Introduction
Diabetic nephropathy (DN), a major complication of
type 1 and 2 diabetes, is the most common cause of advanced kidney
disease, leading to a high number of diabetes-associated
mortalities (1,2). However, investigating the mechanisms
underlying this condition is complex due to its complicated
pathogenesis, and various or numerous symptoms. At present, DN is
widely hypothesized to involve tubulointerstitial fibrosis and
glomerulosclerosis, triggered by oxidative stress, inflammation,
the activation of the renin-angiotensin-aldosterone system,
profibrotic factors, collagen cross-linking and epithelial
mesenchymal transition, amongst other causes (3–5).
However, to the best of our knowledge, the presence of a possible
association between these factors remains to be elucidated.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a member of the ligand-activated transcription factors of the
nuclear hormone receptor superfamily, and is closely associated
with the pathogenesis of numerous diseases, such as diabetes,
obesity and inflammatory diseases, including colitis,
steatohepatitis, chronic obstructive pulmonary disease and
osteoarthritis (6). Regarding the
critical role of PPARγ in regulating diverse biological processes,
such as lipid metabolism, adipogenesis, and insulin sensitization
in diabetes (7), the effects of
PPARγ activation on DN are primarily based on type 2 diabetes
(8–10). However, PPARγ activation prevents
the progression of DN in type 1 diabetes (11). Rosiglitazone and pioglitazone,
thiazolidinediones (PPARγ agonists), ameliorate DN by reducing the
expression level of chemerin receptor 23 in the kidney of
streptozotocin (STZ)-induced type 1 diabetes in rats (11). In addition, low doses of
rosiglitazone halt the progression of experimental nephropathy
induced by type 1 diabetes by decreasing renal oxidative stress
without effecting lipid alteration in diabetic rats (12). Furthermore, a previous study
demonstrated that in Finnish adults with type 1 diabetes, the
mortality associated with diabetes was almost entirely confined to
those with chronic kidney diseases (13). Additionally, evidence indicates
that PPARγ agonists alleviate certain symptoms in various types of
renal disease (10,14,15).
Together, these studies suggested PPARγ activation as an essential
therapeutic strategy for kidney diseases caused by type 2 and 1
diabetes.
Previous studies show that the pathogenesis of type
1 DN is associated with inflammation and oxidative stress (16–18).
Recently, it was proposed that PPARγ agonists exert independent
actions on the kidney functions, which may assist with preventing
diabetic kidney disease, including important effects on inhibition
of inflammation (17,19), oxidative stress (18,20),
and advanced glycation end products and their receptor interaction
(19,20). Therefore, PPARγ may be associated
with oxidative stress, inflammation and other factors during the
pathogenesis of DN. In addition, suppression of cyclooxygenase 2
(COX-2)-mediated prostaglandin E2 production decreases inflammation
and albuminuria in STZ-induced type 1 diabetic mice (21). Notably, previous studies show that,
in addition to inhibition of COX-2, certain nonsteroidal
anti-inflammatory drugs (NSAIDs), such as ibuprofen and
indomethacin, partially activate PPARγ (22). Thus, due to this double action on
inflammation, such NSAIDs may be more efficacious than PPARγ
agonists, thiazolidinediones, in the treatment of diabetic kidney
diseases.
Based on the above-mentioned findings, the present
study aimed to investigate the effects of ibuprofen, a partial
agonist of PPARγ, (a widely used NSAID with fewer and lighter side
effects) on DN, inflammatory response and oxidative stress in
STZ-induced type 1 diabetes in rats. The effects of ibuprofen were
compared with those of thiazolidinediones, and the
thiazolidinedione, pioglitazone served as a positive control.
Materials and methods
Animals
Male Sprague Dawley rats (age, 10 weeks, n=40) were
bred at the Xuzhou Medical College Experimental Animal Centre
(Xuzhou, China). Rats were housed in cages (5 rats per cage, 2
cages per group) at 50±10% humidity (temperature, 24±1°C) under a
12-h light/dark cycle, with free access to water and rodent chow.
All animal experiments were approved by the Animal Ethics Committee
of Xuzhou Medical College before being performed according to the
Guidelines for Ethical Conduct in the Care and Use of Animals
(23). Every effort was made to
minimise stress to the animals.
Experimental design
The rats were fasted for 12 h and subjected to a
single intraperitoneal injection of 60 mg/kg STZ (Sigma-Aldrich,
St. Louis, MO, USA), freshly dissolved in 0.1 mol/l sodium citrate
buffer (pH 4.5; Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China). Age-matched healthy rats (n=10) received sodium citrate
buffer alone. The development of diabetes was assessed in
accordance with non-fasting blood glucose (nFBG) levels using a
reagent kit (Jiancheng Bioengineering Institute, Nanjing, China).
Subsequent to 7 days of STZ treatment, diabetic rats (those with
nFBG ≥16.7 mmol/l) (24) were
successfully obtained, and randomly divided into three groups
(n=10) as follows: Diabetic (DM); ibuprofen-treated (DM + IB; 40
mg/kg); pioglitazone-treated (DM + PI; 25 mg/kg). The administered
volume of ibuprofen (purity >99%; Huayida Technology Co., Ltd.,
Wuhan, China) and pioglitazone (purity >99%; Zhongke Yitong
Chemical Co., Ltd., Jinan, China) was 10 ml/kg, with the
concentrations of 4 and 2.5 mg/ml, respectively, prepared in 1% w/v
sodium carboxymethyl cellulose (Sinopharm Chemical Reagent Co.,
Ltd.). Ibuprofen and pioglitazone were administered orally once per
day for 8 weeks. The rats were weighed weekly and underwent blood
glucose tests, for which the nFBG levels were measured using a
reagent kit (Jiancheng Bioengineering Institute), according to the
manufacturer's instructions, and ultraviolet-visible (UV-Vis)
spectrophotometry (722N UV-Vis spectrophotometer; INESA Analytical
Instrument Co., Ltd., Shanghai, China). Eight weeks later, the rats
were placed in metabolic cages for 24-h urine collection and
consequent albuminuria measurement prior to being sacrificed under
ethyl ether (Sinopharm Chemical Reagent Co., Ltd.) anesthesia,
using cotton balls soaked with ether. The albuminuria levels were
measured using a urine protein test kit (Jiancheng Bioengineering
Institute; cat. no. C035-2), according to the manufacturer's
instructions, and UV-Vis spectrophotometry. Their blood samples (~3
ml) were collected via femoral vein bleeding, and the serum was
collected following centrifugation at 4°C at 1,500 × g for 10 min,
for approximately 1 ml of blood. Bilateral kidneys were removed and
the left kidney was decapsulated and fixed in 4% buffered formalin
(Sinopharm Chemical Reagent Co., Ltd.) for 24 h prior to paraffin
(Beyotime Institute of Biotechnology, Nantong, China) embedding. In
addition, the renal cortex was rapidly isolated. The samples were
stored at −70°C prior to use.
Renal function assessment
Renal function can be evaluated through measurement
of urinary protein and blood urea nitrogen (BUN). Excretion of
urinary protein was quantified using a urine protein test kit
(Jiancheng Bioengineering Institute) through the Coomassie
(Beyotime Institute of Biotechnology) brilliant blue method, while
BUN was examined using a BUN assay kit (Jiancheng Bioengineering
Institute) according to a diacetyl oxime colorimetric method
(25).
Renal pathological changes by periodic
acid-Schiff (PAS) staining and Masson's trichrome staining
Renal PAS staining (Sigma-Aldrich) and Masson's
trichrome (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai,
China) staining were performed, as previously described (26). Kidney tissue samples were fixed in
a 10% buffered formalin solution and embedded in paraffin for
histological analysis. The 3-µm thick paraffin sections were
dewaxed through a series of graded ethanol baths, to displace the
water, and subsequently infiltrated with water. The sections were
stained with PAS or Masson's trichrome, cleared in xylene
(Sinopharm Chemical Reagent Co., Ltd.) and mounted with neutral
balsam (Sinopharm Chemical Reagent Co., Ltd.) prior to examination
under an Olympus BX50 microscope (Olympus Corporation, Tokyo,
Japan). The three typical views of each imperfect section were
analyzed. Linear measurements were obtained using an image analysis
system (Image-Pro Plus 4.0; Media Cybernetics, Inc., Silver Spring,
MD, USA).
PPARγ protein expression in rat kidneys
by immunohistochemistry
Kidney sections (4 µm) were deparaffinized
and endogenous peroxidase was blocked by the addition of 3%
H2O2 (Zhongshan Golden Bridge Biotech Co.,
Ltd.; OriGene Technologies, Inc., Beijing, China) for 10 min. The
sections were incubated overnight at 4°C with a rabbit polyclonal
anti-PPARγ antibody (1:1,000; Bioworld Technology, Inc., St. Louis
Park, MN, USA; cat. no. AP0688), then a polymer helper for 20 min,
followed by a polyclonal horseradish-peroxidase-conjugated goat
anti-rabbit immunoglobulin G antibody (Zhongshan Golden Bridge
Biotech Co., Ltd.; OriGene Technologies, Inc.; cat. no. PV-9001) at
room temperature for 20 min. The peroxidase was visualized through
the addition of 3,3′-diaminobenzidine (Zhongshan Golden Bridge
Biotech Co., Ltd.; OriGene Technologies, Inc.) in the dark for 3
min. The sections were counterstained with hematoxylin (Beyotime
Institute of Biotechnology), dehydrated, and observed under an
Olympus CX22 light microscope (Olympus Corporation). A minimum of 3
relatively intact kidney tissue sections were selected at random
and further incubated with either a primary or a secondary antibody
to determine the binding specificity of PPARγ, without any positive
staining. Five sections were analyzed for each rat, and 10 images
were obtained from a randomly selected site per slide. The optical
density (OD) of PPARγ immunostaining in the cell nucleus was
quantified using Image-Pro Plus 4.0 software.
Protein expression of COX-2 and inducible
nitric oxide synthase (iNOS) in the renal cortex by western
blotting
The preserved renal cortex was weighed and
homogenized using an electronic tissue homogenizer (GF-1;
Kylin-Bell Lab Instruments Co., Ltd., Haimen, China) in 10 vol
(w/v) Tris-buffered saline (50 mmol/l; pH 7.4; Beyotime Institute
of Biotechnology) containing 0.6 mmol/l phenylmethylsulphonyl
fluoride (Beyotime Institute of Biotechnology), 1 mmol/l
Na3VO4 (Sangon Biotech Co. Ltd., Shanghai,
China) and 50 mmol/l NaF (Sinopharm Chemical Reagent Co., Ltd.) in
an ice bath. The resulting homogenates were maintained at 4°C for
≥60 min prior to centrifugation at 4°C for 15 min at 10,000 x g to
obtain the supernatant for western blot analysis. Protein
concentrations in the supernatant were determined using the
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology).
Protein samples (80 µg) were separated by
sodium dodecyl sulphate-polyacrylamide gel electrophoresis (10%
gel; run at 100 volts for 80 min; Beyotime Institute of
Biotechnology) and transferred to BioTrace nitrocellulose membranes
(Merck Millipore Ireland BV, Co Cork, Ireland). The membranes were
blocked with 5% blocking buffer (Beyotime Institute of
Biotechnology) for 120 min and incubated overnight at 4°C with
primary antibodies, including rabbit polyclonal anti-COX-2
(1:1,000; ProteinTech Group, Inc., Chicago, IL, USA; cat. .no.
55070-1-AP), rabbit polyclonal anti-iNOS (1:100; Abcam, Inc.,
Cambridge, UK; cat. .no. ab3523) and rabbit polyclonal anti-β-actin
antibody (1:2,000, Bioworld Technology, Inc.; cat. no. AP0060). The
blots were detected using alkaline phosphatase-conjugated
affinipure goat anti-rabbit secondary antibody (1:1,000; Zhongshan
Golden Bridge Biotech Co., Ltd.; OriGene Technologies, Inc.; cat.
no. ZB-2308). The membranes were exposed to a
5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (NBT)
alkaline phosphatase color developing reagent (Beyotime Institute
of Biotechnology) for 15 min. Signal densities on the blots were
measured with Image J software, version 1.48u (http://imagej.nih.gov/ij/) and normalized against
rabbit anti-β-actin (Bioworld Technology Inc.), which served as an
internal control (ODprotein / ODinternal
control).
Determination of interleukin-1β (IL-1β)
levels in the rat kidney and serum by enzyme linked immunosorbent
assay (ELISA
The level of IL-1β in the rat renal cortex and serum
was estimated using a commercial ELISA kit (ExCell Biology, Inc.,
Shanghai, China) according to the manufacturer's instructions.
Assay of superoxide dismutase (SOD)
activity and reduced glutathione (GSH) level in the rat kidney and
serum
SOD activity was measured using the Total Superoxide
Dismutase (T-SOD) assay kit (Hydroxylamine method; Jiancheng
Bioengineering Institute; cat. no. A001-1) according to the
manufacturer's instructions. Briefly, tissue was prepared in a 10%
solution (100 mg tissue in 1,000 µl normal saline) and mixed
with the reagents of the kit using a vortex mixer. The solution was
incubated at 37°C for 40 min prior to the addition of the
chromogenic agent. Samples were incubated at room temperature for
10 min and the OD was then determined at a wavelength of 550 nm.
SOD activity (U/mg prot) was calculated as follows:
SODactivity =
(ODcontrol−ODtest)/ODcontrol ÷ 50%
× TRV/SV ÷ PrCn, where TRV is the total reaction volume (ml), SV is
the sample volume (ml) and PrCn is the protein concentration of
sample (mg prot/ml). The reduced glutathione (GSH) assay (Jiancheng
Bioengineering Institute; cat. no. A006-2) was performed according
to a previously reported spectrophotometric method (27), using the 722N UV-Vis
spectrophotometer. One unit of SOD was defined as the quantity of
enzyme causing 50% inhibitory rates of NBT reduction. The tissue
GSH level was expressed as nanomoles of GSH per milligram protein,
and the serum GSH level was expressed as micromoles of GSH per
liter.
Statistical analysis
The results are expressed as means ± standard
deviation. Intergroup variation was measured by one-way analysis of
variance followed by Tukey's test. The analysis was performed using
SPSS Statistical Software, version 13.0 (SPSS, Inc., Chicago, IL,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of ibuprofen on nFBG and the body
weight of diabetic rats
The serum glucose levels of experimental rats were
measured at weeks 0 and 8 weeks after treatment with 40 mg/kg
ibuprofen and 25 mg/kg pioglitazone (the positive control).
Compared with the age-matched healthy rats, higher nFBG levels were
observed in the diabetic rats throughout the treatment (P<0.01).
However, nFBG remained at a high level in diabetic rats treated
with either ibuprofen or pioglitazone, without any significant
difference between the treated and untreated groups (Table I). Furthermore, the body weights of
the experimental rats were measured from week 1 to week 8 following
treatment with 40 mg/kg ibuprofen and 25 mg/kg pioglitazone.
Diabetic rats continually demonstrated reduced body weights in
comparison to the age-matched healthy rats following the STZ
injection (P<0.01). Ibuprofen or pioglitazone treatment produced
no obvious effect on the body weights of diabetic rats (Table II).
 | Table IEffects of IB on nFBG of
streptozotocin-induced diabetic rats before and after treatment
with IB. |
Table I
Effects of IB on nFBG of
streptozotocin-induced diabetic rats before and after treatment
with IB.
| Group | n | nFBG (mmol/l)
|
|---|
| Before
treatment | After
treatment |
|---|
| Control | 10 | 6.2±0.5 | 4.7±0.4 |
| DM | 9–10 | 36.8±5.4a | 32.4±4.3a |
| DM + PI | 10 | 36.6±4.6 | 29.9±4.2 |
| DM + IB | 9–10 | 37.1±5.7 | 27.1±6.1 |
 | Table IIEffects of IB on the body weight of
streptozotocin-induced diabetic rats after treatment. |
Table II
Effects of IB on the body weight of
streptozotocin-induced diabetic rats after treatment.
| Group | Body weight (g)
|
|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
|---|
| Control | 245±4 | 275±12 | 312±14 | 337±15 | 356±16 | 382±19 | 393±19 | 409±18 | 391±15 |
| DM | 185±17a | 186±25a | 173±29a | 175±27a | 174±32a | 173±32a | 166±31a | 168±38a | 158±34a |
| DM + PI | 190±18 | 180±28 | 181±27 | 180±28 | 183±33 | 190±39 | 187±36 | 180±37 | 172±32 |
| DM + IB | 183±24 | 182±27 | 164±21 | 175±32 | 174±39 | 178±44 | 163±41 | 168±44 | 168±45 |
Effects of ibuprofen on renal dysfunction
in diabetic rats
Fig. 1 illustrates
the effects of ibuprofen and the positive control, pioglitazone on
rat serum BUN (Fig. 1A), and
urinary protein excretion (Fig.
1B) in the healthy and experimental rats. Significant increases
were observed in urinary protein excretion and serum BUN level in
the diabetic rats compared with the healthy rats (P<0.01).
Chronic treatment with ibuprofen significantly attenuated the
damage resulting from renal function, as evidenced by decreases in
urinary protein excretion (P<0.01) and serum BUN level
(P<0.05) of diabetic rats. This finding was also observed in
pioglitazone-treated diabetic rats (Fig. 1).
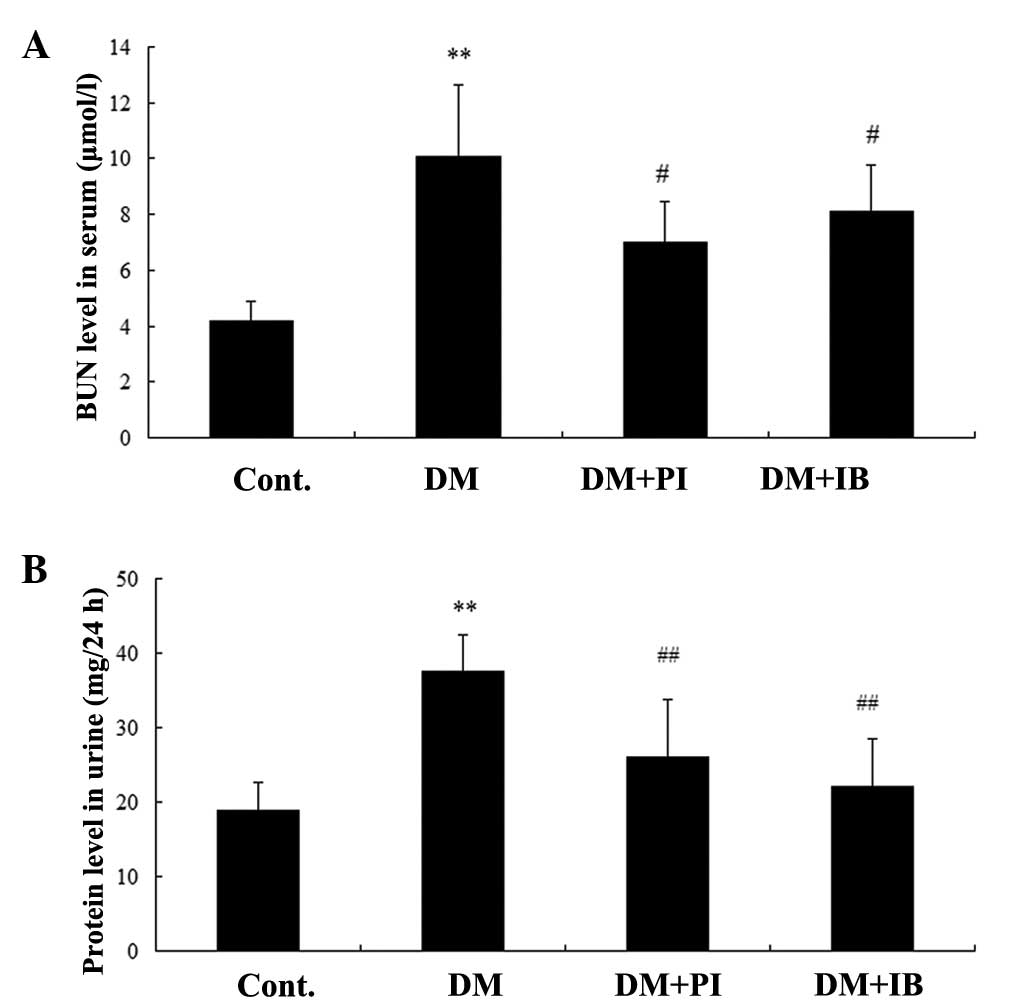 | Figure 1Effects of IB on (A) BUN and (B)
urinary protein excretion. Cont., DM, DM + PI, and DM + IB
represent healthy rats, diabetic rats, and diabetic rats treated
with PI (25 mg/kg) and IB (40 mg/kg), respectively. Data are
presented as means ± standard deviation; n=7.
**P<0.01 vs. Cont.; #P<0.05 and
##P<0.01 vs. DM. BUN, blood urea nitrogen; Cont.,
control; DM, diabetic group; PI, pioglitazone; IB, ibuprofen. |
Effects of ibuprofen on glomerular
basement membrane thickening and renal fibrosis in the kidney of
diabetic rats
Glomerular basement membrane thickening is a
pathological feature of DN. These changes were present in the
kidneys of the healthy (Fig. 2Aa)
and experimental (Fig. 2Ab–d)
rats, and were visualized by PAS staining. Diabetic rats
demonstrated obvious glomerular basement membrane thickening, which
was characterized by a significant increase in the PAS-stained
positive area, when compared with that of the healthy rats
(P<0.01; Fig. 2B). However,
treatment of the diabetic rats with ibuprofen or pioglitazone
significantly reduced glomerular basement membrane thickening
(P<0.01; Fig. 2B).
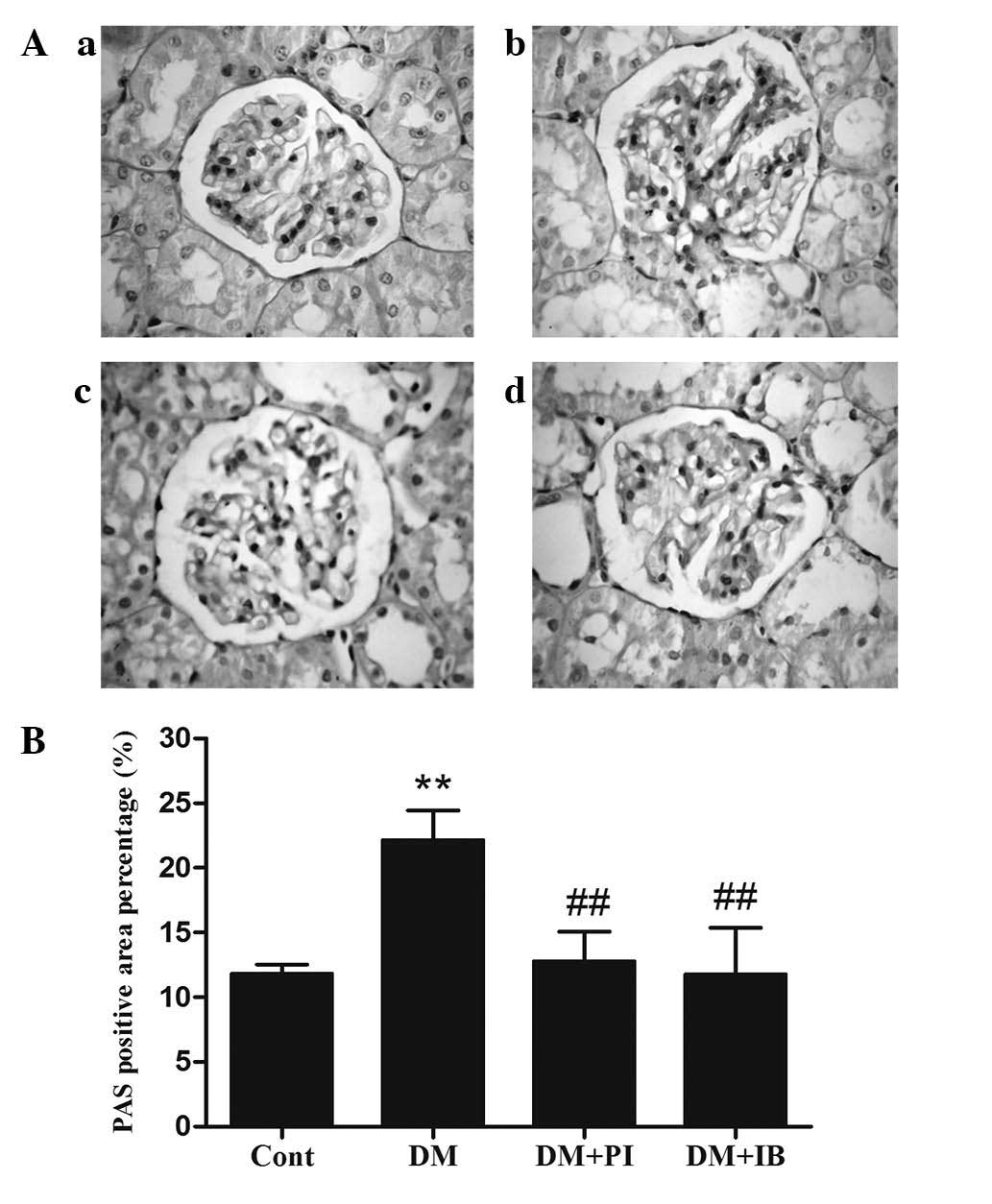 | Figure 2Effects of IB on glomerular basement
membrane thickening in rat kidneys. Glomerular basement membrane
thickening was examined by PAS staining. (A) Typical staining
images (magnification, ×400) of (a) Cont., (b) DM, (c) DM + PI and
(d) DM + IB, and (B) quantitative analysis. The Cont., DM, DM + PI,
and DM + IB groups represent healthy rats, and diabetic rats,
diabetic rats treated with PI (25 mg/kg) and IB (40 mg/kg),
respectively. Data are presented as means ± standard deviation;
n=3. **P<0.01 vs. Cont.; ##P<0.01 vs.
DM. PAS, Periodic acid-Schiff; Cont., control; DM, diabetic group;
PI, pioglitazone; IB, ibuprofen. |
Renal fibrosis is another pathological feature of
DN. Fig. 3 demonstrates the renal
fibrosis of rats from the healthy (Fig. 3Aa) and experimental (Fig. 3Ab–d) groups using Masson's
trichrome staining. Collagen fibers were stained blue, muscle fiber
cytoplasm was stained red and nuclei were stained brown. The
results reveal a marginal quantity of collagen fiber deposition in
the healthy rats (Fig. 3B), but a
significant increase in tubulointerstitial collagen in the diabetic
rats (P<0.01; Fig. 3B).
Diabetic rats that received ibuprofen or pioglitazone treatment
exhibited less tubulointerstitial collagen when compared with the
untreated diabetic rats (P<0.05; Fig. 3B).
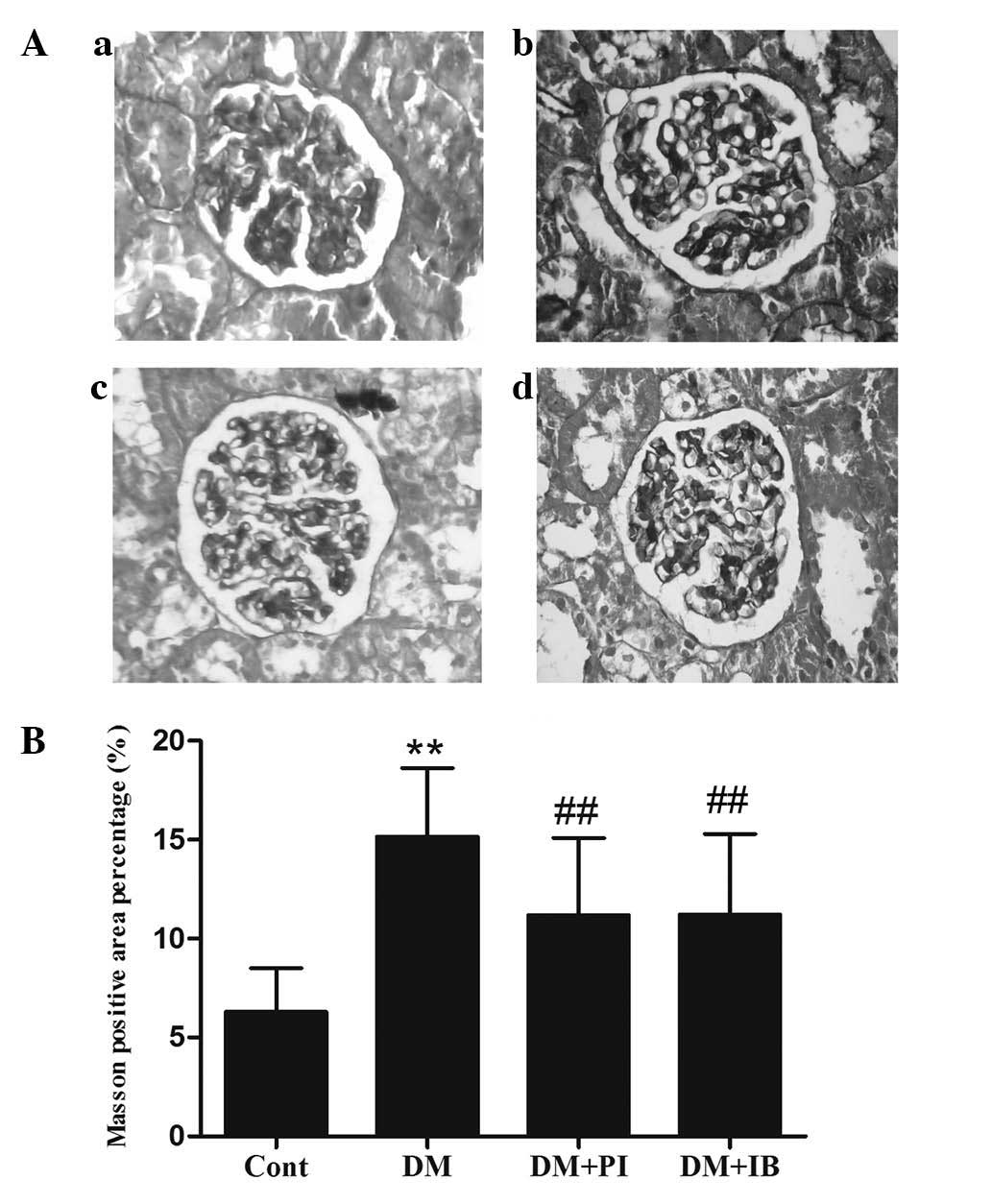 | Figure 3Effects of IB on rat kidney renal
fibrosis, which was assessed by Masson's trichrome staining. (A)
Typical staining images (magnification, ×400) of (a) Cont., (b) DM,
(c) DM + PI and (d) DM + IB, and (B) quantitative analysis. The
Cont., DM, DM + PI, and DM + IB groups represent healthy rats,
diabetic rats, and diabetic rats treated with PI (25 mg/kg) and IB
(40 mg/kg), respectively. Data are presented as means ± standard
deviation (n=3). **P<0.01 vs. Cont.;
##P<0.05 vs. DM. Cont., control; DM, diabetic group;
PI, pioglitazone; IB, ibuprofen. |
Effects of ibuprofen on PPARγ protein
expression in the kidney of diabetic rats
Ibuprofen and pioglitazone activate PPARγ. To
examine the effects of the two therapeutic agents on activation of
PPARγ, the activated form of PPARγ was identified in the kidney
using immunohistochemistry (Fig.
4). The data indicates that the protein expression level of
PPARγ in the cell nuclei was markedly reduced in the kidney of
diabetic rats when compared with that of healthy rats (P<0.01;
Fig. 4B), while chronic ibuprofen
treatment prevented this reduction, with no significant difference
when compared with pioglitazone (Fig.
4B).
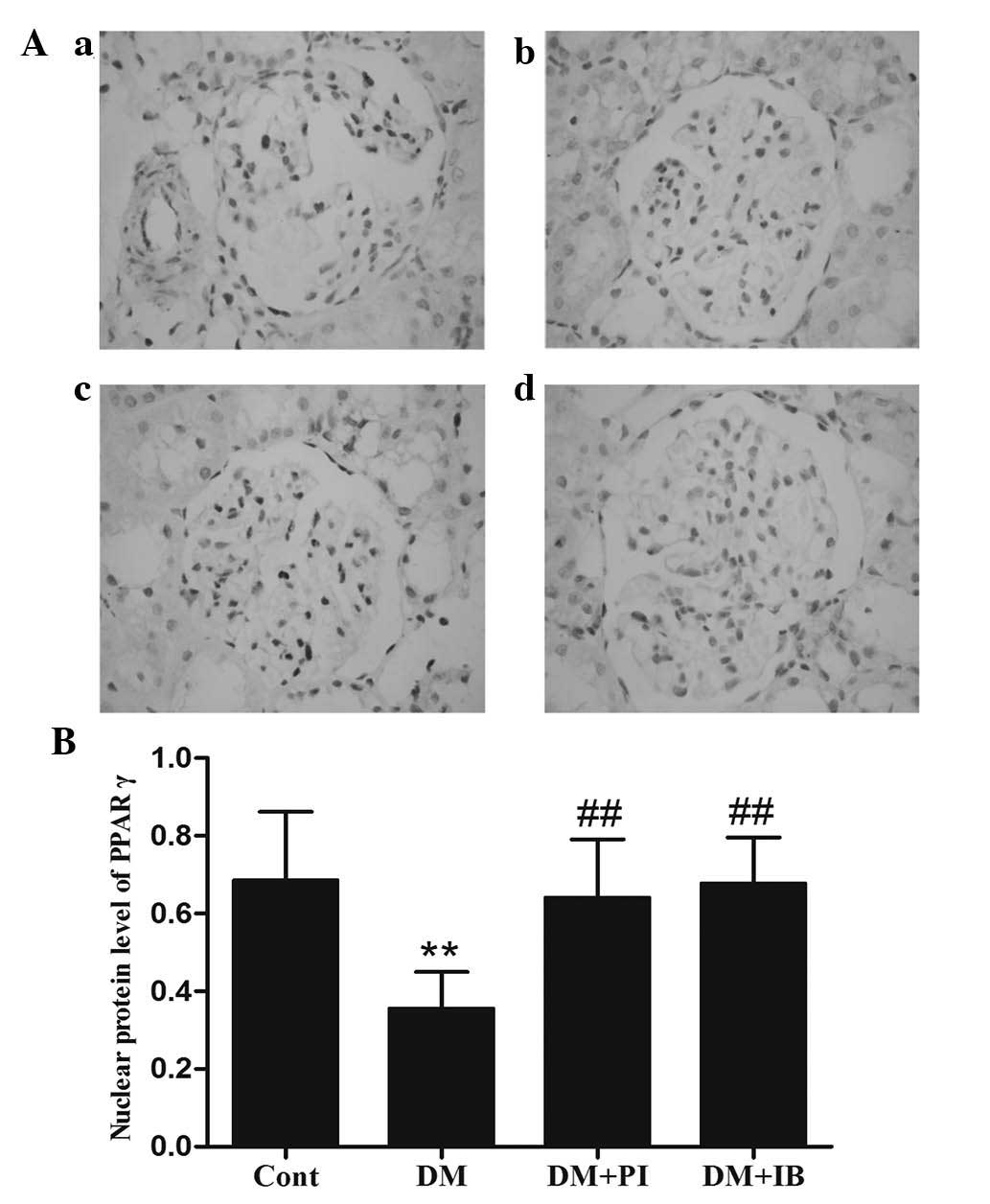 | Figure 4Effects of IB on the activated form
of PPARγ in rat kidneys. Protein expression of PPARγ in the nucleus
was assayed by immunohistochemistry. (A) Typical staining images
(magnification, ×400) of (a) Cont., (b) DM, (c) DM + PI and (d) DM
+ IB, and (B) quantitative analysis. The Cont., DM, DM + PI, and DM
+ IB groups represent healthy rats, diabetic rats, and diabetic
rats treated with PI (25 mg/kg) and IB (40 mg/kg), respectively.
Data are presented as means ± standard deviation; n=3.
**P<0.01 vs. Cont.; ##P<0.01 vs. DM.
PPARγ, peroxisome proliferator-activated receptor γ; Cont.,
control; DM, diabetic group; PI, pioglitazone; IB, ibuprofen. |
Effects of ibuprofen on inflammatory
responses in diabetic rats
The DN rats displayed marked inflammatory responses
in the kidney; protein expressions of COX-2 and iNOS were
significantly increased in the renal cortex of diabetic rats,
compared with those of the healthy rats (P<0.01 and P<0.05,
respectively). Chronic treatment with ibuprofen prevented the
increase of COX-2 and iNOS, while treatment with pioglitazone
significantly decreased the protein expression of iNOS (P<0.01
vs. DM), but not that of COX-2 (Fig.
5A). Furthermore, the level of IL-1β, an important
pro-inflammatory cytokine, was markedly elevated in the renal
cortex and serum (P<0.01) of DN rats compared with that of
healthy rats, which was significantly attenuated following
ibuprofen treatment (P<0.05). By contrast, pioglitazone
treatment prevented the elevated IL-1β level in the serum of DN
rats more effectively (P<0.05) than ibuprofen treatment, and
although PI treatment decreased the IL-1β level in the renal cortex
of DN rats, it exhibited a weaker effect than ibuprofen treatment
(Fig. 5B and C).
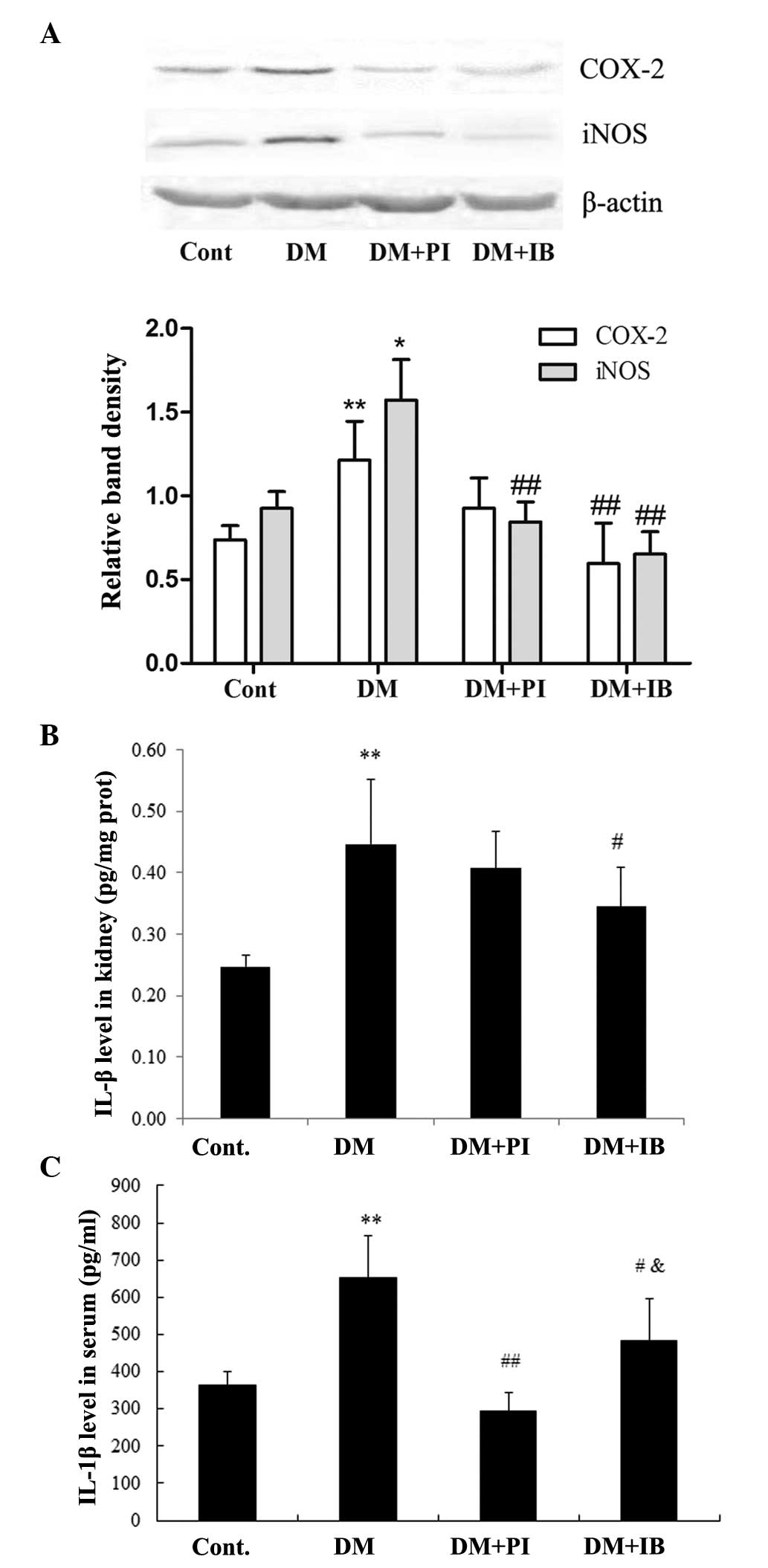 | Figure 5Effects of IB on protein (prot)
expression of COX-2 and iNOS in (A) rat kidneys, and IL-1 levels in
rat (B) kidneys and (C) serum. COX-2 and iNOS protein expression
levels were assayed by western blotting. The IL-1 level was
measured by enzyme linked immunosorbent assay. The Cont., DM, DM +
PI, and DM + IB groups represent healthy rats, diabetic rats, and
diabetic rats treated with PI (25 mg/kg) and IB (40 mg/kg),
respectively. Data are presented as means ± standard deviation; n=4
(COX-2 and iNOS); n=6–8 (IL-1). *P<0.05 and
**P<0.01 vs. Cont.; #P<0.05 and
##P<0.01 vs. DM; &P<0.05 vs. DM +
PI group. COX-2, cyclooxygenase 2; iNOs, inducible nitric oxide
synthase; IL-1, interleukin-1; Cont., control; DM, diabetic group;
PI, pioglitazone; IB, ibuprofen. |
Effects of ibuprofen on anti-oxidative
function in diabetic rats
In addition to inflammatory responses, oxidative
stress damage in DN rats was observed. Diabetes induced significant
decreases in SOD activity (Fig. 6)
and GSH level (Fig. 7) in the
renal cortex of rats compared with healthy rats (P<0.01 and
P<0.05, respectively), which were alleviated by chronic
treatment with ibuprofen and pioglitazone (Figs. 6A and 7A), with no marked difference between the
two. Furthermore, SOD activity and GSH level were markedly reduced
in the serum of diabetic rats (P<0.01), while chronic treatment
with ibuprofen enhanced SOD activity and the GSH level (P<0.01;
Figs. 6B and 7B). Pioglitazone treatment had a similar
effect on SOD activity, but a weaker effect on GSH level
(P<0.05) when compared with ibuprofen treatment (Figs. 6B and 7B).
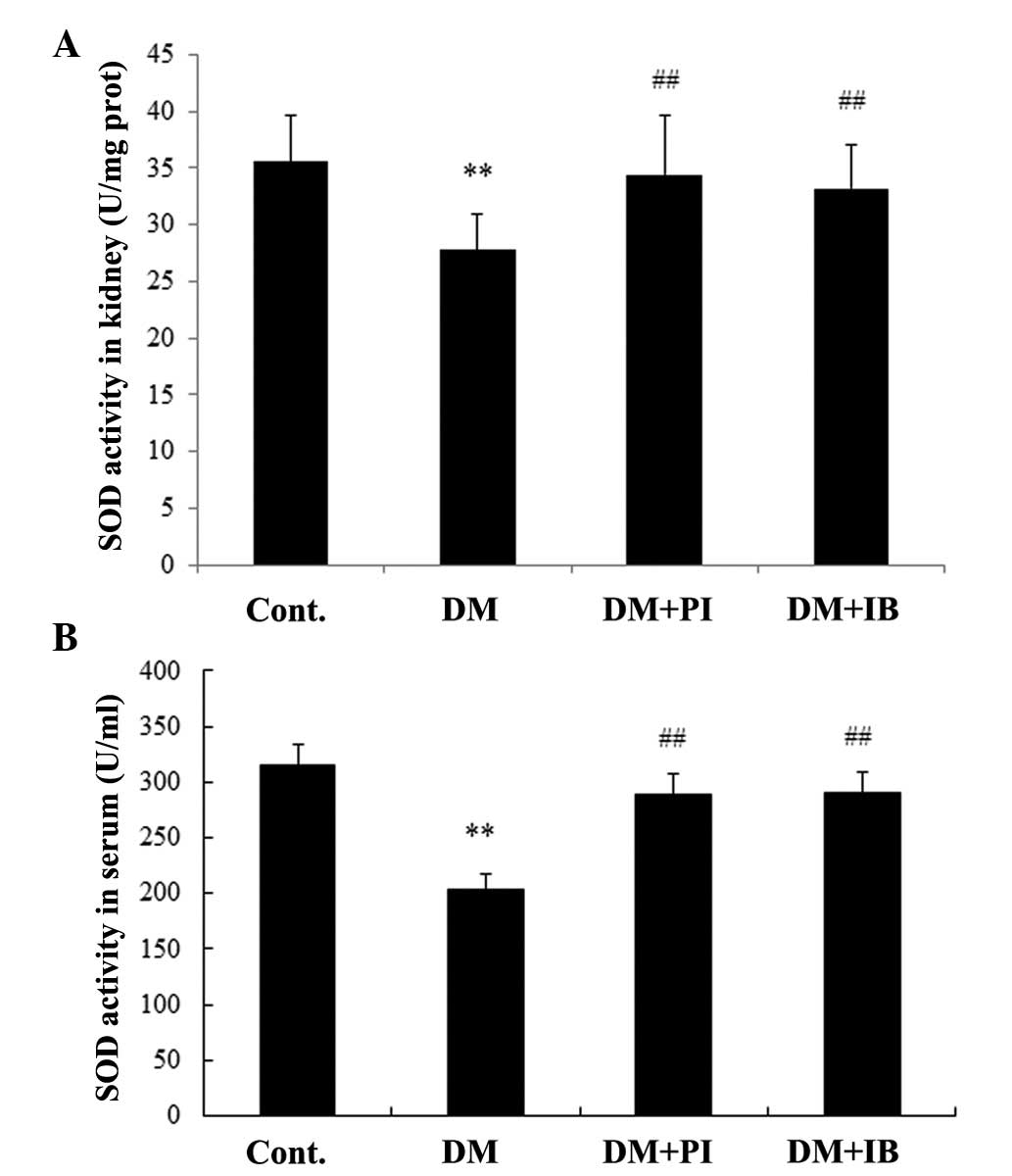 | Figure 6Effects of IB on SOD activity in rat
(A) kidneys and (B) serum. SOD activity was measured using xanthine
oxidase (the hydroxylamine method). The Cont., DM, DM + PI, and DM
+ IB groups represent healthy rats, diabetic rats, and diabetic
rats treated with PI (25 mg/kg) and IB (40 mg/kg), respectively.
Data are presented as means ± standard deviation; n=7–8.
**P<0.01 vs. Cont.; ##P<0.01 vs. DM.
SOD, superoxide dismutase; Cont., control; DM, diabetic group; PI,
pioglitazone; IB, ibuprofen. |
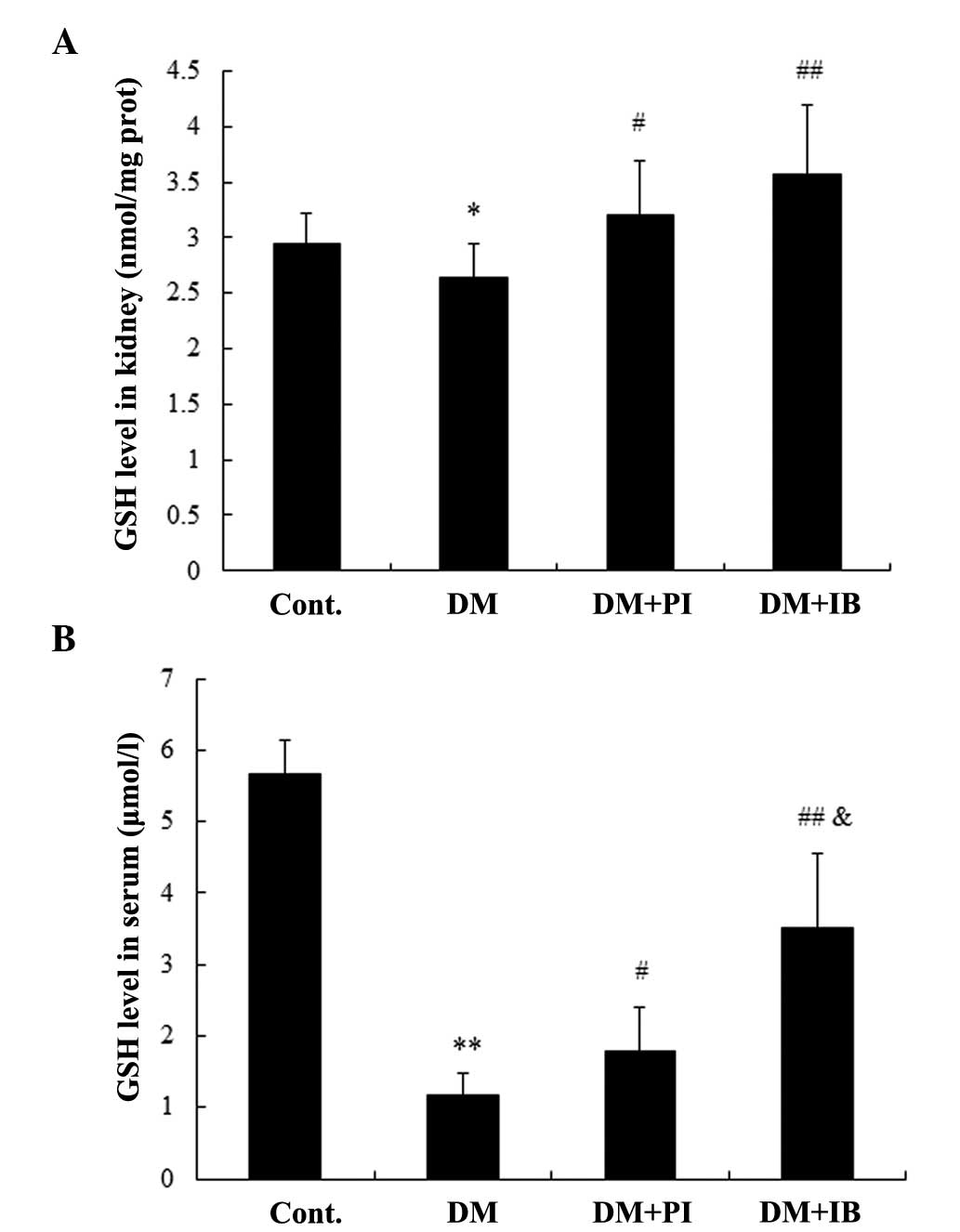 | Figure 7Effects of IB on reduced GSH levels
in rat (A) kidneys and (B) serum. The GSH level was measured by
spectrophotometry. Cont., DM, DM + PI, and DM + IB groups represent
healthy rats, diabetic rats, and diabetic rats treated with PI (25
mg/kg) and IB (40 mg/kg), respectively. Data are presented as means
± standard deviation; n=7–8. *P<0.05 and
**P<0.01 vs. Cont.; #P<0.05 and
##P<0.01 vs. DM; &P<0.05 vs. DM +
PI. GSH, glutathione; Cont., control; DM, diabetic group; PI,
pioglitazone; IB, ibuprofen. |
Discussion
Pathogenesis of DN is complex and multifactorial,
involving different cell types, molecules, and factors, in
particular oxidative stress and inflammatory cytokines (16). The present study demonstrated
significant elevations of urinary protein excretion and BUN level,
as well as glomerular basement membrane thickening and renal
fibrosis in type 1 diabetic rats over a nine-week period. Diabetes
produced large quantities of COX-2, iNOS, and IL-1β protein, but
small quantities of PPARγ protein, SOD activity, and GSH levels in
the kidney of rats. In addition, high IL-1β levels, and low SOD
activity and GSH levels were observed in the serum of diabetic
rats. However, the majority of the changes were markedly attenuated
by treatment with ibuprofen and pioglitazone, which served as a
positive control. These data indicated that ibuprofen prevents the
progression of DN in an experimental rat model of type 1 diabetes,
via suppression of inflammatory and oxidative damage, potentially
through PPARγ activation.
Chronic inflammation is significant in the
development of diabetes and its late complications, including DN
(28–30). Inflammatory markers are well known
to be associated with the development of renal disease in diabetes
(31). Anti-inflammatory
therapeutic agents have been reported to prevent renal injury in
diabetic rats by reducing macrophage infiltration and the protein
expression of transforming growth factor-β, type IV collagen and
intercellular adhesion molecule 1 in renal cells (32). Furthermore, chronic COX inhibition
by NSAIDs (non-selective COX inhibitor, ibuprofen and selective
COX-2 inhibitor, NS-398) reduced diabetes-induced hyperfiltration,
proteinuria and fibrosis in the kidney of Akita DN mice (33); with the effects of ibuprofen
observed to be similar to, if not more beneficial than, COX-2
inhibition by NS-398. In the present study, chronic ibuprofen
treatment at a low dose (40 mg/kg) attenuated proteinuria and renal
fibrosis of diabetic rats, which was coupled with the reduced
protein expression of COX-2 and iNOS (common inducible proteins in
the presence of inflammation) in the renal cortex. In a previous
study, IL-1 was hypothesized to increase vascular permeability,
mesangial cell proliferation and extracellular matrix deposition,
as well as glomerular basement membrane thickening (34). The present study identified that
glomerular basement membrane thickening occurred in the kidney of
diabetic rats, with increased IL-1β levels observed in the serum
and renal cortex. However, ibuprofen prevented glomerular basement
membrane thickening, and decreased the IL-1β level in the blood and
kidney. The above-mentioned studies and the results of the present
study suggest that anti-inflammatory therapy alleviates functional
and morphological impairments of diabetic animals. A previous study
demonstrated that PPARγ agonists attenuated renal injury and
inflammation in a mouse model of unilateral ureteral obstruction
(35). Ohga et al (6) reported that pioglitazone ameliorated
renal injury through the inhibition of nuclear factor
κ-light-chain-enhancer of activated B cells activation,
intercellular adhesion molecule 1 expression and macrophage
infiltration in STZ-induced diabetic rats. The present findings
showed that ibuprofen, a typical NSAID and partial agonist of
PPARγ, decreased the level of inflammatory markers in the renal
cortex, and elevated the activated PPARγ level in the kidneys of
diabetic rats. These results demonstrated that the
anti-inflammatory effect, possibly via PPARγ activation, was
responsible for the alleviation of DN by ibuprofen in type 1
diabetes.
In addition to chronic inflammation, oxidative
stress was hypothesized to be vital in the pathological process of
chronic diabetic complications, including DN (36,37).
A unifying hypothesis has been proposed, where over-production of
reactive oxygen species in mitochondria, in response to chronic
hyperglycemia, may be the key initiator of various pathogenic
pathways in diabetic complications (38). Gumieniczek (39) reported that GSH reductase activity
and the GSH level were diminished in the kidney of alloxan-induced
diabetes in rabbits, and pioglitazone restored them to the control
values. In the current study, SOD activity and GSH level, two
important endogenous antioxidants, were significantly declined in
the renal cortex and serum of STZ-induced diabetes in rats.
Furthermore, oxidative stress and renal fibrosis in the kidney of
STZ-induced diabetes in mice have previously been associated with
decreased protein expression of PPARγ and its coactivator PGC-1α,
which may be attenuated by telmisartan (18). The present findings indicated that
ibuprofen and pioglitazone relieved the oxidative damage in the
kidney of diabetic rats, in combination with a marked increase in
protein expression of nuclear PPARγ in the kidney. Notably, a
previous study indicated that ibuprofen disrupted the signaling
cascades that lead to microglial nicotinamide adenine dinucleotide
phosphate-oxidase activation independently of COX inhibition,
preventing oxidative damage in the brain (40). These results indicate that
enhancement of anti-oxidation function, potentially via PPARγ
activation, contributes to the renal protection of ibuprofen in
type 1 diabetes.
Although in the current study ibuprofen or
pioglitazone treatment did not influence the nFBG level and the
body weight of diabetic rats, ibuprofen and pioglitazone showed
significant attenuation on renal function and morphological changes
in diabetic rats. There have been similar studies regarding these
effects of PPARγ agonist; in experimental models of diabetes, PPARγ
agonists have been shown to attenuate renal damage and reduce
albuminuria, independent to blood glucose lowering (41). Previous studies have indicated that
a low dose of pioglitazone ameliorated renal fibrosis and preserved
renal function in an animal model of metabolic syndrome,
independently of hyperglycemic control or effects on body weight
(12,42). A recent review demonstrated that
PPARγ agonist-mediated renal protection resulted from numerous
other efficacies beyond the glucose lowering effect (43). Together, these findings further
confirmed the key role of anti-inflammatory and anti-oxidative
actions of ibuprofen via PPARγ activation in the prevention and
treatment of type 1 diabetes-induced nephropathy.
In conclusion, the present study demonstrated that
ibuprofen markedly attenuated the functional and morphological
changes in the kidney of rats with STZ-induced type 1 diabetes,
which was realized by anti-inflammatory and anti-oxidative action,
potentially via COX-2 suppression and PPARγ activation, suggesting
that ibuprofen may serve as a multi-target therapeutic agent for
DN.
Acknowledgments
The present study was funded by the China
Postdoctoral Science Foundation (grant no. 201150M1576), the Zhen
Xing Project of XZMC, and a project funded through the Priority
Academic Program Development of Jiangsu Higher Education
Institutions.
References
|
1
|
Biesenbach G: Highest mortality during the
last year before and the first year after start of dialysis
treatment in type 2 diabetic patients with nephropathy. Curr
Diabetes Rev. 3:123–126. 2007. View Article : Google Scholar
|
|
2
|
Schernthaner G: Kidney disease in
diabetology: Lessons from 2007. Nephrol Dial Transplant.
23:1112–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonson MS: Phenotypic transitions and
fibrosis in diabetic nephropathy. Kidney Int. 71:846–854. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qian Y, Feldman E, Pennathur S, Kretzler M
and Brosius FC III: From fibrosis to sclerosis: Mechanisms of
glomerulosclerosis in diabetic nephropathy. Diabetes. 57:1439–1445.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swaminathan S and Shah SV: Novel
approaches targeted toward oxidative stress for the treatment of
chronic kidney disease. Curr Opin Nephrol Hypertens. 17:143–148.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohga S, Shikata K, Yozai K, Okada S, Ogawa
D, Usui H, Wada J, Shikata Y and Makino H: Thiazolidinedione
ameliorates renal injury in experimental diabetic rats through
anti-inflammatory effects mediated by inhibition of NF-kappaB
activation. Am J Physiol Renal Physiol. 292:F1141–1150. 2007.
View Article : Google Scholar
|
|
7
|
Willson TM, Lambert MH and Kliewer SA:
Peroxisome proliferator-activated receptor gamma and metabolic
disease. Annu Rev Biochem. 70:341–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baylis C, Atzpodien EA, Freshr G and
Engels K: Peroxisome proliferator-activated receptor [gamma]
agonist provides superior renal protection versus
angiotensin-converting enzyme inhibition in a rat model of type 2
diabetes with obesity. J Pharmacol Exp Ther. 307:854–860. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Zhang D, Li J, Zhang X, Fan F and
Guan Y: Role of PPARgamma in renoprotection in Type 2 diabetes:
Molecular mechanisms and therapeutic potential. Clin Sci (Lond).
116:17–26. 2009. View Article : Google Scholar
|
|
10
|
Yang J, Zhou Y and Guan Y: PPARgamma as a
therapeutic target in diabetic nephropathy and other renal
diseases. Curr Opin Nephrol Hypertens. 21:97–105. 2012. View Article : Google Scholar
|
|
11
|
Hu W, Yu Q, Zhang J and Liu D:
Rosiglitazone ameliorates diabetic nephropathy by reducing the
expression of Chemerin and ChemR23 in the kidney of
streptozotocin-induced diabetic rats. Inflammation. 35:1287–1293.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arora MK, Reddy K and Balakumar P: The low
dose combination of fenofibrate and rosiglitazone halts the
progression of diabetes-induced experimental nephropathy. Eur J
Pharmacol. 636:137–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Groop PH, Thomas MC, Moran JL, Wadèn J,
Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K,
Heikkilä O, et al: The presence and severity of chronic kidney
disease predicts all-cause mortality in type 1 diabetes. Diabetes.
58:1651–1658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fogo AB: PPARgamma and chronic kidney
disease. Pediatr Nephrol. 26:347–351. 2011. View Article : Google Scholar
|
|
15
|
Iglesias P and Díez JJ: Peroxisome
proliferator-activated receptor gamma agonists in renal disease.
Eur J Endocrinol. 154:613–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012. View Article : Google Scholar
|
|
17
|
Gumieniczek A: Effect of the new
thiazolidinedione-pioglitazone on the development of oxidative
stress in liver and kidney of diabetic rabbits. Life Sci.
74:553–562. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lakshmanan AP, Watanabe K, Thandavarayan
RA, Sari FR, Harima M, Giridharan VV, Soetikno V, Kodama M and
Aizawa Y: Telmisartan attenuates oxidative stress and renal
fibrosis in streptozotocin induced diabetic mice with the
alteration of angiotensin-(1–7) mas receptor expression associated
with its PPAR-gamma agonist action. Free Radic Res. 45:575–584.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsui T, Yamagishi S, Ueda S, Nakamura K,
Imaizumi T, Takeuchi M and Inoue H: Telmisartan, an angiotensin II
type 1 receptor blocker, inhibits advanced glycation end-product
(AGE)-induced monocyte chemoattractant protein-1 expression in
mesangial cells through downregulation of receptor for AGEs via
peroxisome proliferator-activated receptor-gamma activation. J Int
Med Res. 35:482–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui T, Yamagishi S, Takeuchi M, Ueda S,
Fukami K and Okuda S: Nifedipine inhibits advanced glycation end
products (AGEs) and their receptor (RAGE) interaction-mediated
proximal tubular cell injury via peroxisome proliferator-activated
receptor-gamma activation. Biochem Biophys Res Commun. 398:326–330.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohamed R, Jayakumar C, Ranganathan PV,
Ganapathy V and Ramesh G: Kidney proximal tubular
epithelial-specific overexpression of netrin-1 suppresses
inflammation and albuminuria through suppression of COX-2-mediated
PGE2 production in streptozotocin-induced diabetic mice. Am J
Pathol. 181:1991–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaradat MS, Wongsud B, Phornchirasilp S,
Rangwala SM, Shams G, Sutton M, Romstedt KJ, Noonan DJ and Feller
DR: Activation of peroxisome proliferator-activated receptor
isoforms and inhibition of prostaglandin H(2) synthases by
ibuprofen, naproxen and indomethacin. Biochem Pharmacol.
62:1587–1595. 2001. View Article : Google Scholar
|
|
23
|
Couto M: Laboratory guidelines for animal
care. Methods Mol Biol. 770:579–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haidara MA, Mikhailidis DP, Rateb MA,
Ahmed ZA, Yassin HZ, Ibrahim IM and Rashed LA: Evaluation of the
effect of oxidative stress and vitamin E supplementation on renal
function in rats with streptozotocin-induced Type 1 diabetes. J
Diabetes Complications. 23:130–136. 2009. View Article : Google Scholar
|
|
25
|
Wybenga DR, Di Giorgio J and Pileggi VJ:
Manual and automated methods for urea nitrogen measurement in whole
serum. Clin Chem. 17:891–895. 1971.PubMed/NCBI
|
|
26
|
Liu YW, Zhu X, Zhang L, Lu Q, Wang JY,
Zhang F, Guo H, Yin JL and Yin XX: Up-regulation of glyoxalase 1 by
mangiferin prevents diabetic nephropathy progression in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 721:355–364.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YW, Zhu X, Yang QQ, Lu Q, Wang JY, Li
HP, Wei YQ, Yin JL and Yin XX: Suppression of methylglyoxal
hyperactivity by mangiferin can prevent diabetes-associated
cognitive decline in rats. Psychopharmacology (Berl). 228:585–594.
2013. View Article : Google Scholar
|
|
28
|
Chow FY, Nikolic-Paterson DJ, Atkins RC
and Tesch GH: Macrophages in streptozotocin-induced diabetic
nephropathy: Potential role in renal fibrosis. Nephrol Dial
Transplant. 19:2987–2996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nguyen D, Ping F, Mu W, Hill P, Atkins RC
and Chadban SJ: Macrophage accumulation in human progressive
diabetic nephropathy. Nephrology (Carlton). 11:226–231. 2006.
View Article : Google Scholar
|
|
30
|
Rivero A, Mora C, Muros M, García J,
Herrera H and Navarro-González JF: Pathogenic perspectives for the
role of inflammation in diabetic nephropathy. Clin Sci (Lond).
116:479–492. 2009. View Article : Google Scholar
|
|
31
|
Tuttle KR: Linking metabolism and
immunology: Diabetic nephropathy is an inflammatory disease. J Am
Soc Nephrol. 16:1537–1538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yozai K, Shikata K, Sasaki M, Tone A, Ohga
S, Usui H, Okada S, Wada J, Nagase R, Ogawa D, et al: Methotrexate
prevents renal injury in experimental diabetic rats via
anti-inflammatory actions. J Am Soc Nephrol. 16:3326–3338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nasrallah R, Robertson SJ and Hébert RL:
Chronic COX inhibition reduces diabetes-induced hyperfiltration,
proteinuria and renal pathological markers in 36-week
B6-Ins2(Akita) mice. Am J Nephrol. 30:346–353. 2009. View Article : Google Scholar
|
|
34
|
Elmarakby AA, Abdelsayed R, Yao Liu J and
Mozaffari MS: Inflammatory cytokines as predictive markers for
early detection and progression of diabetic nephropathy. EPMA J.
1:117–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawai T, Masaki T, Doi S, Arakawa T,
Yokoyama Y, Doi T, Kohno N and Yorioka N: PPAR-gamma agonist
attenuates renal interstitial fibrosis and inflammation through
reduction of TGF-beta. Lab Invest. 89:47–58. 2009. View Article : Google Scholar
|
|
36
|
Ha H and Lee HB: Oxidative stress in
diabetic nephropathy: Basic and clinical information. Curr Diab
Rep. 1:282–287. 2001. View Article : Google Scholar
|
|
37
|
Forbes JM, Coughlan MT and Cooper ME:
Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes. 57:1446–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gumieniczek A: Effects of pioglitazone on
hyperglycemia-induced alterations in antioxidative system in
tissues of alloxan-treated diabetic animals. Exp Toxicol Pathol.
56:321–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilkinson BL, Cramer PE, Varvel NH,
Reed-Geaghan E, Jiang Q, Szabo A, Herrup K, Lamb BT and Landreth
GE: Ibuprofen attenuates oxidative damage through NOX2 inhibition
in Alzheimer's disease. Neurobiol Aging. 33:197.e21–32. 2012.
View Article : Google Scholar
|
|
41
|
Isshiki K, Haneda M, Koya D, Maeda S,
Sugimoto T and Kikkawa R: Thiazolidinedione compounds ameliorate
glomerular dysfunction independent of their insulin-sensitizing
action in diabetic rats. Diabetes. 49:1022–1032. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toblli JE, Ferrini MG, Cao G, Vernet D,
Angerosa M and Gonzalez-Cadavid NF: Antifibrotic effects of
pioglitazone on the kidney in a rat model of type 2 diabetes
mellitus. Nephrol Dial Transplant. 24:2384–2391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sugawara A, Uruno A, Kudo M, Matsuda K,
Yang CW and Ito S: PPARγ agonist beyond glucose lowering effect.
Korean J Intern Med. 26:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|





















