Introduction
Soft drinks consumption (SDC) has increased
worldwide in the past two-three decades (1). Their effects on health are unclear,
although epidemiological studies pointed toward its associations
with obesity, kidney, liver diseases and osteoporosis (2,3).
Soft drinks contain predominantly water, phosphoric acid, caffeine,
sugar in the form of sucrose and other chemicals in the form of
preservatives, colorings and flavors (2). The rate of SDC is alarming,
particularly in the affluent countries (4). Numerous various health problems are
associated with regular consumption of soft drinks (2). It has been shown that ~25 separate
harmful effects are associated with SDC (2,3).
Contents of soft drinks have a bad effect on human health (4). Caffeine in carbonated beverages is
deliberately added to make individuals addicted, and is readily
absorbed rapidly compared with any other drink (5).
A correlation exists between SDC and the incidence
of multiple diseases, including obesity, dental/bone problems,
diabetes mellitus and cardiovascular disease (6–9).
Additionally, SDC is notbaly associated with kidney health and a
high risk of kidney stone formation (10,11).
SDC causes bone fracture, disruption in bone formation,
affects serum or urinary calcium metabolism markers and
hypocalcemia, both in clinical and experimental settings (4,12–16).
However, no significant association between SDC and the reduction
of bone mineral density were reported in previous studies (17,18).
Therefore, the effect of SDC on bone, liver and renal health
remains unknown and proper evidence is still required. The liver is
the predominant organ in the human body that serves an essential
role in the metabolism of food and drugs, and any alteration in its
function induces deleterious effects. Additionally, the kidney is
an organ whose functions in either the removal of metabolic waste
products from the blood and water, or the regulation of
electrolytes. The majority of published papers focused on
biochemical alterations induced by soft drinks on the serum levels
of hepatic and renal biomarkers.
In Saudi Arabia and the Middle East, individuals are
used to drinking soft drinks on average 3 times per day with meals.
Therefore, the present study aimed to examine the effect of chronic
SDC (Coca-cola, Pepsi and 7-up) on oxidative stress biomarkers,
histopathology of bone, liver and kidney and the expression of
certain genes associated with liver and kidney functions, in order
to outline its effect on the health of Wistar rats.
Materials and methods
Chemicals and kits
Ethidium bromide for agarose preparation and agarose
were purchased from Sigma-Aldrich (St. Louis, MO, USA). A total of
40 male Wistar rats were purchased from King Abdel-Aziz University,
King Fahd Center for Scientific Research (Jeddah, Saudi Arabia).
Catalase, malondialdehyde (MDA), glutathione peroxidase (GSH-Px),
glutathione reductase (GR), creatinine and urea kits were purchased
from Bio Diagnostic Co. (Dokki, Egypt). Coca-Cola, Pepsicola and
7-Up were purchased from Taif markets in Saudi Arabia. The 100 bp
DNA ladder marker was purchased from Fermentas (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Oligo dT primer and Qiazol
reagent were purchased from Qiagne (Valencia, CA, USA).
Experimental animals, design and
sampling
The present study was approved by the Ethics
Committee for the College of Applied Medical Sciences (Taif
University; no. 3792/34/1). A total of 40 male Wistar rats
(12-weeks-old; weight, 200–280 g). The rats were monitored daily
and were maintained under observation for 1 week for complete
acclimatization. The rats were maintained at 12 h light/dark cycles
and received free access to food and water for the first week. The
rats were then divided into four groups: i) Control without any
treatment; ii) Coca-cola group; iii) Pepsi group; iv) 7-Up group.
The rats in groups 2–4 received free access to the respective soft
drinks for three consecutive months. At the end of experiment, all
rats were anesthetized by diethyl ether inhalation. Blood and
tissues were collected from anesthetized rats in sterilized
vacutainer tubes. The serum was extracted following centrifugation
of clotted blood for 15 min at 3,000 × g and was maintained at
−20°C until use. For gene expression analysis, hepatic and renal
tissues were maintained at −80°C in Qiazol reagent for RNA
extraction. For histopathological examination, sections from the
bone, liver and kidney were immersed overnight in neutral buffered
formalin (NBF; 10%) at room temperature.
Serum biochemical assays
MDA, GSH-Px, GSH-reductase (GSH-R), catalase,
glucose, alkaline phosphatase (ALP), urea, bilirubin, creatinine,
alanine transaminase (ALT), uric acid, aspartate transaminase
(AST), creatine kinase (CK) and CKM were assayed
spectrophotometrically using commercial kits purchased from Bio
Diagnostic Co., according to the manufacturer's protocol. The
levels of osteocalcin levels were assayed using enzyme-linked
immunosorbent assay kits purchased from MyBioSource, LLC, San
Diego, CA, USA). Various minerals, triglycerides (TG), cholesterol
and high-dentisty lipoproteins (HDL) were assayed
spectrophotometrically using kits imported from HUMAN Gesellschaft
für Biochemica und Diagnostica mbH (Wiesbaden, Germany), according
to the manufacturer's protocol. Serum lipase was measured using a
lipase activity assay kit imported from Abcam (Cambridge, MA,
USA).
cDNA preparation, synthesis and gene
expression assessments
The total RNA from tissue samples was extracted, as
previously described (19). The
integrity of RNA was visualized and confirmed following running on
a denatured agarose gel (1.5%) stained with ethidium bromide. Oligo
dT primer (0.5 ng) was added to 2 µg total RNA to induce
denaturation and was subsequently used for cDNA synthesis (19). For semi-quantitative gene
expression analysis, specific primers were designed for certain
genes, as illustrated in Table I,
using Oligo 4 computer program (version 7; Molecular Biology
Insights, Inc., Colorado Springs, CO, USA) and synthesized by
(Macrogen Company, Geumcheongu. Korea). PCR was performed in a
total volume of 25 µl (1 µl cDNA, 1 µl 10 pM
each primer, 12.5 µl PCR master mix and 9.5 µl
sterilized, deionized water). A Bio-Rad T100™ Thermal Cycle machine
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for PCR,
which was performed at 95°C for 4 min, followed by 33 cycles of
denaturation at 95°C for 50 sec, annealing as shown in Table I and extension at 72°C for 1
minute, with an additional final extension at 72°C for 5 min. The
expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
used as internal standard and as a reference gene. The PCR products
were electrophoresed in a 1.5% agarose gel, followed by staining
with ethidium bromide in Tris-Borate-EDTA buffer. The bands were
visualized using an InGenius 3.0 gel documentation system (Syngene,
Frederick, MD, USA) under UV light.
 | Table IPrimers used for polymerase chain
reaction. |
Table I
Primers used for polymerase chain
reaction.
| Primer
(product) | Sequence
(5′-3′) | Annealing
conditions (°C) |
|---|
| IL-1β (550 bp) |
| Forward |
TTCAAATCTCACAGCAGCATCT | |
| Reverse |
TGTGCAGACTCAAACTCCACTT | 60°C for 1 min |
| AGP (325 bp) |
| Forward |
GCTCCTGTCTGTTTCCTTAGTT | |
| Reverse |
GGCTTTTTGTTGTTTGCTTCTATTTC | 55°C for 1 min |
| α2-MG (230 bp) |
| Forward |
GCTCCTGTCTGTTTCCTTAGTT | |
| Reverse |
ATTGGCCTTTCGTGGTTTAG | 56°C for 1 min |
| GST (575 bp) |
| Forward |
GCTGGAGTGGAGTTTGAAGAA | |
| Reverse |
GTCCTGACCACGTCAACATAG | 55°C for 1 min |
| SOD (410 bp) |
| Forward |
AGGATTAACTGAAGGCGAGCAT | |
| Reverse |
TCTACAGTTAGCAGGCCAGCAG | 55°C for 1 min |
| HO-1 (250 bp) |
| Forward |
CTTGCAGAGAGAAGGCTACATGA | |
| Reverse |
AGAGTCCCTCACAGACAGAGTTT | 54°C for 1 min |
| AGT (263 bp) |
| Forward |
TTGTTGAGAGCTTGGGTCCCTTCA | |
| Reverse |
CAGACACTGAGGTGCTGTTGTCCA | 57°C for 1 min |
| AT1 (440 bp) |
| Forward |
GCACAATCGCCATAATTATCC | |
| Reverse |
CACCTATGTAAGATCGCTTC | 54°C for 1 min |
| GAPDH (309 bp) |
| Forward |
AGATCCACAACGGATACATT | |
| Reverse |
TCCCTCAAGATTGTCAGCAA | 52°C for 1 min |
Histopathology of bone, liver and
kidney
Tissues from the bone, liver and kidney were
dissected following diethyl ether inhalation and the sacrificing of
the rats. The samples were immersed and fixed overnight in a 10%
NBF solution. Bone, liver and kidney tissues were processed, washed
and preserved in ethanol (70%). The samples were subsequently
dehydrated in ethanol solution in ascending grades, cleared with
xylene, paraffin wax embedded, casted and cut into 5 µm
sections using microtome (Leica Microsystems, Inc., Buffalo Grove,
IL, USA). Mayer's hematoxylin and eosin, and Masson's trichrome
staining were used to stain the slides with the fixed tissue
samples (20). The slides with
tissue were examined using a Wolfe S9-0982 microscope (Carolina
Biological Supply Company, Burlington, NC, USA) and images were
captured using a Canon Power-Shot SX500 IS digital camera.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Analysis of variance was used to analyze data together
with post hoc descriptive tests using SPSS 11.5 for Windows
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. The intensities of
the bands were quantified densitometrically using Image J software
(version 1.47) and were matched compared to the control group and
expressed as a percent (http://imagej.en.softonic.com/).
Results
Effect of SDC for 3 months on the serum
levels of MDA, GSH-Px, catalase and GSH-R in Wistar rats
SDC for 3 months revealed an increase in MDA levels
(Table II) with a highly
significant effect on Pepsi-administered rats compared with the
control rats. By contrast, the antioxidant activity of GSH-Px,
catalase and GSH-R in the rats were decreased significantly in all
SDC groups (Table II).
 | Table IISerum changes in MDA, GSH-Px, GSH-R
and catalase levels in rats following chronic consumption of soft
drinks. |
Table II
Serum changes in MDA, GSH-Px, GSH-R
and catalase levels in rats following chronic consumption of soft
drinks.
| Condition | MDA
(nmol/g protein) | GSH-Px
(U/g protein) | GSH-R
(U/g protein) | Catalase
(U/g protein) |
|---|
| Control | 32.7±2.7 | 86.1±4.8 | 75.4±4.2 | 44.7±2.2 |
| Cola | 63.7±7.6a | 57.9±5.2a | 54.7±4.6a | 28.6±1.4a |
| Pepsi | 96.7±9.8a | 36.3±5.8a | 34.2±6.0a | 17.3±1.9a |
| 7-Up | 59.1±3.7a | 55.3±3.2a | 43.8±6.3a | 25.5±2.5a |
Effect of SDC for 3 months on the blood
levels of glucose, hepatic and renal biomarkers in Wistar rats
SDC for 3 months, significantly increased the serum
levels of glucose and moderately increased uric acid and bilirubin
in the Coca-Cola, Pepsi and 7-up groups compared with the control
group (Table III).
 | Table IIISerum changes in glucose, urea, uric
acid, creatinine, and total and direct bilrubin levels in rats
following chronic consumption of soft drinks. |
Table III
Serum changes in glucose, urea, uric
acid, creatinine, and total and direct bilrubin levels in rats
following chronic consumption of soft drinks.
| Condition | Glucose
(mg/dl) | Urea
(mg/dl) | Uric acid
(mg/dl) |
Creatinine
(mg/dl) | Total
bilirubin
(mg/dl) | Direct
bilirubin
(mg/dl) |
|---|
| Control | 85.0±1.9 | 36.3±3.5 | 1.6±0.1 | 0.40±0.01 | 0.03±0.007 | 0.024±0.004 |
| Cola | 106.5±3.7a | 31.9±1.8 | 1.9±0.2 | 0.39±0.01 | 0.06±0.006 | 0.043±0.002 |
| Pepsi | 120.8±5.1a | 32.8±1.9 | 2.3±0.3a | 0.33±0.02 | 0.10±0.009 | 0.041±0.009 |
| 7-Up | 132.8±13.8a | 29.3±3.3 | 2.4±0.3a | 0.27±0.01 | 0.08±0.006 | 0.028±0.007 |
Effect of SDC for 3 months on serum
levels of hepatic biomarkers in Wistar rats
SDC for 3 months significantly increased the levels
of liver biomarkers, with a more marked effect on AST and a
moderate effect on ALT. Additionally, ALP increased in all groups
compared with the control rats (Table
IV).
 | Table IVSerum changes in AST, ALT and ALP
levels in rats following chronic consumption of soft drinks. |
Table IV
Serum changes in AST, ALT and ALP
levels in rats following chronic consumption of soft drinks.
| Condition | AST (U/l) | ALT (U/l) | ALP (U/l) |
|---|
| Control | 130.6±2.6 | 55.4±2.8 | 155.5±2.6 |
| Cola | 158.1±9.5a | 68.6±3.0a | 246.1±20.5a |
| Pepsi | 212.7±31.4b | 84.9±3.5b | 196.8±20b |
| 7-Up | 145.9±12.1 | 60.4±3.7 | 202.5±11.2 |
Effect of SDC for 3 months on the serum
levels of minerals and osteocalcin in Wistar rats
SDC decreased both calcium and iron levels in the
rats. By contrast soft drinks increased serum phosphorous levels.
In addition, soft drinks revealed no effect sodium and chloride
levels. Notably, soft drinks increased the levels of osteocalcin
(Table V).
 | Table VSerum changes in mineral levels in
rats following chronic consumption of soft drinks. |
Table V
Serum changes in mineral levels in
rats following chronic consumption of soft drinks.
| Condition | Ca
(mg/dl) | P
(mg/dl) | Cl
(nmol/l) | Fe
(µg/dl) | K
(nmol/l) | Mg
(mg/dl) | Na
(nmol/l) |
Osteocalcin
(ng/ml) |
|---|
| Control | 11.6±0.3 | 5.3±0.2 | 88.7±1.2 | 342.6±34.2 | 9.8±0.1 | 2.7±0.1 | 134.8±2.1 | 39.1±2.9 |
| Cola | 9.3±0.2a | 6.8±0.6 | 96.1±0.9a | 233.4±17.8a | 9.9±0.6 | 2.2±0.1 | 132.3±0.5a | 60.5±4.6 |
| Pepsi | 9.4±0.0a | 7.9±0.4a | 96.2±0.2a | 201.1±21.7a | 11.7±1.0a | 2.4±0.1 | 131.3±1.4b | 80.4±7.8 |
| 7-Up | 9.6±0.3a | 7.4±0.8a | 96.0±0.6a | 256.4±21.9a | 12.7±0.2a | 2.3±0.1 | 130.3±0.6 | 60.8±4.8 |
Effect of SDC for 3 months on the serum
levels of lipase and lipid profiles in Wistar rats
SDC increased serum lipase levels, which resulted in
a decrease in cholesterol and TG levels in the SDC groups compared
with the control (Table VI). By
contrast, HDL was decreased in the Coca-Cola and Pepsi groups
compared with the control group; however, 7-Up caused no
effect.
 | Table VISerum changes in lipid profiles and
lipase levels in rats following chronic consumption of soft
drinks. |
Table VI
Serum changes in lipid profiles and
lipase levels in rats following chronic consumption of soft
drinks.
| Condition | Lipase (U/l) | Cholesterol
(mg/dl) | TG (mg/dl) | HDL-C (mg/dl) |
|---|
| Control | 4.6±0.6 | 59.9±2.0 | 182.3±8.3 | 44.6±1.6 |
| Cola | 5.9±0.9a | 41.4±2.9a | 115.3±11.8a | 29.1±3.9a |
| Pepsi | 5.8±0.1a | 43.2±2.7a | 78.6±8.3a | 33.1±2.9a |
| 7-Up | 5.4±0.2 | 56.2±1.0 | 92.4±14.9a | 43.8±1.8 |
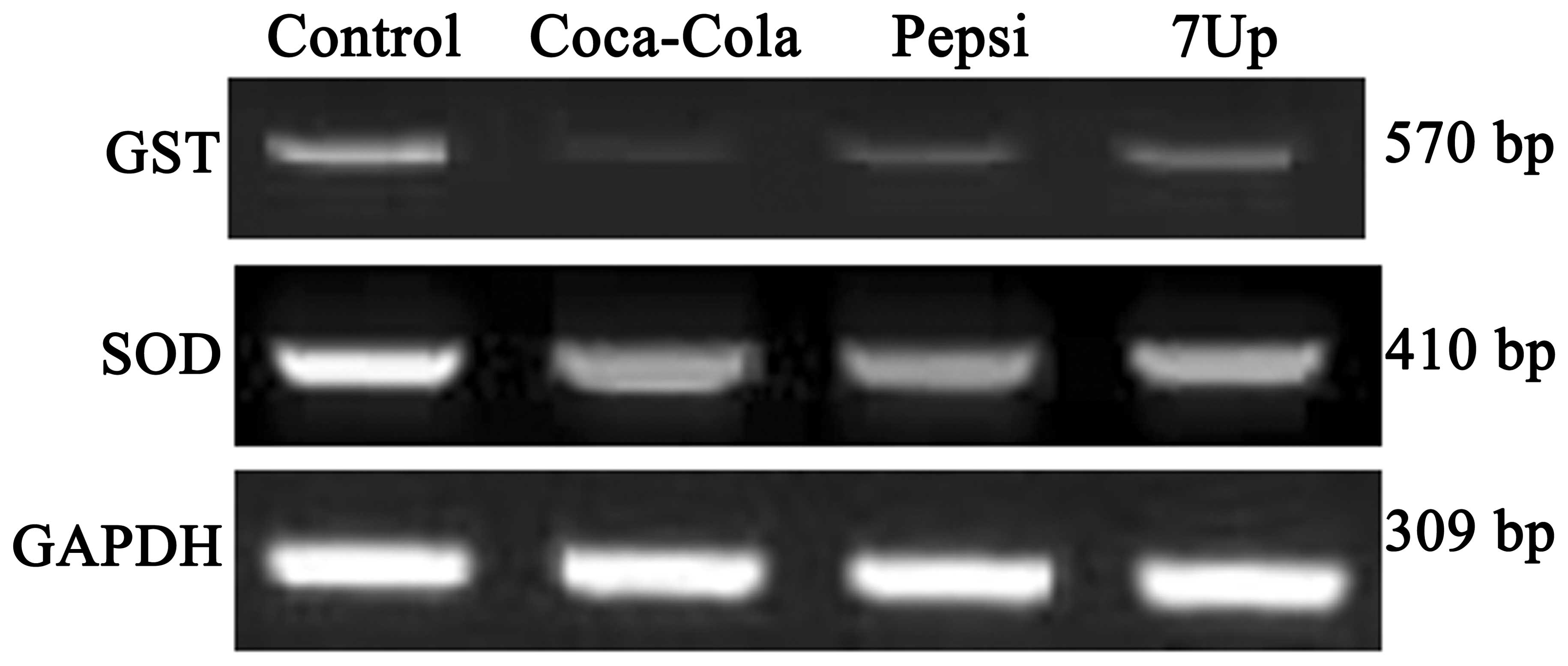
Effect of SDC for 3 months on the mRNA
expression levels of GST and SOD in the liver of Wistar rats
SDC for 3 months downregulated the gene expression
levels of GST and SOD (Fig. 1).
The expression percentage of GST was ~80, 60 and 40% decreased in
Coca-Cola, Pepsi and 7-Up groups, respectively. All groups
exhibited a slight downregulation in the mRNA expression of SOD in
hepatic tissue at a percentage of 30, 18 and 15%, respectively,
compared with the control rats.
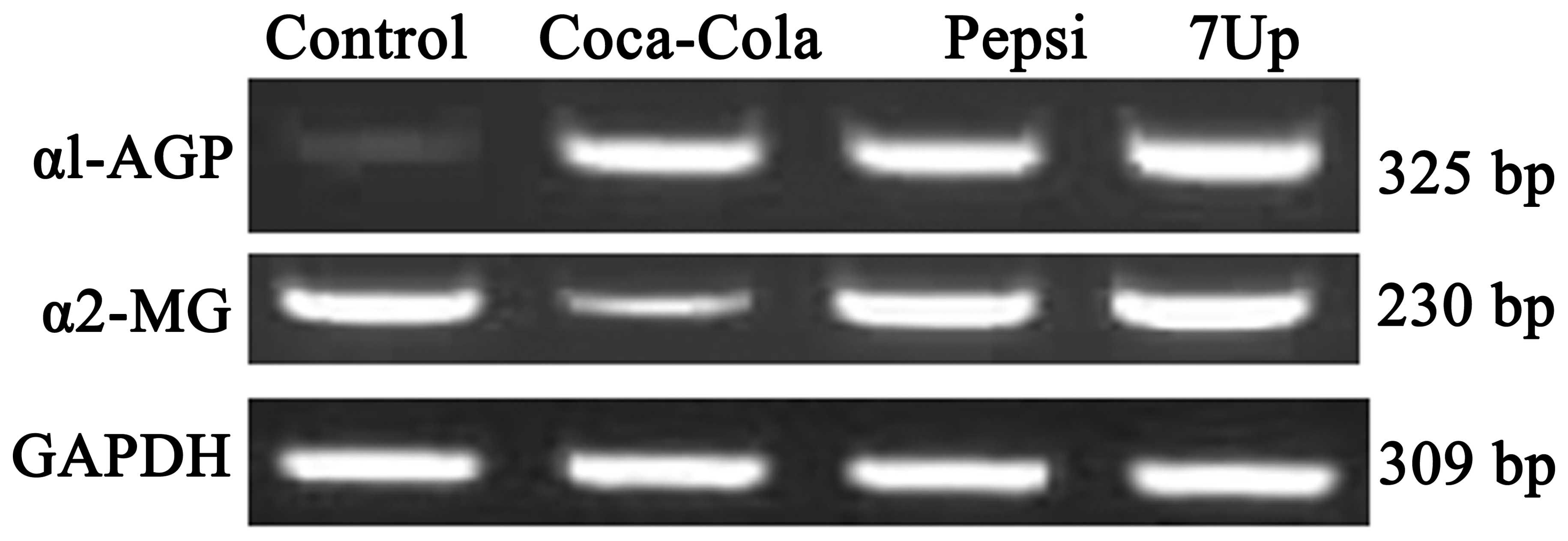
Effect of SDC for 3 months on the mRNA
expression levels of α1-AGP and α2-MG in the liver of Wistar
rats
SDC significantly upregulated the mRNA expression of
α1-AGP in the liver tissues (Fig.
2). The percentage of upregulation in the expression of α1-AGP
was 66, 70 and 75% for Coca-Cola, Pepsi and 7-Up, respectively. By
contrast, the expression of α2-MG was downregulated by Coca-Cola
(45%) and moderately upregulated in the Pepsi and 7-Up groups by 15
and 20%, respectively, compared with the control rat (Fig. 2).
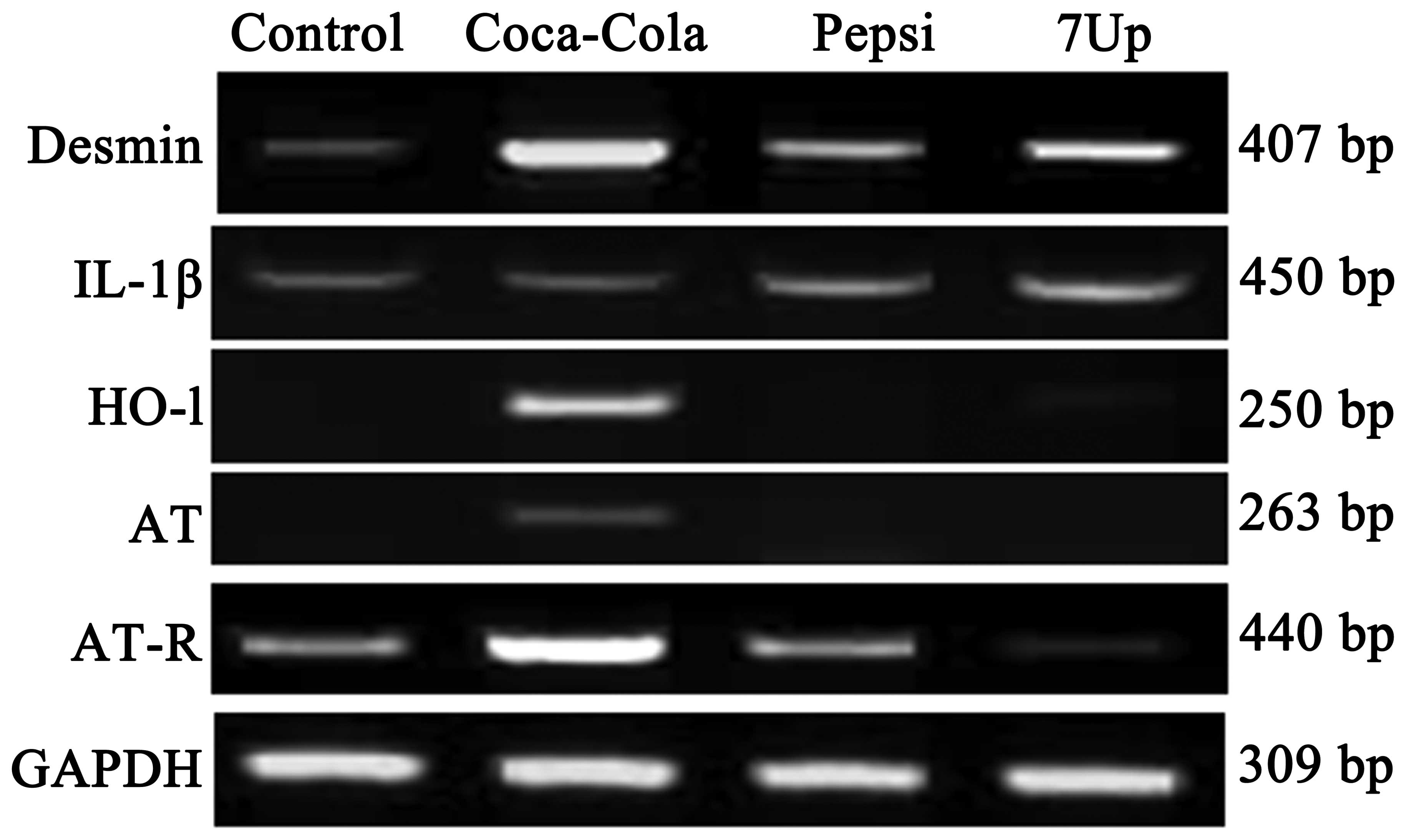
Effect of SDC for 3 months on the mRNA
expression levels of genes associated with renal function
biomarkers and stability in Wistar rats
Next, the present study tested the effect of SDC on
the mRNA expression levels of certain genes associated with normal
kidney function and stability. The expression of desmin was
upregulated in the Coca-Cola-, Pepsi- and 7-Up-administered rats at
a percentage of 75, 50 and 60%, respectively (Fig. 3). Interleukin (IL)-1β was only
increased in the Pepsi and 7-Up groups by 30 and 50%, respectively.
The expression of homooxygenase (HO)-1 was only increased in the
Coca-Cola and 7-Up groups by 90 and 25%, respectively. The present
study also examined the genes associated with blood pressure,
including angiotensinogen (AT) and its receptor (AT-R). The
expression of AT was upregulated in the Coca-Cola group (55%
increase compared with the control). The expression of AT-R was
also upregulated by 64 and 10% for Cola and Pepsi, respectively,
compared with the control and 7-Up groups (Fig. 3).
Effect of SDC for 3 months on bone, liver
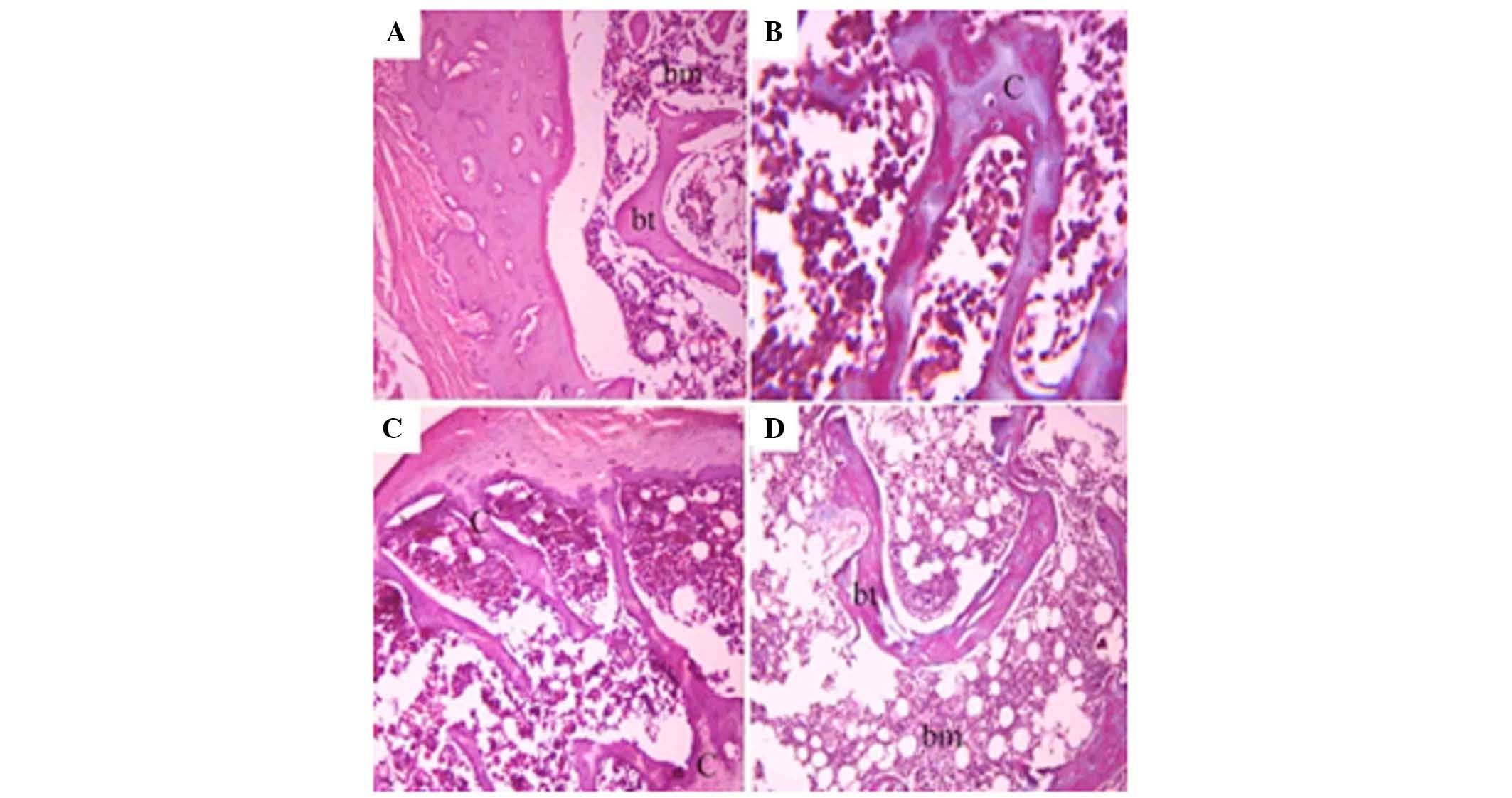
and kidney histopathology
The bone consisted of numerous bony lamellae, and
bone marrow extended between these lamella. The blood progenitor
cells occupied the architecture of the bone marrow. The structure
unit of the bone consisted of osteocytes arranged around the
haversian canal (Fig. 4A). In the
Coca-Cola and Pepsi groups, the bone lamella exhibited dark
acidophilic areas in the bone architecture due to calcium
withdrawal, while the other parts exhibited faint basophilic
patches (Fig. 4B and C). In the
7-Up group, the bone lamella exhibited little histological changes
in the form of small patches of faint basophilic areas (Fig. 4D).
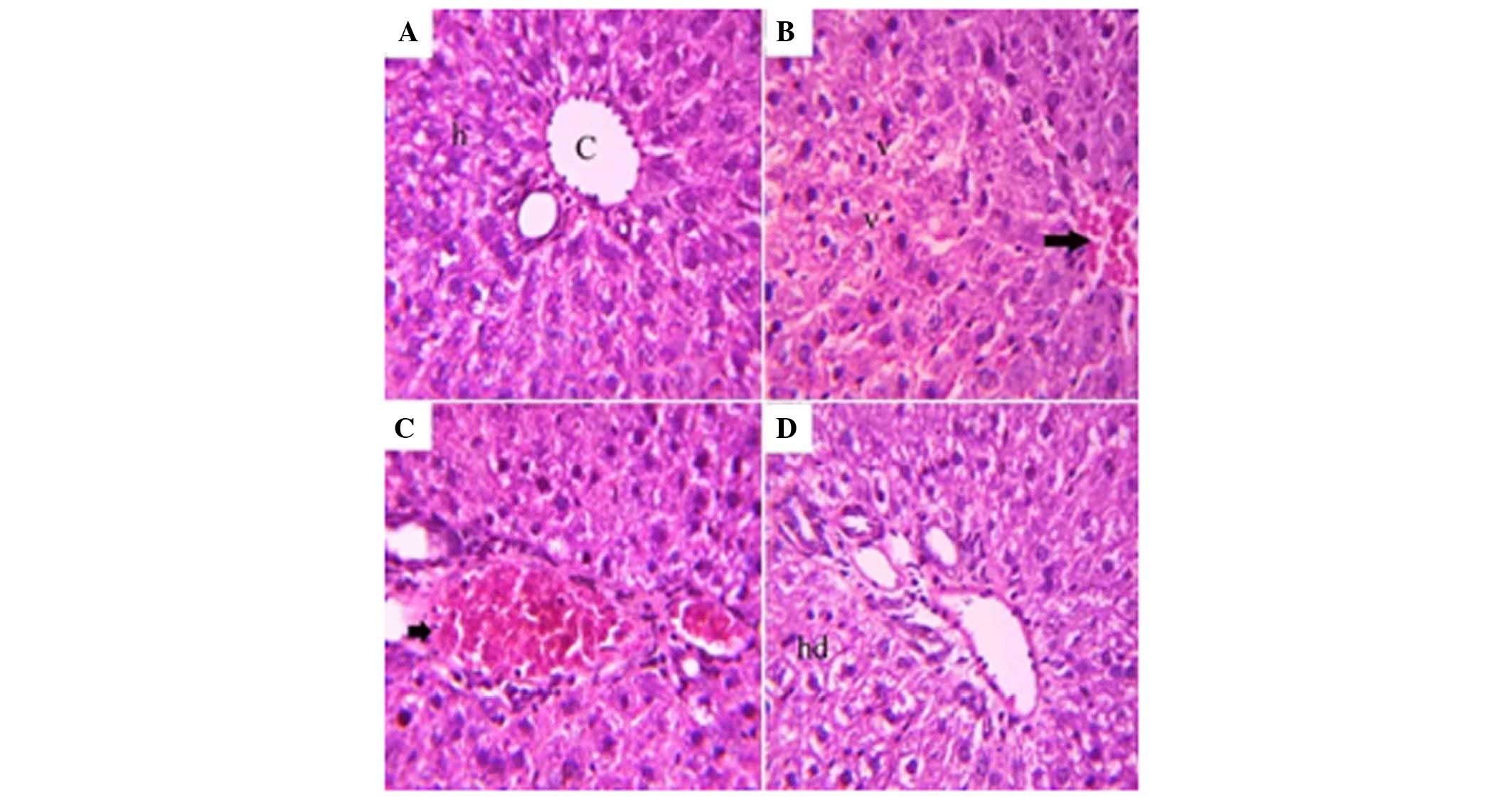
The liver consisted of a central vein, which was
surrounded by numerous hepatic cords. The hepatic cords were
synthesized from polygonal hepatic cells with acidophilic cytoplasm
and basophilic nuclei, which were centrally located. Von Kupffer
cells were located between the hepatic cords (Fig. 5A). In the Coca-Cola group, the
liver exhibited activation of Von Kupffer cells and congestion in
the blood vessels (Fig. 5B). The
liver of the Pepsi group exhibited congestion in blood vessels
(Fig. 5C). In the 7-up group, the
liver exhibited hydropic degeneration, in addition to congestion of
the blood vessels (Fig. 5D).
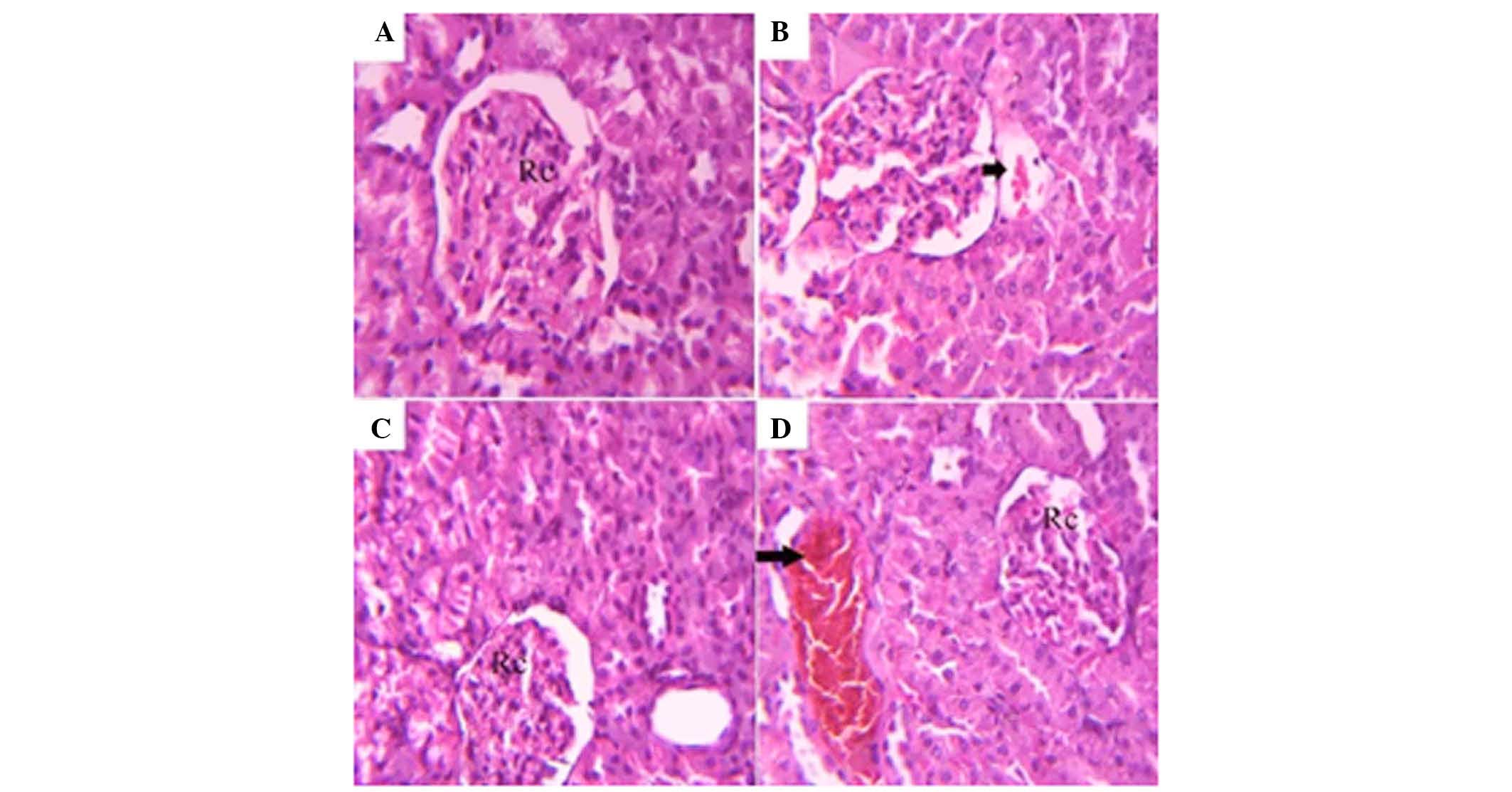
The kidney of the control group consisted of renal
corpuscle, surrounded by proximal and distal convoluted tubules
(Fig. 6A). In all soft drink
groups, the renal tissues exhibited mild congestion in the renal
blood vessels (Fig. 6B–D).
Discussion
The results of the present study confirmed that
chronic SDC induced oxidative stress, metabolic alterations and
changes in gene expression in Wistar rats. Oxidative stress has
been associated with the etiology and pathogenesis of various
chronic diseases and serves a vital role in the aging process
(21). High levels of free
radicals or reactive oxygen species (ROS) can cause direct damage
to lipids inside cells and induce peroxidation (21). The primary sources of endogenous
ROS production are the mitochondria, plasma membrane, endoplasmic
reticulum and peroxisomes through a variety of mechanisms,
including enzymatic reactions and/or auto-oxidation of several
compounds, including catecholamines and hydroquinone (22). Chronic SDC-induced oxidative
stress, which may cause hepatic toxicity, is indicated by the
increase in MDA and the decrease in GSH-Px, GSH-R and catalase
levels, as well as the decrease in the mRNA expression levels of
GST and SOD. Soft drinks are the predominant source of sugar
worldwide and are associated with obesity in children and
adolescents. Soft drinks favor the incidence of insulin resistance
and inflammation, and other diseases, including obesity, type-2
diabetes, dental caries, osteoporosis and low nutrient level
(23–25).
The present study hypothesized that SDC is
associated with lower bone mineral density, as indicated by the
disturbance in minerals levels that is likely due to its caffeine
contents, which is a predisposing factor for osteoporosis incidence
(5,26,27).
In addition, phosphoric acid interferes with absorption of calcium
and increases the loss of calcium (5). Additionally, high fructose may
negatively affect bone stability (28). The increase in glucose reported in
the present results is due to presence of caffeine in soft drinks.
Caffeine causes the release of adrenaline and the increase in blood
glucose to counteract the requirement for energy during emergency
(3,5). SDC caused liver injuries that are
followed by the increase in the activities of GPT and GOT (29). Alkaline phosphatase increase is
considered as a bone remodeling marker. The increase in AP is due
to the presence of caffeine in soft drinks (30). Caffeine affects the biosynthesis of
AP and inhibits the formation of a competent extracellular matrix,
essential for bone remodling (30,31).
Additionally, the increase in osteocalcin that serves a role in
mineralization of bone and calcium homeostasis cannot be neglected
(32). It has been previously
shown that heavy SDC is associated with hypocalcemia, and
alterations in liver enzymes and histology in non-alcoholic fatty
liver disease, both in clinical and experimental settings (5,12,13,33,34).
The explanation of mineral metabolism disorders is
due to high phosphate intake as exogenous phosphate loads inhibits
renal 1α-hydroxylase and reduces vitamin D biosynthesis (35). Therefore, the increase in phosphate
hinders both intestinal absorption and renal calcium re-absorption
causing hypocalcemia (36).
Consequently, hyperparathyroidism is developed, however, this is
not enough to prevent sustained hypocalcemia (36). This explains why heavy SDC is
characterized by hypocalcemia, hyperparathyroidism and reduced
1α-25-dihy droxyvitamin D level (5,12).
In parallel, SDC decreased iron absorption in the intestine, as
explained in the present and a previous study (37). In addition, acidemia occurred when
SDC increases bone resorption and calcium mobilization, and reduces
1α-hydroxylase activity and vitamin D production in kidney
(38–40).
α1-AGP is a protein of acute phase stage,
synthesized predominantly in hepatic cells and is regulated by
pregnancy, drugs and certain diseases, and is increased during
inflammation (41). The present
study reported that SDC increased the mRNA expression of α1-AGP, as
a result of hepatic cells injury. Additionally, α-2MG is slightly
decreased in response to Coca-Cola and increased in response to
Pepsi and 7-Up. α-2MG upregulation is probably a reversed mechanism
to prohibit plasmin biosynthesis and to facilitate the transport of
growth factors, which assist in the regeneration of hepatocytes due
to hepatic congestion reported in histopathological findings
(42). Renal podocytes are
essential components of the kidney, which maintain normal
glomerular structure. Podocytes regulate filtration flow through
the intracellular spaces, and they are situated at the basement of
glomeruli and act as ultrafilteration barrier (43,44).
Focal segmental glomerulosclerosis and chronic renal diseases are
caused by podocyte injury (45).
SDC upregulated the expression levels of desmin and
HO-1 and angiotensinogen. Desmin upregulation is indicative for
inflammation in the intrafilament proteins (46). HO-1 assists in the oxidation of
heme producing active molecules (carbon monoxide, biliverdin and
ferrous ion) that serve as a second messenger to control various
cellular functions (47).
Upregulation of HO-1 causes inhibition of either effectors of
immune functions or adaptive response to various body injuries
(48). The upregulation in the
expression of IL-1β is proceeded by an increase in the expression
of HO-1. Development of hypertension, renal oxidative stress and
tissue injury are due to an increase in AGT biosynthesis, secretion
and excretion (49). Of interest,
the changes reported in the expression levels of AT-1 and AGT were
due to changes in oxidative stress and renal injuries resulting
from SDC, as confirmed by renal congestion. Bone is the most
examined organ affected histopathologically due to minerals
metabolism disorder, as reported in the present and other previous
studies (16,18,26,27).
The kidney and liver histopathological alterations reported are
moderate degenerative changes, which resulted in the changes in
gene expression of both the kidney and liver. Taken together, these
data confirmed that SDC induced biochemical and genetic alterations
in examined organs, although less histopathological changes
reported in the bone, liver and kidney.
Acknowledgments
The present study was supported by a grant in aid
for Mohamed Mohamed Soliman of the Deans of Scientific Research
Affairs, Taif University, Saudi Arabia (no. 3792–1436).
References
|
1
|
Nielsen SJ and Popkin BM: Changes in
beverage intake between 1977 and 2001. Am J Prev Med. 27:205–210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adjene JO, Ezeoke JC and Nwose EU:
Histological effects of chronic consumption of soda pop drinks on
kidney of adult Wister rats. N Am J Med Sci. 2:215–217.
2010.PubMed/NCBI
|
|
3
|
Amato D, Maravilla A, García-Contreras F
and Paniagua R: Soft-drinks and health. Rev Invest Clin.
49:387–395. 1997.In Spanish.
|
|
4
|
Amato D, Maravilla A, Montoya C, Gaja O,
Revilla C, Guerra R and Paniagua R: Acute effects of soft drink
intake on calcium and phosphate metabolism in immature and adults
rats. Rev Invest Clin. 50:185–189. 1998.PubMed/NCBI
|
|
5
|
Rapuri PB, Gallagher JC, Kinyamu HK and
Ryschon KL: Caffeine intake increases the rate of bone loss in
elderly women and interacts with vitamin D receptor genotypes. Am J
Clin Nutr. 74:694–6700. 2001.PubMed/NCBI
|
|
6
|
Fung TT, Malik V, Rexrode KM, Manson JE,
Willett WC and Hu FB: Sweetened beverage consumption and risk of
coronary heart disease in women. Am J Clin Nutr. 89:1037–1042.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmer JR, Boggs DA, Krishnan S, Hu FB,
Singer M and Rosenberg L: Sugar-sweetened beverages and incidence
of type 2 diabetes mellitus in African American women. Arch Intern
Med. 168:1487–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swinburn BA, Caterson I, Seidell JC and
James WP: Diet, nutrition and the prevention of excess weight gain
and obesity. Public Health Nutr. 7:123–146. 2004.PubMed/NCBI
|
|
9
|
Yip HH, Wong RW and Hägg U: Complications
of orthodontic treatment: Are soft drinks a risk factor? World J
Orthod. 10:33–40. 2009.PubMed/NCBI
|
|
10
|
Ferraro PM, Taylor EN, Gambaro G and
Curhan GC: Soda and other beverages and the risk of kidney stones.
Clin J Am Soc Nephrol. 8:1389–1395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Passman CM, Holmes RP, Knight J, Easter L,
Pais V and Assimos DG: Effect of soda consumption on urinary stone
risk parameters. J Endourol. 23:347–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernando GR, Martha RM and Evangelina R:
Consumption of soft drinks with phosphoric acid as a risk factor
for the development of hypocalcemia in postmenopausal women. J Clin
Epidemiol. 52:1007–1010. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazariegos-Ramos E, Guerrero-Romero F,
Rodríguez-Morán M, Lazcano-Burciaga G, Paniagua R and Amato D:
Consumption of soft drinks with phosphoric acid as a risk factor
for the development of hypocalcemia in children: A case control
study. J Pediatr. 126:940–942. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petridou E, Karpathios T, Dessypris N,
Simou E and Trichopoulos D: The role of dairy products and
non-alcoholic beverages in bone fractures among schoolage children.
Scand J Soc Med. 25:119–125. 1997.PubMed/NCBI
|
|
15
|
Smith S, Swain J, Brown EM, Wyshak G,
Albright T, Ravnikar VA and Schiff I: A preliminary report of the
short term effect of carbonated beverage consumption on calcium
metabolism in normal women. Arch Intern Med. 149:2517–2519. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teófilo JM, Leonel DV and Lamano T: Cola
beverage consumption delays alveolar bone healing: A histometric
study in rats. Braz Oral Res. 24:177–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Morton DJ and Barrett-Connor EL:
Carbonated beverage consumption and bone mineral density among
older women: The Rancho Bernardo study. Am J Public Health.
87:276–279. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
García-Contreras F, Paniagua R, Avila-Díaz
M, Cabrera-Muñoz L, Martínez-Muñiz I, Foyo-Niembro E and Amato D:
Cola beverage consumption induces bone mineralization reduction in
ovariectomized rats. Arch Med Res. 31:360–365. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soliman MM, Baiyoumi AA and Yassin MH:
Molecular and histopathological study on the ameliorative effects
of curcumin against lead acetate-induced hepatotoxicity and
nephrototoxicity in Wistar rats. Biol Trace Elem Res. 167:91–102.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bancroft JD and Gamble M: Theory and
practice of histological techniques. 6th edition. Churchill
Livingstone Elsevier; Philadelphia: pp. 126–127. 2008
|
|
21
|
Moldovan L and Moldovan NI: Oxygen free
radicals and redox biology of organelles. Histochem Cell Biol.
122:395–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP, Tuttle TD and Higgins CL:
Energy beverages: Content and safety. Mayo Clin Proc. 85:1033–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaby AR: Adverse effects of dietary
fructose. Altern Med Rev. 10:294–306. 2005.PubMed/NCBI
|
|
24
|
Assy N, Nasser G, Kamayse L, Nseir W,
Beniashvili Z, Djibre A and Grosovski M: Soft drink consumption
linked with fatty liver in the absence of traditional risk factors.
Can J Gastroeterol. 22:811–816. 2008. View Article : Google Scholar
|
|
25
|
Vartaman LR, Schwartz MB and Brownwell KD:
Effects of soft drink consumption on nutrition and health: A
systematic review and meta-analysis. Am J Public Health.
97:667–675. 2007. View Article : Google Scholar
|
|
26
|
Hernández-Avila M, Stampfer MJ, Ravnikar
VA, Willett WC, Schiff I, Francis M, Longcope C, McKinlay SM,
McKinlay SM and Longscope C: corrected to Longcope C. Caffeine and
other predictors of bone density among pre- and perimenopausal
women. Epidemiology. 4:128–134. 1993. View Article : Google Scholar
|
|
27
|
Massey LK and Whiting SJ: Caffeine,
urinary calcium, calcium metabolism and bone. J Nutr.
123:1611–1614. 1993.PubMed/NCBI
|
|
28
|
Milne DB and Nielsen FH: The interaction
between dietary fructose and magnesium adversely affects
macromineral homeostasis in men. J Am Coll Nutr. 19:31–37. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeroh E, Awhin EP, Osadem L and Awire EI:
Effect of carbonated drinks on the activities of Alanine
Aminotransferase (ALT) and Aspartate Aminotransferase (AST) in
serum and kidney in Rattus novergicus. Asian J Biochem. 7:59–62.
2012. View Article : Google Scholar
|
|
30
|
Casiglia E, Spolaore P, Ginocchio G and
Ambrosio GB: Unexpected effects of coffee consumption on liver
enzymes. Eur J Epidemiol. 9:293–297. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tassinari MS, Gerstenfeld LC, Stein GS and
Lian JB: Effect of caffeine on parameters of osteoblast growth and
differentiation of a mineralized extracellular matrix in vitro. J
Bone Miner Res. 6:1029–1036. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanazawa I, Yamaguchi T, Yamamoto M,
Yamauchi M, Yano S and Sugimoto T: Serum osteocalcin/bone-specific
alkaline phosphatase ratio is a predictor for the presence of
vertebral fractures in men with type 2 diabetes. Calcif Tissue Int.
85:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abid A, Taha O, Nseir W, Farah R,
Grosovski M and Assy N: Soft drink consumption is associated with
fatty liver disease independent of metabolic syndrome. J Hepatol.
51:918–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohta M, Cheuk G, Thomas KA,
Kamagata-Kiyoura Y, Wink CS, Yazdani M, Falster AU, Simmons WB and
Nakamoto T: Effect of caffeine on the bones of aged, ovariectomized
rats. Ann Nutr Metab. 43:52–59. 1999. View Article : Google Scholar
|
|
35
|
Portale AA, Halloran BP, Murphy MM and
Morris RC Jr: Oral intake of phosphorus can determine the serum
concentration of 1,25-dihydroxyvitamin D by determining its
production rate in humans. J Clin Invest. 77:7–12. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haussler MR and McCain TA: Basic and
clinical concepts related to vitamin D metabolism and action (first
of two parts). N Engl J Med. 297:974–983. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hallberg L and Rossander L: Effect of
different drinks on the absorption of non-heme iron from composite
meals. Hum Nutr Appl Nutr. 36:116–123. 1982.PubMed/NCBI
|
|
38
|
Lemann J Jr and Lennon EJ: Role of diet,
gastrointestinal tract and bone in acid base homeostasis. Kidney
Int. 1:275–279. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hintz HF: Calcium, cola, calamity. Cornell
Vet. 70:3–9. 1980.PubMed/NCBI
|
|
40
|
Celec P, Pálffy R, Gardlík R, Behuliak M,
Hodosy J, Jáni P, Bozek P and Sebeková K: Renal and metabolic
effects of three months of decarbonated cola beverages in rats. Exp
Biol Med (Maywood). 235:1321–1327. 2010. View Article : Google Scholar
|
|
41
|
Anderson SP, Cattley RC and Corton JC:
Hepatic expression of acute-phase protein genes during
carcinogenesis induced by peroxisome proliferators. Mol Carcinog.
26:226–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lyoumi S, Tamion F, Petit J, Déchelotte P,
Dauguet C, Scotté M, Hiron M, Leplingard A, Salier JP, Daveau M and
Lebreton JP: Induction and modulation of acute-phase response by
protein malnutrition in rats: Comparative effect of systemic and
localized inflammation on interleukin-6 and acute-phase protein
synthesis. J Nutr. 128:166–174. 1998.PubMed/NCBI
|
|
43
|
Fries JW, Sandstrom DJ, Meyer TW and
Rennke HG: Glomerular hypertrophy and epithelial cell injury
modulate progressive glomerulosclerosis in the rat. Lab Invest.
60:205–218. 1989.PubMed/NCBI
|
|
44
|
Kriz W, Gretz N and Lemley KV: Progression
of glomerular diseases: Is the podocyte the culprit? Kidney Int.
54:687–697. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
DePianto D and Coulombe PA: Intermediate
filaments and tissue repair. Exp Cell Res. 301:68–76. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Omary MB, Coulombe PA and McLean WH:
Intermediate filament proteins and their associated diseases. N
Engl J Med. 351:2087–2100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bach FH: Heme oxygenase-1 as a protective
gene. Wien Klin Wochenschr. 114(Suppl 4): S1–S3. 2002.
|
|
49
|
Lara LS, McCormack M, Semprum-Prieto LC,
Shenouda S, Majid DS, Kobori H, Navar LG and Prieto MC: AT1
receptor-mediated augmentation of angiotensinogen, oxidative
stress, and inflammationin ANG II-salt hypertension. Am J Physiol
Renal Physiol. 302:F85–F94. 2012. View Article : Google Scholar
|