Introduction
Sepsis and septic shock, which are caused by
infection with gram-negative and gram-positive bacteria, fungi,
viruses and parasites, have become increasingly important over the
past few decades. Septic shock is a complex pathophysiological
process, which is characterized by low blood pressure, systemic
inflammatory reaction and multiple organ dysfunction. Patients
suffering from sepsis utilize numerous medical resources, and the
mortality rate is high and continuing to rise (1). The heart is considered a main 'target
organ' of sepsis-induced multiple organ dysfunction, and the
occurrence of myocardial dysfunction further promotes the
development and deterioration of sepsis, which significantly
contributes to the mortality of patients with septic shock
(2). The pathogenesis of sepsis is
very complex, and to date, studies regarding septic shock have
concentrated on the generation of inflammatory mediators, including
tumor necrosis factor (TNF)-α and interleukin-1β, and damage to the
liver, lungs and kidneys (3–6). In
addition, previous studies have demonstrated that the heart is a
potential site for the generation of inflammatory mediators
(7,8). Excessive production and release of
proinflammatory mediators has an important role in the process of
sepsis-induced myocardial dysfunction (SIMD) (9). Excessive production of TNF-α,
activation of the p38-mitogen-activated protein kinase (MAPK)
pathway, and TNF-α-mediated apoptosis have been reported to have an
important role in the progression of SIMD; however, the mechanism
by which numerous toxic products are released due to the generation
and release of inflammatory mediators and biological active
substances has yet to be elucidated. Our previous study detected
marked inflammatory damage in the myocardial tissue of a rat model
of sepsis (10). In addition,
alterations to nuclear factor (NF)-κB activity are closely
associated with the production and release of inflammatory
mediators in sepsis. The TNF-α/p38-MAPK/caspase-3 pathway has also
been shown to be involved in the pathophysiological process of
myocardial depression and myocardial cell apoptosis (11). These results suggested that
inhibition of the expression of NF-κB, the production of TNF-α, and
activation of the TNF-α/p38-MAPK/caspase-3 signaling pathway may
have a positive role in the treatment of SIMD.
Oxymatrine (OMT) is an alkaloid extracted from
Sophora flavescens. OMT exhibits various biological
activities, and its anti-inflammatory effects have been reported in
experimental animal models and clinical studies (12,13).
It has previously been demonstrated that OMT exerts inhibitory
effects on Escherichia coli, Staphylococcus aureus,
Salmonella typhimurium, Streptococcus agalactiae and
Pasteurella multocida, and its effects are positively
correlated with concentration (14). Furthermore, it has been reported
that OMT may reduce the generation of oleic acid in a rat model of
acute lung injury by inhibiting the p38-MAPK signaling pathway and
the expression of TNF-α (15). In
addition, by regulating the expression of lipopolysaccharide (LPS)
recognition receptors [cluster of differentiation (CD)14 and
scavenger receptor-A], OMT is able to reduce endotoxin-induced
pathological lung damage in mice (16). However, it remains unclear whether
OMT is able to prevent myocardial apoptosis associated with
infectious shock by inhibiting activation of the
TNF-α/p38-MAPK/caspase-3 signaling pathway. The present study used
a cecal ligation and puncture (CLP) rat model to examine the
effects of OMT on inhibition of the TNF-α/p38-MAPK/caspase-3
signaling pathway in response to septic shock-induced cardiac
muscle injury.
Materials and methods
Chemicals and reagents
OMT was purchased from Ningxia Qi Yuan
Pharmaceutical Co., Ltd. (Yinchuan, China). The terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit was
obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Immunoprecipitation cell lysis buffer and the bicinchoninic acid
(BCA) protein concentration assay kit were purchased from Jiangsu
Green Biotechnology Co., Ltd. (Xuzhou, China). The 125I
TNF-α radioimmunoassay kit was obtained from Beijing Chemclin
Biotech Co., Ltd. (Beijing, China). TRIzol® was
purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Antibodies targeting lipopolysaccharide binding protein
(LBP; goat polyclonal; cat. no. sc-70072), CD14 (goat polyclonal;
cat. no. sc-5749), NF-κB (rabbit poly-clonal; cat. no. sc-372),
phosphorylated (p)-NF-κB inhibitor (IκB)-α (goat polyclonal; cat.
no. sc-7977), p-p38-MAPK (mouse monoclonal; cat. no. sc-7973) and
caspase-3 (mouse monoclonal; cat. no. sc-7272)were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-β-actin
(cat. no. A4700) was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Animals
The animal experimental procedure used in the
present study was approved by the Ningxia Medical University Animal
Care Committee (Yinchuan, China). Male Sprague-Dawley
specific-pathogen-free rats [Animal Center, Ningxia Medical
University, Yinchuan, China; SCXK (Ning) 2005-001], weighing
200–250 g, were randomly divided into six groups (n=8/group): Sham
operation (CON) group, OMT control group, CLP model group, and CLP
+ OMT (high dose, 52 mg/kg; medium dose, 26 mg/kg; low dose, 13
mg/kg) groups. The septic shock model was induced by CLP, as
previously described (17). The
rats were maintained in light- and temperature-controlled
conditions (12 h light/dark cycle; 22±2°C) and were given ad
libitum access to food and water. Briefly, rats were
anesthetized with 40 mg/kg pentobarbital (2%; Sigma-Aldrich) by
intraperitoneal injection. A 2–3 cm midventral abdominal incision
was made to expose the intestines. The ileocecal valve was ligated
with 3–0 silk and three perforations were made in the cecum using a
20-gauge needle. To ensure consistent cecal damage among the
animals, the perforated cecum was squeezed until 50–80 µl
feces extruded onto both surfaces, the bowel was reinserted into
the abdomen and the incision was closed. In the sham group, the
abdomen was opened to expose the intestines, and then closed. A
single post-operative saline bolus was provided (30 ml/kg
subcutaneous) for fluid support. Caudal artery blood pressure was
monitored; once it had decreased to 2/3 basic blood pressure and
<20 mmHg, the model was considered successful. Rats received the
drugs via tail vein injection (volume, 5 ml/kg). In the sham and
OMT control groups, rats received intravenous injection of normal
saline and OMT (26 ml/kg), respectively, and the CLP group received
normal saline (26 ml/kg). After treatment, the cecum was exposed
under sterile conditions. The rats' tail artery pressure dropped to
2/3 of the baseline blood pressure and pulse pressure was reduced
to <20 mmHg as a judgment of sepsis model standards.
Cardiac function and histological
analyses
To examine the cardiac function of mice in response
to sepsis, the right common carotid artery was used to determine
heart rate (HR), mean arterial pressure (MAP), left
intraventricular pressure change rate (LVdp/dtmax), left
ventricular end systolic pressure (LVESP) and left ventricular end
diastolic pressure (LVEDP) using cardiac catheterization. Rats were
anesthetized with sodium pentobarbital (40 mg/kg, i.p.), and were
then sacrificed by cervical dislocation. Subsequently, the heart
was removed for histological analyses. Apical tissue blocks (~2
mm3) were collected, fixed in 10% formalin, and embedded
in paraffin. After staining with hematoxylin and eosin,
pathological alterations to the myocardial tissue were observed
under a light microscope (CHC-212; Olympus Corporation, Tokyo,
Japan). Other myocardial tissues (~2 mm3) were placed in
2% glutaraldehyde, and were sectioned as electron microscopy
specimens, in order to observe ultrastructural changes.
RT-PCR analysis
Cardiac tissues (~100 mg) were collected and
homogenized using a Polytron PT1200E (Kinematica, Shanghai, China).
Total RNA was extracted using TRIzol® reagent. The PCR
reaction volume was 25 µl, and RT-PCR was conducted using
the Promega One-Step RT-PCR kit (Promega Corporation, Madison, WI,
USA). According to the protocol, 5 µl DEPC water, 5
µl AMV/TfI 5X buffer, 0.5 µl dNTP mixture, 1
µl 25 mM MgSO4, 0.5 µl Tf1 DNA polymerase,
0.5 µl AMV-RT, 12.5 µl Master Mix, 2.5 µl
upstream primer (50 pmol), 2.5 µl downstream primer (50
pmol) and RNA (0.5–1 µg) were mixed in a microcentrifuge
tube. RT-PCR was performed using GeneAmp 9700 thermal cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). First strand cDNA synthesis was conducted as follows: 1 cycle
at 48°C for 45 min for RT, 1 cycle at 94°C for 2 min for AMV-RT
inactivation and RNA/cDNA/primer denaturation. Second strand cDNA
synthesis and PCR amplification were conducted as follows: 35
cycles at 94°C for 30 sec for denaturation, annealing at the
primer-specific temperatures for 1 min, 68°C for 2 min for
extension, and 1 cycle at 68°C for 7 min for final extension.
β-actin was used as an internal control. The primers and annealing
temperatures used are listed in Table
I. Primers were designed using Primer 5.0 software (Premier
Biosoft, Palo Alto, CA, USA), and were synthesized by Baisheng
Company (Beijing, China). The PCR products were analyzed by 2%
agarose gel electrophoresis using a Gel Doc™ EZ system purchased
from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). The results
were semi-quantified and expressed as relative optical density x
surface area (mm2). Relative mRNA expression levels were
expressed as optical density normalized to β-actin.
 | Table IPrimer sequences, annealing
temperature and product size of LBP, CD14, NF-κB (p65), TNF-α,
p38-MAPK, caspase-3 and β-actin genes. |
Table I
Primer sequences, annealing
temperature and product size of LBP, CD14, NF-κB (p65), TNF-α,
p38-MAPK, caspase-3 and β-actin genes.
| Gene | Sequences | Size (bp) | Annealing temperature
(°C) |
|---|
| LBP | F
5′ACTACAGTTTGGTGGCG3′ | 500 | 55.3 |
| R
5′TTGTTGAAAGTTATTGAGGC3′ | | |
| CD14 | F
5′ACATCTTGAACCTCCGCAAC3′ | 500 | 59.2 |
| R
5′AGGGTTCCTATCCAGCCTGT3′ | | |
| NF-κB (p65) | F
5′TGATGTGCATCAAGTGG3′ | 296 | 58 |
| R
5′GAAGTTGAGTTTCGGGTAGGC3′ | | |
| TNF-α | F
5′CAATGGCATGGATCTCAAAG3′ | 355 | 60 |
| R
5′CAGAGCAATGACTCCAAAGT3′ | | |
| p38-MAPK | F
5′CGTTGTTTCCTGGTACAGACC3′ | 430 | 58 |
| R
5′CCATTTCTICTTGGTCAAGGG3 | | |
| Caspase-3 | F
5′ATGGACAACAACGAAACCTCCGTG3′ | 277 | 56 |
| R
5′CCACTCCCAGTCATTCCTTTAGTG3′ | | |
| β-actin | F
5′AGGTGAGAGGGAAATCGTGCG3′ | 662 | 55 |
| R
5′CCACTCCCAGTCATTCCTTTAGTG3′ | | |
Western blotting
Cardiac tissues (~100 mg) were collected and
homogenized in radioimmunoprecipitation assay buffer (Biovision,
Inc., Wuhan, China). Total protein concentration was measured using
the BCA kit. Equal amounts of protein (40 µg) were separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
and were then transferred to 0.45 µm polyvinylidene
difluoride membranes (KeHaoJia Company, Beijing, China). Blots were
soaked in blocking buffer (5% non-fat milk) and were then incubated
with the various primary antibodies (LBP, 1:200; anti-CD14, 1:200;
NF-κB, 1:500; p-IκB-α, 1:200; p-p38-MAPK, 1:300; caspase-3, 1:300;
β-actin, 1:400) at 4°C overnight. After thorough washing with
Tris-buffered saline containing 0.1% Tween 20, the membranes were
incubated with horseradish peroxidase-conjugated anti-goat (cat.
no. BA1060), anti-rabbit (cat. no. BA1054) and anti-mouse (cat. no.
BA1051) secondary antibodies (1:10,000; Wuhan Boster Biological
Technology Co., Ltd. (Wuhan, China), and immune complexes were
visualized using an enhanced chemiluminescence detection system
(Pierce Biotechnology, Inc., Rockford, IL, USA). The results were
analyzed using a Gel Doc™ EZ system from Bio-Rad Laboratories, Inc.
Expression levels were semi-quantified relative to the optical
density of β-actin.
Electron microscopy
Apical sections of rat myocardial tissue (~2
mm3) were collected and fixed in 2% glutaraldehyde for
electron microscopy (H-600; Hitachi, Tokyo, Japan) in order to
observe ultrastructural alterations to the myocardial tissue.
Radioimmunoassay
The levels of TNF-α in the myocardial tissue were
determined by radioimmunoassay, according to the manufacturer's
protocol. Myocardial tissue (100 mg) was mixed with a threefold
volume of phosphate-buffered saline and was homogenized.
Subsequently, the homogenate was centrifuged at 35,616 × g for 20
min at 4°C, and the protein levels of TNF-α in the supernatant were
quantified using the radioimmunoassay assay kit.
TUNEL assay
The TUNEL assay was conducted according to the
manufacturer's protocol. Briefly, myocardial tissue was fixed in 4%
paraformaldehyde for 1 h, and was processed for antigen retrieval
with 0.1% Triton X-100 and 0.1% sodium citrate for 2 min on ice.
The tissue was subsequently incubated with a TUNEL reaction mixture
containing terminal deoxynucleotidyl transferase in a humidified
chamber for 1 h at 37°C. Apoptosis of myocardial cells was
characterized as reduced cell volume and visible brown particles
within the nuclei. Myocardial cell apoptotic index (AI) was
determined according to the following equation: AI = (total number
of apoptotic cells/total number of cells) × 100%.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (n=3). Data were analyzed by one-way analysis of variance
followed by Student-Newman-Keuls post-hoc test for multiple
comparisons. Statistical analyses were conducted using SPSS 11.5
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Behavior and morphological
alterations
Once the rats awoke from anesthesia, they started
drinking and cleaning their fur. Rats in the CON and OMT groups
exhibited a normal manner and appearance, as evidenced by a smooth
and shiny coat and regular food intake. In addition, the rats in
these groups did not appear dispirited or restless, and
piloerection or chills were not observed. The abdominal organs also
appeared normal. Rats in the CLP group gradually appeared listless,
and exhibited reduced activity, food intake and responsiveness. In
addition, the coat of the CLP rats appeared to have lost its
luster. After 2–4 h, sickness gradually increased and the rats
appeared slumped and restless, with increased piloerection, chills,
diarrhea and eye secretions. After 9 h, their condition worsened.
Following an intraperitoneal cesarean section, there was visible
liquid turbidity in the abdominal cavity; and the cecum emitted a
foul-smelling odor, alongside swelling, necrosis, adhesion, and
jejunal bowel distension. These findings are consistent with the
literature (18), thus indicating
that a rat model of sepsis was successfully generated. Rats in the
CLP + OMT high and medium dose groups exhibited increased activity,
and reduced dispiritedness, restlessness, piloerection and chills,
as compared with the CLP group, following a laparotomy. In
addition, there was no obvious liquid abnormality in the abdominal
cavity, no cecal swelling, and no observation of gangrene and
adhesion; however, improvement was not as obvious in the CLP + OMT
low dose group.
Cardiac function assay
The CLP rat model was used to evaluate the effects
of OMT on myocardial diastolic dysfunction, which is an important
characteristic of septic shock in rats. As shown in Fig. 1, there was no difference in the
cardiac function parameters, including HR, MAP, LVESP, LVEDP,
LVdp/dtmax and -LVdp/dtmax indices, between
the OMT control group and the CON group. In the CLP group, each
index was significantly altered compared with the CON group. HR was
increased by 18%, MAP was reduced by 37%, LVESP was reduced by 27%,
LVEDP was increased by 39%, LVdp/dtmax was reduced by
38%, and -LVdp/dtmax was reduced by 37%. Following
treatment with various doses of OMT, cardiac function improved.
Compared with the CLP group, CLP + high dose OMT reduced HR by 10%
(P<0.05), increased MAP by 35% (P<0.01), increased LVESP by
28% (P<0.01), reduced LVEDP by 25% (P<0.05), increased
LVdp/dtmax by 45% (P<0.01), and increased
-LVdp/dtmax by 29% (P<0.01). CLP + medium dose OMT
reduced HR by 8% (P<0.05), increased MAP by 22% (P<0.05),
increased LVESP by 16% (P<0.05), reduced LVEDP by 20%
(P<0.01), increased LVdp/dtmax by 32% (P<0.01),
and increased-LVdp/dtmax by 23% (P<0.01). CLP + low
dose OMT reduced HR by 4% (P>0.05), increased MAP by 11%
(P<0.05), increased LVESP by 4% (P>0.05), reduced LVEDP by 7%
(P>0.05), increased LVdp/dtmax by 13% (P<0.05),
and increased -LVdp/dtmax by 10% (P<0.05).
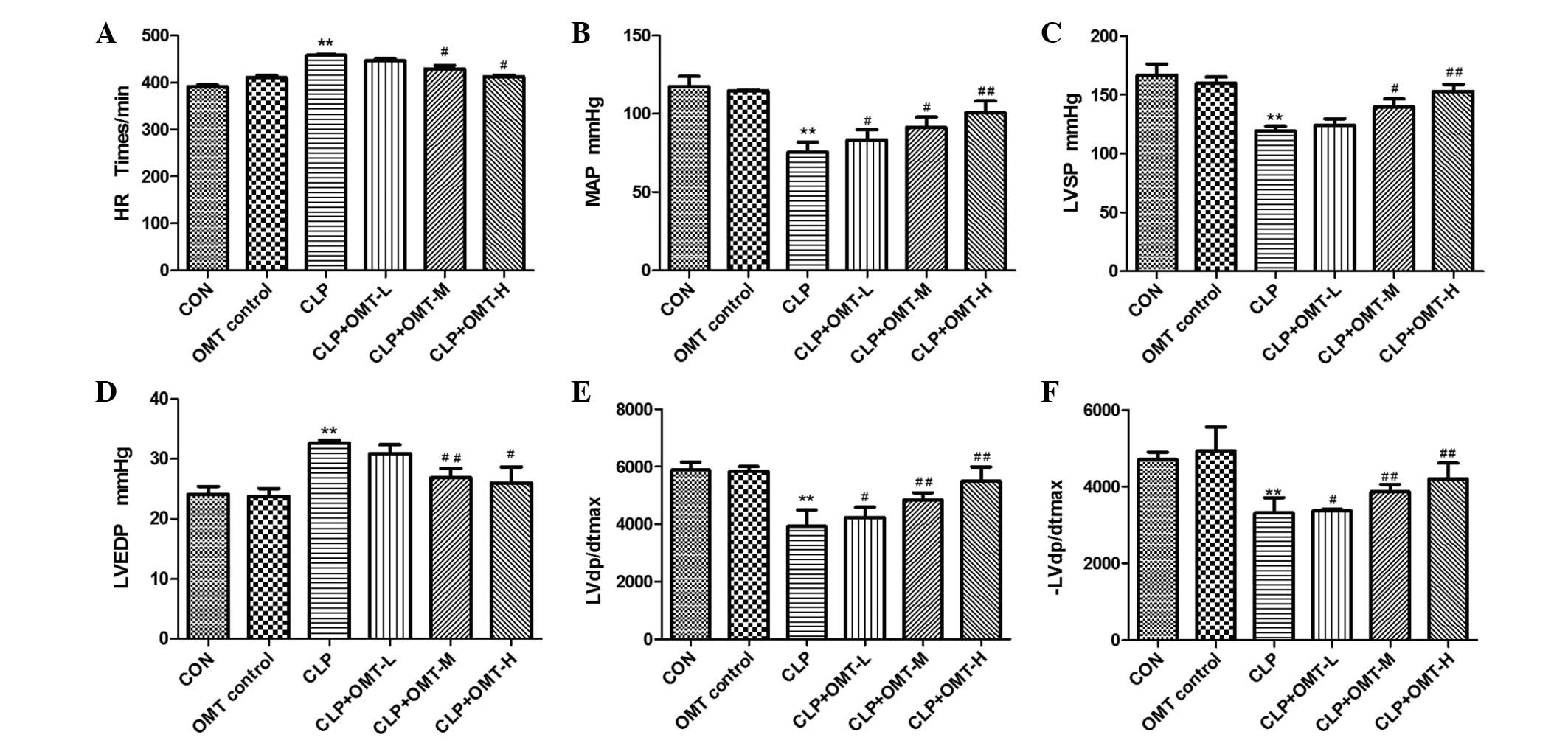 | Figure 1Effects of oxymatrine (OMT) on cardiac
function in rats with septic shock (n=8). The following indices
were measured: (A) Heart rate (HR), (B) mean arterial pressure
(MAP), (C) left ventricular end systolic pressure (LVESP), (D) left
ventricular end diastolic pressure (LVEDP), (E) left
intraventricular pressure change rate (LVdp/dtmax) and
(F) -LVdp/dtmax by cardiac catheterization in all
groups, including the control (CON), OMT control, cecal ligation
and puncture (CLP), CLP + OMT low dose (L), CLP + OMT medium dose
(M), CLP + OMT high dose (H) groups. Data are presented as the mean
± standard error of the mean. **P<0.01 compared with
the CON group; #P<0.05 and ##P<0.01
compared with the CLP group. |
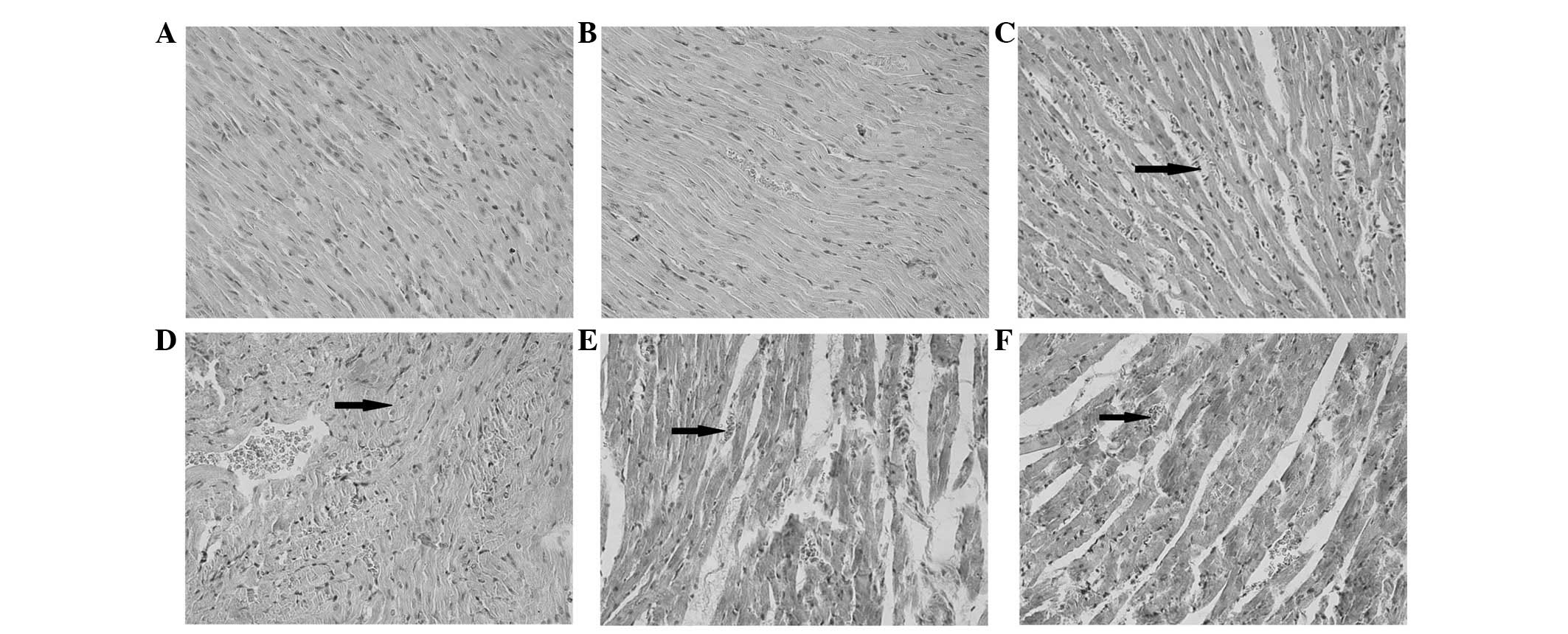
Myocardial histological analysis
Myocardial histology appeared no different between
the CON and OMT control groups. The endocardial membrane was
complete, and edema and fibrous connective tissue hyperplasia were
not detected. The myocardial stripes were clear and the nucleus was
centered, with no vasodilation or inflammatory cell infiltration
detected. Epicardial membrane integrity was intact, and was not
covered with inflammatory exudate (Fig. 2A and B). The myocardial tissue of
the CLP group exhibited an extensively disordered subendocardial
structure compared with the CON group. A wide range of inflammatory
cells infiltrated the tissue, and large numbers of mononuclear
cells, intermingled with a few lymphocytes and neutrophils were
detected. In addition, telangiectasia and bleeding were observed
(Fig. 2C). The CLP group also
displayed interstitial edema and fibroblast proliferation, and
various degrees of cell necrosis and fibrosis. Myocardial tissue
damage in the CLP + OMT low dose group was reduced to some degree;
however, the myocardial structure remained slightly disorganized,
with inflammatory cell infiltration, telangiectasia, bleeding, cell
necrosis and fibrosis all detected (Fig. 2D). Myocardial tissue damage in the
CLP + OMT medium and high dose groups was markedly reduced, and
normal basic cardiac structure was observed. Edema, degeneration
and necrosis were notably reduced; however, a small amount of
inflammatory cell infiltrate and a few exudative changes remained
(Fig. 2E and F). These results
indicate that OMT may reduce myocardial injury and exert protective
effects on cardiac structure and function in rats with septic
shock.
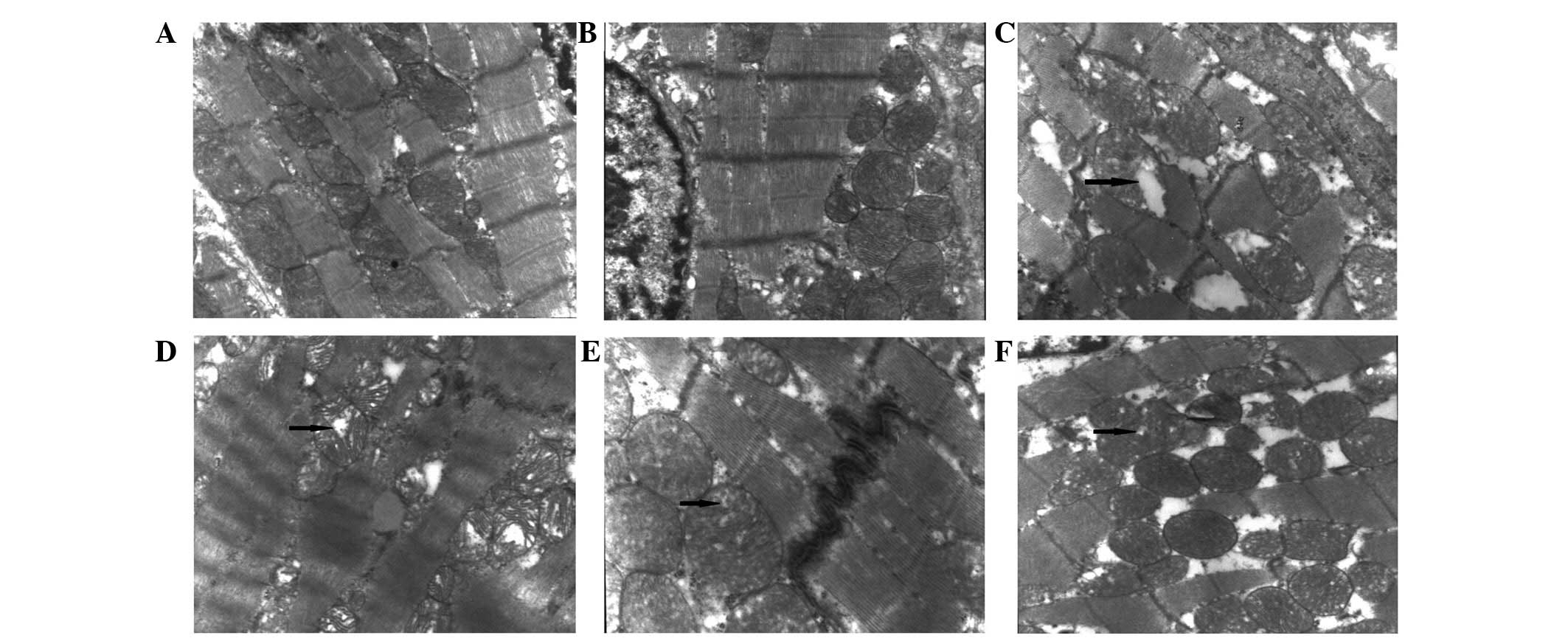
Myocardial ultramicrohistological
analysis
The myocardial tissue, including myofilaments,
sarcomeres, capillaries, mitochondria, sarcoplasmic reticulum and
nuclei, was not altered in the OMT control group compared with the
CON group, and all structures appeared normal (Fig. 3A and B). The myofilament and
sarcomere arrangement was normal, and the capillaries opened well.
In addition, mitochondrial structures were normal, and mitochondria
exhibited complete membranes, dense ridges and clear matrix. The
structure of the intercalated disks was continuous. Sarcoplasmic
reticulum was smooth and continuous. Nuclear structure was clear,
with slightly visible nuclear pyknosis and uniform chromatin.
Conversely, the ultramicrohistological structure of the CLP
myocardial tissue was markedly degraded. As shown in Fig. 3C, mitochondria appeared markedly
swollen, resulting in damaged membranes and disordered cristae.
Disordered myofilaments and sarcomeres subsequently dissolved,
leading to the generation of vacuoles. The nuclei shrunk in size
and the chromatin was marginated. The structure of the intercalated
disks was not continuous, and dissolution was observed. However,
the ultramicrohistological injuries of the myocardial tissue were
markedly improved in the CLP + OMT groups (Fig. 3D–F). The myocardial fibers were
normally arranged, and the majority of mitochondria exhibited a
complete structure with ordered dense ridges. Although the
structure of the mitochondria cristae appeared vague, arrangement
remained regular. In addition, mitochondrial swelling was reduced;
however, in some cases mitochondrial damage was not yet fully
recovered.
Effects of OMT on LBP, CD14, NF-κB (p65),
TNF-α, p38-MAPK and caspase-3 mRNA expression
The mRNA expression levels of LBP, CD14, NF-κB
(p65), TNF-α, p38-MAPK and caspase-3 were similar in the myocardial
tissue of the OMT control and CON groups. However, expression
levels were significantly increased in the CLP group (P<0.05).
Compared with the CLP group, the mRNA expression levels of LBP,
CD14, NF-κB (p65), TNF-α, p38-MAPK and caspase-3 were markedly
decreased in the CLP + OMT groups (P<0.05; Fig. 4).
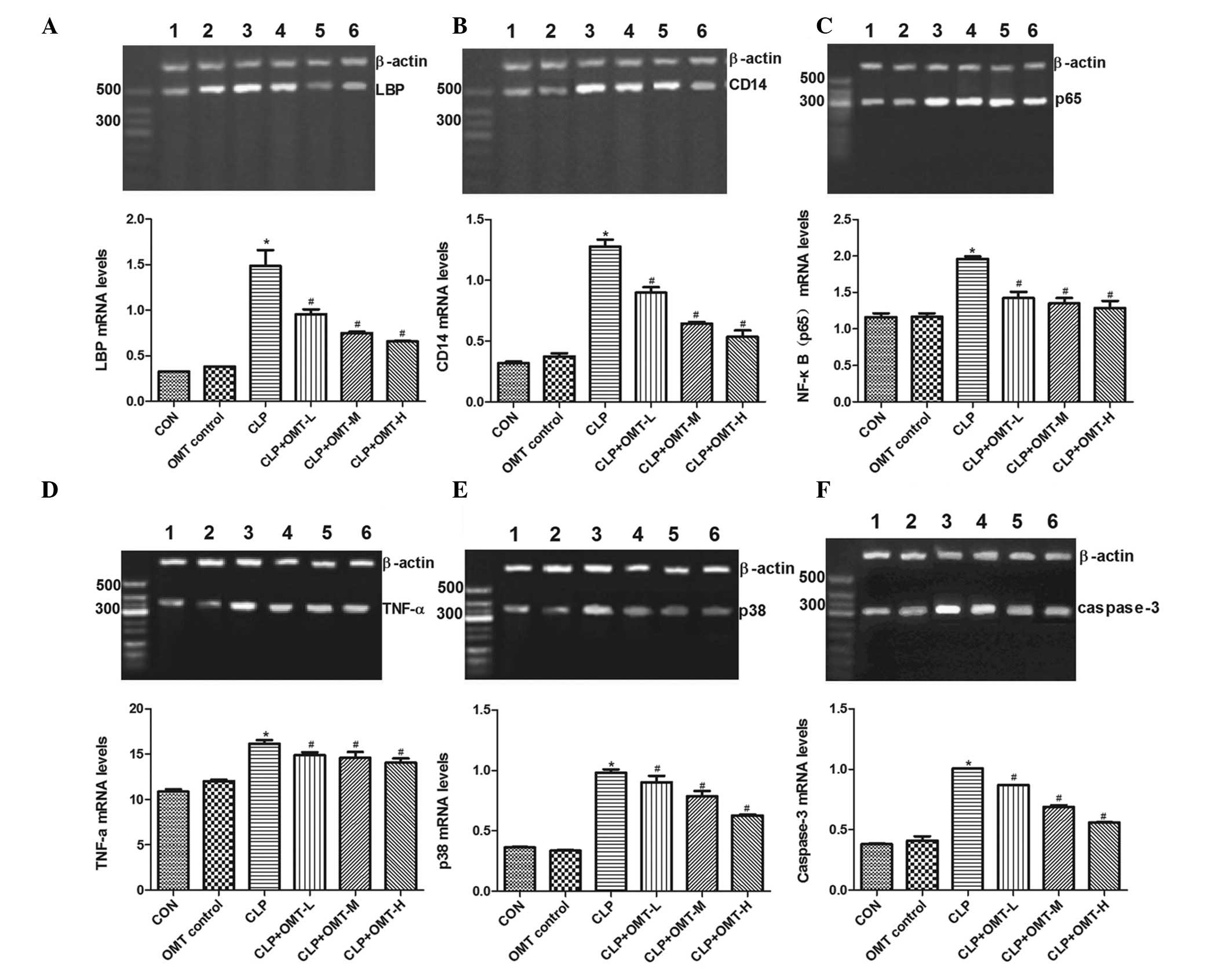 | Figure 4Effects of oxymatrine (OMT) on (A)
lipopolysaccharide binding protein (LBP), (B) cluster of
differentiation (CD)14, (C) nuclear factor-κB (p65), (D) tumor
necrosis factor (TNF)-α, (E) p38-mitogen-activated protein kinase
and (F) caspase-3 mRNA expression in rat cardiac muscle with septic
shock. Lane 1, Control (CON) group; lane 2, OMT control group; lane
3, cecal ligation and puncture (CLP) group; lane 4, CLP + OMT low
dose (L) group; lane 5, CLP + OMT medium dose (M) group; lane 6,
CLP + OMT high dose (H) group. Polymerase chain reaction results
were quantified, and bar graphs present the mRNA expression levels
relative to the internal control (β-actin). Data are presented as
the mean ± standard error of the mean. *P<0.05
compared with the CON group; #P<0.05 compared with
the CLP group. |
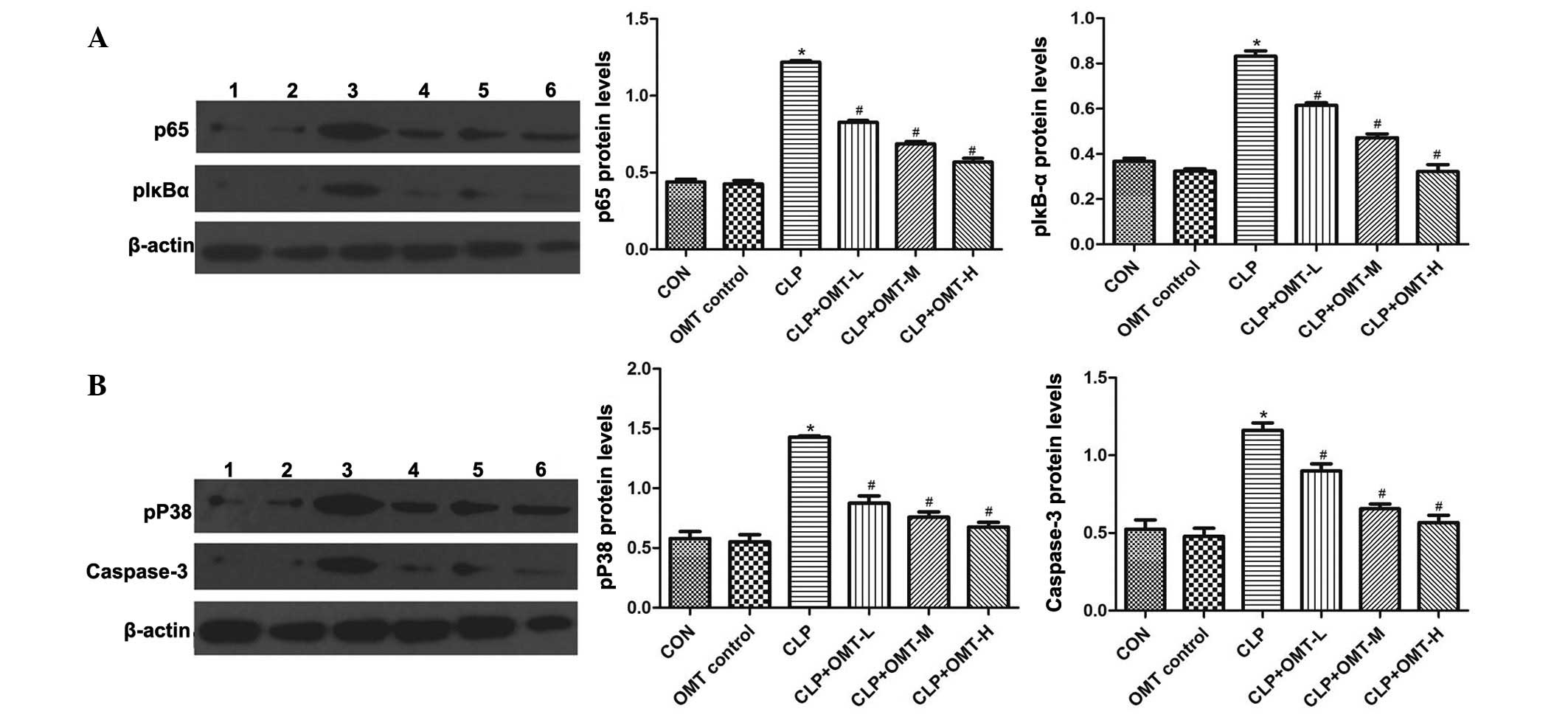
Effects of OMT on NF-κB (p65), p-IκB-α,
p-p38-MAPK and caspase-3 protein expression
Western blotting results indicated that NF-κB (p65),
p-IκB-α, p-p38-MAPK and caspase-3 protein expression levels were
similar in the myocardial tissue of the OMT control and CON groups.
However, expression levels were significantly increased in the CLP
group (P<0.05). Conversely, NF-κB (p65), p-IκB-α, p-p38-MAPK and
caspase-3 protein expression levels were markedly decreased in the
CLP + OMT groups compared with in the CLP group (P<0.05;
Fig. 5).
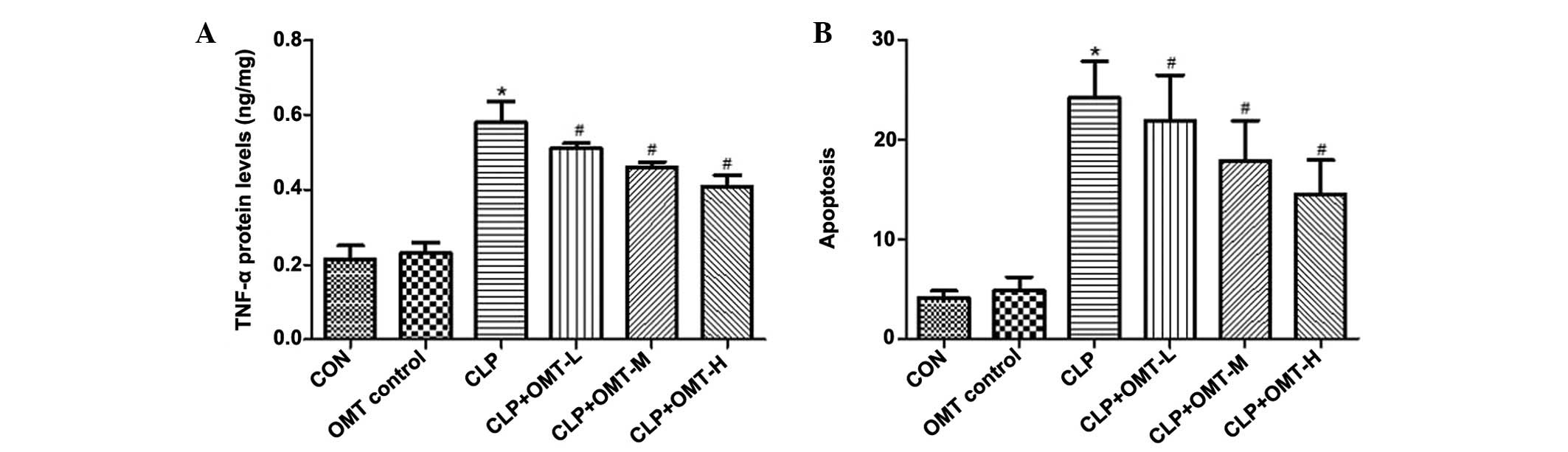
Effects of OMT on TNF-α expression
The results of a radioimmunoassay demonstrated that
TNF-α levels were significantly increased in the CLP group compared
with in the CON and OMT control groups (P<0.05). However,
compared with the CLP group, the TNF-α levels were significantly
decreased in the CLP + OMT groups (P<0.05) (Fig. 6A).
Effects of OMT on myocardial cell
apoptosis
No significant differences were detected in
myocardial cell AI between the CON and OMT control groups. Compared
with the CON group, myocardial AI was significantly increased in
the CLP group (P<0.01). Conversely, myocardial AI was
significantly reduced following treatment with various doses of OMT
compared with in the CLP group (P<0.05) (Fig. 6B).
Discussion
OMT, which is a type of alkaloid extracted from
Sophora flavescens Ait., has previously been reported to
exert positive pharmacological effects on regulation of the immune
response, reduction of hypersensitive reactions, and inhibition of
histamine release in vitro and in vivo (19–21).
Furthermore, OMT has been demonstrated to inhibit LPS-induced
inflammation by downregulating Toll-like receptor (TLR)4/NF-κB in
macrophages (22). OMT also exerts
protective effects on LPS-induced mastitis, an inflammatory
reaction of the mammary gland, the anti-inflammatory mechanism of
which has been shown to be associated with inhibition of the NF-κB
and MAPK signaling pathways (23).
However, the pharmacological effects of OMT on cardiac tissue and
the underlying mechanisms have not been well elucidated. Zhang
et al (24) demonstrated
that OMT was able to ameliorate left ventricular hypertrophy and
dysfunction in rats with heart failure. In addition, a combination
of sodium ferulate and OMT has been reported to exert protective
effects against CLP-induced lethal sepsis in mice (25). Accordingly, the present study used
the CLP-induced model of sepsis to investigate the
anti-inflammatory effects of OMT, and aimed to determine its
protective effects and possible underlying mechanisms on
sepsis-induced cardiac injury.
Sepsis is a complex pathophysiological process that
is characterized by hypotension, hypoxia, metabolic acidosis,
systemic inflammatory response and multiple organ dysfunction. The
heart is considered a potential site for the generation of
inflammatory mediators, and the generation and release of
proinflammatory mediators, such as TNF-α, and activation of the
p38-MAPK pathway has been reported to have an important role in
sepsis-induced cell apoptosis and cardiac dysfunction (26). A previous study confirmed that the
three pathways: Janus kinase/signal transducers and activators of
transcription, NF-κB and MAPK are important in the regulation of
inflammatory signal transduction (27). NF-κB is an important cell
transcription factor associated with immune and inflammatory
reactions, which has a core position in the convergence of these
three intracellular signaling pathways (28). In the resting state, NF-κB proteins
combine with the IκB family proteins, and thus exist in the
cytoplasm in their inactive form. Inactive NF-κB complex and active
NF-κB are in dynamic equilibrium between the cytoplasm and
nucleus.
When gram-negative bacteria infect the body and die
they release LPS. LPS is the main pathogenic gram-negative
bacteria-associated molecular pattern, which is recognized by a
series of receptors in the innate immune system, including LBP,
CD14 and TLR4, resulting in activation of effector cells. LPS is
known to first combine with LBP and CD14 in the circulating blood,
thus forming the LPS-LBP-CD14 complex. This complex is then
transported to the cell membrane, where it is anchored to the LPS
receptor complex, and induces dimerization of TLR4 and two
molecules of the extracellular adapter protein MD-2. The
cytoplasmic region of TLR4 then interacts with the adaptor protein
myeloid differentiation factor 88, inducing the activation of
NF-κB-inducing kinase (NIK) (29).
NIK is a serine/threonine protein kinase, which can phosphorylate
IκB kinase and induce its activation, consequently leading to IκB-α
phosphorylation. Phosphorylation of IκB-α induces IκB and NF-κB
dissociation, thus resulting in nuclear localization of NF-κB
through the nuclear pore where it binds to corresponding
DNA-specific sequences, so as to play its role in the regulation of
gene transcription. NF-κB can bind to the fixed nucleotide sequence
of subregions of numerous cytokines, and the promoters of
inflammatory mediators, thus initiating gene expression of
cytokines such as TNF-α (30).
Upregulation of TNF-α eventually activates caspase-3 and induces
myocyte apoptosis via the NF-κB signaling pathway.
The present study detected the mRNA expression
levels of LBP, CD14, NF-κB (p65), TNF-α, p38-MAPK and caspase-3,
and the protein expression levels of NF-κB (p65), p-IκB-α, p-p38
MAPK, caspase-3 and TNF-α in the myocardial tissue of rats with
CLP-induced sepsis. The expression of all of these factors was
significantly increased in the CLP group. In addition, HR and LVEDP
were markedly increased, whereas MAP, LVESP, LVdp/dtmax
and -LVdp/dtmax were decreased. These results suggested
that cardiac structure and function were markedly damaged, and the
underlying mechanism may be associated with activation of LBP and
CD14 by toxins, such as LPS, on the myocardial cell membrane.
Activation of the NF-κB (p65)/IκB-α signaling pathway may result in
increased production of TNF-α and other inflammatory mediators, and
activation of the p38-MAPK/caspase-3 pathway may induce myocardial
cell apoptosis.
Following intervention with various doses of OMT,
the mRNA expression levels of LBP, CD14, NF-κB (p65) and TNF-α were
significantly decreased in myocardial tissue compared with in the
CLP group, thus indicating that OMT exerts marked inhibitory
effects on the LBP, CD14 and NF-κB pathway in the myocardial tissue
of rats with sepsis. The underlying mechanism of these effects may
be associated with suppression of NF-κB (p65) expression and NIK
activation, thereby inhibiting the expression of proinflammatory
cytokines such as TNF-α. In addition, the mRNA expression levels of
p38-MAPK and caspase-3 mRNA were significantly decreased, which was
consistent with the decreased protein expression levels of
p-p38-MAPK and caspase-3 detected in the myocardial tissue.
Furthermore, following OMT treatment, cardiac function, myocardial
tissue structure and ultrastructural injury were all markedly
improved, and the myocardial AI was significantly reduced. These
results indicated that OMT may increase myocardial contractility
and compliance, correct increases in LVEDP caused by apoptosis of
myocardial cells, and decrease preload, thus improving myocardial
function. The underlying mechanisms of these effects may involve
inhibition of TNF-α expression and reduced TNF-α-induced myocardial
apoptosis, which is mediated by the p38-MAPK/caspase-3 pathway.
The present study is the first, to the best of our
knowledge, to demonstrate that sepsis-induced cardiac injuries are
associated with the TNF-α/p38-MAPK/caspase-3 signaling pathway, and
that OMT has the ability to suppress activation of the
p38-MAPK/caspase-3 pathway. Therefore, OMT may be considered a
potential therapeutic agent for the treatment of septic shock.
In conclusion, the present study provides valuable
information regarding the mechanisms underlying the
cardioprotective effects of OMT, thus providing a theoretical basis
for further clinical studies focusing on the treatment of
sepsis.
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of Ningxia (grant nos. NZ13068 and
NZ14057), the National Natural Science Foundation of China (grant
nos. 31260243 and 31460257), and the Ningxia Higher School
Scientific Research Project (grant no. NGY2013081). Additional
funding was provided to Dr Yin Wang by the Program for New Century
Excellent Talents in University.
References
|
1
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al Surviving Sepsis Campaign Guidelines Committee including
The Pediatric Subgroup: Surviving Sepsis Campaign: International
guidelines for management of severe sepsis and septic shock, 2012.
Intensive Care Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nowak RM, Nanayakkara P, DiSomma S, Levy
P, Schrijver E, Huyghe R, Autunno A, Sherwin RL, Divine G and Moyer
M: Noninvasive hemodynamic monitoring in emergency patients with
suspected heart failure, sepsis and stroke: The PREMIUM registry.
West J Emerg Med. 15:786–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Celes MR, Prado CM and Rossi MA: Sepsis:
Going to the heart of the matter. Pathobiology. 80:70–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang MH, Li GZ, Xu H, Zhang J and Cao J:
Effect of oxymatrine on NF-kappaB and other cell factors in rats
lung tissue with septic shock. Zhongguo Zhong Yao Za Zhi.
33:2390–2394. 2008.In Chinese.
|
|
5
|
Zhang MH, Xu H, Wang F, Yang XL, Zhang J,
Li GZ and Cao J: The preventive and therapeutic effects of
oxymatrine on lung injury in a rat model of septic shock. Ningxia
Yixueyuan Xuebao. 30:421–423. 2008.In Chinese.
|
|
6
|
Wang XY, Zhang MH, Yang ML, Jiang YD, Li
GZ, Yang XL, Xu H and Cao J: Effect of oxymatrine on JAK2/STAT3
signaling in renal tissues of rats with septic shock. Zhongguo
Zhong Yao Za Zhi. 38:2696–2700. 2013.In Chinese. PubMed/NCBI
|
|
7
|
Belperio J, Horwich T, Abraham WT, Fonarow
GC, Gorcsan J III, Bersohn MM, Singh JP, Sonel A, Lee LY, Halilovic
J, et al: Inflammatory mediators and clinical outcome in patients
with advanced heart failure receiving cardiac resynchronization
therapy. Am J Cardiol. 117:617–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pecoraro M, Del Pizzo M, Marzocco S,
Sorrentino R, Ciccarelli M, Iaccarino G, Pinto A and Popolo A:
Inflammatory mediators in a short-time mouse model of
doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol.
293:44–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moussa MD, Santonocito C, Fagnoul D,
Donadello K, Pradier O, Gaussem P, De Backer D and Vincent JL:
Evaluation of endothelial damage in sepsis-related ARDS using
circulating endothelial cells. Intensive Care Med. 41:231–238.
2015. View Article : Google Scholar
|
|
10
|
Zhang M, Wang X, Wang X, Hou X, Teng P,
Jiang Y, Zhang L, Yang X, Tian J, Li G, et al: Oxymatrine protects
against myocardial injury via inhibition of JAK2/STAT3 signaling in
rat septic shock. Mol Med Rep. 7:1293–1299. 2013.PubMed/NCBI
|
|
11
|
Drosatos K, Lymperopoulos A, Kennel PJ,
Pollak N, Schulze PC and Goldberg IJ: Pathophysiology of
sepsis-related cardiac dysfunction: Driven by inflammation, energy
mismanagement, or both? Curr Heart Fail Rep. 12:130–140. 2015.
View Article : Google Scholar :
|
|
12
|
Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M and
Liu Z: The effects of the combination of sodium ferulate and
oxymatrine on lipopolysaccharide-induced acute lung injury in mice.
Inflammation. 35:1161–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui HL, Wang YF, Li XL and Kang QX:
Clinical observation of matrine injection in the treatment of 51
cases of various types of cancers. Shanxi Yiyao Zazhi. 22:232–233.
1993.In Chinese.
|
|
14
|
Zheng P, Niu FL, Liu WZ, Shi Y and Lu LG:
Anti-inflammatory mechanism of oxymatrine in dextran sulfate
sodium-induced colitis of rats. World J Gastroenterol.
11:4912–4915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ
and Yu SQ: Attenuation of acute lung injury in mice by oxymatrine
is associated with inhibition of phosphorylated p38
mitogen-activated protein kinase. J Ethnopharmacol. 98:177–183.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Y, Zhou Y and Liu Q: Antiendotoxic
effects of Sophora alopecuroides L. Zhong Yao Cai. 29:1066–1068.
2006.In Chinese.
|
|
17
|
Jing HM: Cecal ligation puncture of rat
model of sepsis. Zhongguo Chaosheng Yixue Zazhi. 2:126–127. 1990.In
Chinese.
|
|
18
|
Zhang L, Yao J, Wang X, Li H, Liu T and
Zhao W: Poly (ADP-ribose) synthetase inhibitor has a heart
protective effect in a rat model of experimental sepsis. Int J Clin
Exp Pathol. 8:9824–9835. 2015.PubMed/NCBI
|
|
19
|
Fan DL, Zhao WJ, Wang YX, Han SY and Guo
S: Oxymatrine inhibits collagen synthesis in keloid fibroblasts via
inhibition of transforming growth factor-β1/Smad signaling pathway.
Int J Dermatol. 51:463–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Lu W, Ma Z and Li Z: Oxymatrine
attenuates bleomycin-induced pulmonary fibrosis in mice via the
inhibition of inducible nitric oxide synthase expression and the
TGF-β/Smad signaling pathway. Int J Mol Med. 29:815–822.
2012.PubMed/NCBI
|
|
21
|
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP
and Wu BY: Oxymatrine liposome attenuates hepatic fibrosis via
targeting hepatic stellate cells. World J Gastroenterol.
18:4199–4206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Yan R and Hu Y: Oxymatrine
inhibits lipopolysaccharide-induced inflammation by down-regulating
Toll-like receptor 4/nuclear factor-kappa B in macrophages. Can J
Physiol Pharmacol. 93:253–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Yin R, Cong Y, Yang Z, Zhou E, Wei
Z, Liu Z, Cao Y and Zhang N: Oxymatrine lightened the inflammatory
response of LPS-induced mastitis in mice through affecting NF-κB
and MAPKs signaling pathways. Inflammation. 37:2047–2055. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Zhang J, Liu YK, Liu J, Wang X,
Xu Q, Wang Y, Xu X and Dai G: Cardioprotective effects of
oxymatrine on isoproterenol-induced heart failure via regulation of
DDAH/ADMA metabolism pathway in rats. Eur J Pharmacol. 745:29–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu M, Wang W, Pei X, Sun S, Xu M and Liu
Z: Protective effects of the combination of sodium ferulate and
oxymatrine on cecal ligation and puncture-induced sepsis in mice.
Exp Ther Med. 7:1297–1304. 2014.PubMed/NCBI
|
|
26
|
Ailawadi S, Wang X, Gu H and Fan GC:
Pathologic function and therapeutic potential of exosomes in
cardiovascular disease. Biochim Biophys Acta. 1852:1–11. 2015.
View Article : Google Scholar
|
|
27
|
Park SY, Bae YS, Ko MJ, Lee SJ and Choi
YW: Comparison of anti-inflammatory potential of four different
dibenzocy-clooctadiene lignans in microglia; action via activation
of PKA and Nrf-2 signaling and inhibition of MAPK/STAT/NF-κB
pathways. Mol Nutr Food Res. 58:738–748. 2014. View Article : Google Scholar
|
|
28
|
Kim BH, Lee JM, Jung YG, Kim S and Kim TY:
Phytosphingosine derivatives ameliorate skin inflammation by
inhibiting NF-κB and JAK/STAT signaling in keratinocytes and mice.
J Invest Dermatol. 134:1023–1032. 2014. View Article : Google Scholar
|
|
29
|
Park OJ, Han JY, Baik JE, Jeon JH, Kang
SS, Yun CH, Oh JW, Seo HS and Han SH: Lipoteichoic acid of
Enterococcus faecalis induces the expression of chemokines via TLR2
and PAFR signaling pathways. J Leukoc Biol. 94:1275–1284. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuebler U, Zuccarella-Hackl C, Arpagaus A,
Wolf JM, Farahmand F, von Känel R, Ehlert U and Wirtz PH:
Stress-induced modulation of NF-κB activation,
inflammation-associated gene expression and cytokine levels in
blood of healthy men. Brain Behav Immun. 46:87–95. 2015. View Article : Google Scholar : PubMed/NCBI
|