPS-stimulated RAW264.7 cells, and decreases cellular ROS levels
and phagocytosis by inhibiting the MAPKs and NF-κB signaling
pathways. The results suggest that EPREGO exhibits an
anti-inflammatory role in macrophage cells, and may serve as a
therapeutic agent for macrophage-mediated inflammation.
Acknowledgments
The present study was supported by the Scientific
Research Foundation of the Heilongjiang Provincial Education
Department of China (no. 1251H010) and the Ministry of Education
Scientific Research Foundation for Returned Overseas Students
(46th).
Abbreviations:
|
LPS
|
lipopolysaccharide
|
|
NO
|
nitric oxide
|
|
iNOS
|
inducible nitric oxide synthase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin-6
|
|
NADPH
|
nicotinamide adenine dinucleotide
phosphate
|
|
EPREGO
|
16α,
17α-epoxypregnenolone-20-oxime
|
References
|
1
|
Underhill DM and Ozinsky A: Phagocytosis
of microbes: Complexity in action. Annu Rev Immunol. 20:825–852.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li W, Ashok M, Li J, Yang H, Sama AE and
Wang H: A major ingredient of green tea rescues mice from lethal
sepsis partly by inhibiting HMGB1. PLoS One. 2:e11532007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ehlting C, Ronkina N, Böhmer O, Albrecht
U, Bode KA, Lang KS, Kotlyarov A, Radzioch D, Gaestel M, Häussinger
D and Bode JG: Distinct functions of the mitogen-activated protein
kinase-activated protein (MAPKAP) kinases MK2 and MK3: MK2 mediates
lipopolysaccharide-induced signal transducers and activators of
transcription 3 (STAT3) activation by preventing negative
regulatory effects of MK3. J Biol Chem. 286:24113–24124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011. View Article : Google Scholar
|
|
8
|
Shin JS, Noh YS, Lee YS, Cho YW, Baek NI,
Choi MS, Jeong TS, Kang E, Chung HG and Lee KT: Arvelexin from
Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene
expression by inhibiting activation of IκB kinase. Br J Pharmacol.
164:145–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JH, Kim DH, Baek SH, Lee HJ, Kim MR,
Kwon HJ and Lee CH: Rengyolone inhibits inducible nitric oxide
synthase expression and nitric oxide production by down-regulation
of NF-kappaB and p38 MAP kinase activity in LPS-stimulated RAW
264.7 cells. Biochem Pharmacol. 71:1198–1205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Mutairi MS, Cadalbert LC, McGachy HA,
Shweash M, Schroeder J, Kurnik M, Sloss CM, Bryant CE, Alexander J
and Plevin R: MAP kinase phosphatase-2 plays a critical role in
response to infection by Leishmania mexicana. PLoS Pathog.
6:e10011922010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu HE, Chang AS, Teng CM, Chen CC, Tsai
AC and Yang CR: Potent anti-inflammatory effects of denbinobin
mediated by dual inhibition of expression of inducible no synthase
and cyclooxygenase 2. Shock. 35:191–197. 2011. View Article : Google Scholar
|
|
12
|
Liou HC: Regulation of the immune system
by NF-kappaB and IkappaB. J Biochem Mol Biol. 35:537–546. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karin M and Delhase M: The I kappaB kinase
(IKK) and NF-kappa B: Key elements of proinflammatory signalling.
Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baeuerle PA and Baltimore D: NF-kappaB:
Ten years after. Cell. 87:13–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun HN, Jin MH, Han B, Feng L, Han YH,
Shen GN, Yu YZ, Jin CH, Lian ZX, Lee DS, et al: 16α,
17α-Epoxypregnenolone-20-oxime prevent LPS-induced NO production
and iNOS expression in BV-2 microglial cells by inhibiting JNK
phosphorylation. Biol Pharm Bull. 37:1096–1102. 2014. View Article : Google Scholar
|
|
16
|
Liu Y, Hao W, Letiembre M, Walter S,
Kulanga M, Neumann H and Fassbender K: Suppression of microglial
inflammatory activity by myelin phagocytosis: Role of
p47-PHOX-mediated generation of reactive oxygen species. J
Neurosci. 26:12904–12913. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha- and RANKL-mediated
osteoclastogenesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chowdhury P, Das AM and Goswami P:
Synthesis of some new steroidal [16alpha, 17alpha-d]-isoxazolines.
Steroids. 70:494–498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banerjee T, Shukla A, Shinde K and Patil
S: Mixed culture bioconversion of 16-dehydropregnenolone acetate to
androsta-1,4-diene-3,17-dione: Optimization of parameters.
Biotechnol Prog. 19:662–664. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pålsson-McDermott EM and O'Neill LA:
Signal transduction by the lipopolysaccharide receptor, Toll-like
receptor-4. Immunology. 113:153–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao W, Bao C, Padalko E and Lowenstein CJ:
Acetylation of mitogen-activated protein kinase phosphatase-1
inhibits Toll-like receptor signaling. J Exp Med. 205:1491–1503.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH
and Kang JJ: Shikonin derivatives inhibited LPS-induced NOS in RAW
264.7 cells via downregulation of MAPK/NF-kappaB signaling. J
Ethnopharmacol. 120:264–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie C, Kang J, Ferguson ME, Nagarajan S,
Badger TM and Wu X: Blueberries reduce pro-inflammatory cytokine
TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB
activation and the MAPK pathway. Mol Nutr Food Res. 55:1587–1591.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu X, Ci X, He J, Wei M, Yang X, Cao Q,
Li H, Guan S, Deng Y, Pang D and Deng X: A novel anti-inflammatory
role for ginkgolide B in asthma via inhibition of the ERK/MAPK
signaling pathway. Molecules. 16:7634–7648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HG, Kim NR, Gim MG, Lee JM, Lee SY, Ko
MY, Kim JY, Han SH and Chung DK: Lipoteichoic acid isolated from
Lactobacillus plantarum inhibits lipopolysaccharide-induced
TNF-alpha production in THP-1 cells and endotoxin shock in mice. J
Immunol. 180:2553–2561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin S, Park JY, Hong JM, Kim TH, Shin HI,
Park EK and Kim SY: Inhibitory effect of (−)-epigallocatechin
gallate on titanium particle-induced TNF-α release and in vivo
osteolysis. Exp Mol Med. 43:411–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Zhong X, He Z, Wen M, Li J, Peng X,
Liu G, Deng J, Zhang J and Bai J: Effect of erythromycin on
cigarette-induced histone deacetylase protein expression and
nuclear factor-κB activity in human macrophages in vitro. Int
Immunopharmacol. 12:643–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YQ, Zhang ZX, Xu YJ, Ni W, Chen SX,
Yang Z and Ma D: N-Acetyl-L-cysteine and pyrrolidine
dithiocarbamate inhibited nuclear factor-kappaB activation in
alveolar macrophages by different mechanisms. Acta Pharmacol Sin.
27:339–346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korobowicz A: Biology of tumor necrosis
factor type alpha (TNF-alpha). Pol Merkur Lekarski. 21:358–361.
2006.In Polish.
|
|
31
|
Shuai K and Liu B: Regulation of JAK-STAT
signalling in the immune system. Nat Rev Immunol. 3:900–911. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohmori Y and Hamilton TA: Requirement for
STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol.
69:598–604. 2001.PubMed/NCBI
|
|
33
|
Simon AR, Rai U, Fanburg BL and Cochran
BH: Activation of the JAK-STAT pathway by reactive oxygen species.
Am J Physiol. 275:C1640–C1652. 1998.PubMed/NCBI
|